
Chinese herbal medicine Jia Wei Jing Xie Yin (JWJXY) ameliorates psoriasis via suppressing the Th17 cell response
Introduction
Psoriasis is a chronic autoimmune disease that affects 2–3% of the population (1,2). This disease, which is called “bai bi” in traditional Chinese medicine (TCM), is caused by a combination of genetic and environmental factors and is known in clinical practice for its easy recurrence and persistence. Psoriasis affects the sufferers’ physical and mental health, and no definite treatment is currently available. The disease is characterized by distinctive skin symptoms, the most common being erythema covered with silvery layered scales. Although the etiology of psoriasis is not fully understood, recent studies have shown that pathological interactions between immune cells and keratinocytes, activated by a combination of genetic and environmental factors, drive the development and progression of psoriasis, in which T helper (Th)17 cells and effector cytokine interleukin-17A (IL-17A) play a core part (1,3). Th17 cell response can inhibit neutrophil apoptosis and induce chemotaxis by secreting inflammatory cytokines, which initiates and spreads inflammation and changes epidermal structure, resulting in the formation of psoriatic lesions (3,4). In one study, Rizzo et al. found that IL-17A knockout mice injected with recombinant mouse IL-23 showed little epidermal hyperplasia (5). Therefore, Th17 may be a critical part of the mechanism of action of psoriasis.
The Chinese herbal medicine Jia Wei Jing Xie Yin (JWJXY) has its origins in Jing Xie Yin, a medicine created by Wu Jun, an experienced TCM doctor (6-8). JWJXY has the effect of dispelling blood stasis and wind and eliminating dampness. In previous clinical studies, we found that JWJXY could significantly reduce the Psoriasis Area Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores in patients with psoriasis vulgaris of blood-heat syndrome characterized by bright red skin lesions, increasing or rapidly expanding new rashes, red or purple tongue, and rapid pulse and so on (8). Experimental studies using imiquimod (IMQ)-induced model mice have shown that a high-dose intervention of JWJXY significantly reduced the thickness of the left ear, decreased the infiltration of inflammatory cells in the left ear, and downregulated serum IL-17 expression (9). Following previous studies, the present study investigated the effect of JWJXY on Th17 cell expression in IMQ-induced mice so as to provide a foundation for the medical use of JWJXY in psoriasis treatment. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-967/rc).
Methods
Laboratory animals
Female BALB/c mice, 7–8 weeks old and weighing 20–22 g, were provided by Chengdu Dashuo Biotechnology Co., Ltd. (certificate No. SCXK [Sichuan] 2008-24). These animals were housed in an animal barrier system (temperature: 22±1 ℃; humidity: 55%±5%; 12-hour light-dark cycle) and were used after 1 week of housing. Nutrition and water were supplied freely after disinfection. A protocol was prepared before the study without registration. The animal experiment was approved by Medical Ethics Committee of Yunnan Provincial Hospital of Traditional Chinese Medicine (No. S2020-010), in compliance with institutional guidelines for the care and use of animals.
Drugs and reagents
The composition of JWJXY (60 kg adult dose) is as follows: buffalo horn 40 g, radix rehmanniae 30 g, lithospermum 30 g, radix scutellariae 15 g, cortex dictamni 15 g, caulis spatholobi 15 g, oldenlandia diffusa 30 g, radix rubiae 10 g, radix clematidis 10 g, rhizoma atractylodis 10 g, sophora flavescens 15 g, radix rhapontied 15 g, and tripterygium 30 g. The drug was produced at the pharmacy of Kunming Municipal Hospital of TCM. The procedure was as follows: add 10 times water, soak for 30 minutes, boil for 30 minutes, decoct 3 times, combine the decocted liquid, filter with gauze, centrifuge, evaporate with a rotary evaporator to concentrate the extract, weigh the mass, and convert 9.17 g of raw medicine into 1 g of extract of the original medicine. Based on the conventional dose of JWJXY used in the clinical treatment of adult psoriasis and the validation of previous studies, the concentration and dose of JWJXY were determined according to the conversion formula of body surface area between adult and mouse and the conversion ratio of drug dose. IMQ cream was purchased from UK 3M Health Care Limited (batch No. GTI080A, Loughborough, UK). Methotrexate tablets were purchased from Tonghua Maoxiang Pharmaceutical Co., Ltd. (batch No. 190902, Tonghua, China). APC anti-mouse IL-17A (TC11-18H10.1), fluorescein isothiocyanate (FITC) rat anti-mouse interferon gamma (IFN-γ) antibody, FITC rat anti-mouse cluster of differentiation 4 (CD4) antibody (GK1.5), 488 anti-mouse/rat/human forkhead box P3 (FOXP3) antibody (150D), and a FOXP3/Transcription Factor Staining Buffer Set were purchased from Invitrogen (Waltham, MA, USA). Anti-cluster of differentiation 11B (CD11b) and anti-CD4 antibodies were purchased from Abcam (Cambridge, UK). Phycoerythrin (PE)/Cyanine7 anti-mouse CD4 antibody (Gk1.5), PE rat anti-mouse CD4 (H129.19), APC anti-mouse CD3 (145-2C11), and PE rat anti-mouse CD45R/B220 (RA3-6B2) were purchased from BioLegend (San Diego, CA, USA). Fetal bovine serum (FBS) was purchased from BI Israel (Tel Aviv, Israel). Dulbecco’s phosphate-buffered saline (DPBS) was purchased from Gibco (USA). SYBR Premix Ex Taq II and a reverse transcription kit were purchased from Takara Biotechnology (Dalian) Co., Ltd. (Dalian, China). Trizol lysate (Buffer RZ) and an RNA extraction kit were purchased from Tiangen Biotech Co., Ltd. (Beijing, China).
IMQ-induced psoriatic BALB/c mice
Fifty female BALB/c mice, with an average body weight of 18–20 g, were randomly divided into 5 groups: a vehicle group; a low-, medium-, and high-dose JWJXY group (8, 16, and 32 g/kg, respectively); and a methotrexate-positive control group (MTX group), with 10 mice per group. All mice were housed in clean animal feeding rooms. The hair from the central area of the ear of the mouse was carefully shaved, the surface hair was removed with a mild depilatory cream, and moisturizer was applied to the surface for later use. If the hair grew out again during the test, hair removal was repeated 1–2 times. IMQ cream of 5% was evenly applied to the alopecia area at a dose of 50 mg/cm2, once a day for 6 days. The vehicle group was administered 0.4 mL of normal saline by i.g., and the dose group was administered 0.4 mL of the corresponding concentration of the drug solution by i.g., once a day for 6 days.
Hematoxylin and eosin (HE) staining
Six days after drug administration, the mice in each group were sampled, and the samples were fixed in 4% paraformaldehyde solution. Transparent tissue sections were made by embedding, sectioning, drying, dewaxing, hydrating, and HE staining. The tissue structure and inflammatory cell infiltration of the skin of the mice in each group were observed under an inverted light microscope, and images were collected with a digital camera.
Immunohistochemistry
The sections were routinely dewaxed in water, washed with phosphate buffer saline (PBS), incubated, mixed with the primary antibody, mixed with the secondary antibody, stained, dehydrated, and then mounted to make transparent sections. The infiltration of CD11b and CD4 cells in the skin of the mice in each group was observed under an inverted optical microscope, using images that had been composed with a digital camera.
Flow cytometry
Samples were acquired using a flow cytometer (FACSCanto II, BD Biosciences, Franklin Lakes, NJ, USA), and the number and proportion of the lymphocyte subsets CD3, CD4, and B220 were analyzed using FlowJo software (BD Life Sciences). The splenocytes of the mice were harvested and blocked with rat anti-mouse CD16/CD32. Cells were fluorescently characterized and incubated with related antibodies at 4 ℃ for 15 minutes. In the presence of brefeldin A, spleen cells were restimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 4 hours. After incubation, cells were composed, blocked with rat anti-mouse CD16/CD32, and stained with PE/Cyanine7 anti-mouse CD4 antibody. Cells were fixed, permeated, and stained with fluorochrome-conjugated anti-mouse IL-17A and anti-mouse IFN-γ. The samples were acquired using the flow cytometer, and CD4+IL-17A+ cells, CD4+FOX3+ cells, and CD4+IFN-γ+ cells as a percentage of CD4+ T cells were analyzed using FlowJo software.
RNA extraction and real-time quantitative PCR
Mouse skin cells were collected, and RNA was isolated using a RNeasy kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was manufactured from 1 µg of total RNA and detected by quantitative polymerase chain reaction (q-PCR) using a SYBR Premix Ex Taq II kit. The expression of IL-17A, IL-17F, IL-1β, IL-23, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), retinoic acid receptor-related orphan receptor gamma t (RORγt), and chemokine receptor 6 (CCR6) messenger RNA (mRNA) in the mouse skin cells was determined, and the relative expression amount of β-actin in each sample was calculated by applying the 2−∆∆CT approach. The primer sequences of specific genes were shown in Table 1.
Table 1
| Gene name | Primer sequences (5'-3') |
|---|---|
| Mouse β-actin | Forward: 5'-GGCTGTATTCCCCTCCATCG-3' |
| Reverse: 5'-CCAGTTGGTAACAATGCCATGT-3' | |
| Mouse IL-17A | Forward: 5'-TTTAACTCCCTTGGCGCAAAA-3' |
| Reverse: 5'-CTTTCCCTCCGCATTGACAC-3' | |
| Mouse IL-17F | Forward: 5'-CTGTTGATGTTGGGACTTGCC-3' |
| Reverse: 5'-TCACAGTGTTATCCTCCAGG-3' | |
| Mouse IFN-γ | Forward: 5'-ATGAACGCTACACACTGCATC-3' |
| Reverse: 5'-CCATCCTTTTGCCAGTTCCTC-3' | |
| Mouse RoRγt | Forward: 5'-TGTCCTGGGCTACCCTACTGA-3' |
| Reverse: 5'-CACATTACACTGCTGGCTGC-3' | |
| Mouse IL-1β | Forward: 5'-GCAACTGTTCCTGAACTCAACT-3' |
| Reverse: 5'-ATCTTTTGGGGTCCGTCAACT-3' | |
| Mouse CCR6 | Forward: 5'-CCTGGGCAACATTATGGTGGT-3' |
| Reverse: 5'-CAGAACGGTAGGGTGAGGACA-3' | |
| Mouse IL-23p19 | Forward: 5'-ATGCTGGATTGCAGAGCAGTA-3' |
| Reverse: 5'-ACGGGGCACATTATTTTTAGTCT-3' |
IL, interleukin; IFN, interferon; RoRγt, retinoic acid receptor-related orphan receptor gamma t.
Statistical methods
Statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± standard deviation (SD) of indicated experiments. A chi-square test was used to analyze the data, and a t-test was used to compare the 2 samples. P<0.05 was considered to be statistically significant.
Results
Efficacy of JWJXY on IMQ-induced psoriasis (ear thickness, HE staining)
IMQ is a toll-like receptor 7/8 (TLR7/8) agonist that induces psoriasis in mouse models with similar changes to human psoriasis, such as epidermal thickening, aberrant differentiation of keratinocytes, inflammatory cell infiltration, and associated inflammatory cytokines (10). It is generally accepted that lesions in this model are mediated principally via the IL-23/IL-17 axis (11). We established a mouse model of psoriasis induced via IMQ, in which normal saline, MTX (2 mg/kg), and low, medium, and high doses of JWJXY (8, 16, and 32 g/kg, respectively) were administered for 6 days. Ear thickness (Table 2, Figure 1A) and body weight (Table 3, Figure 1B) were measured on day 1, day 3, and day 5. The ear thickness of the mice reflected the severity of the rash. On the 5th day, the severity of the skin rashes in the medium dose JWJXY group was significantly lower than that in the vehicle group. Body weight in the MTX group decreased markedly and arrived on the top till 5th day. The variation in body weight in the vehicle group and the MTX control group was statistically significant. Other groups showed no significant change in body weight. Histopathological examination of the lesions revealed a significant decrease in pathological lesions in the medium and high dose JWJXY group and in the MTX group (Figure 1C). These results confirm that JWJXY can treat experimental psoriasis in mice.
Table 2
| Group | Dose | Day 1 thickness (mm) | Day 3 thickness (mm) | Day 5 thickness (mm) |
|---|---|---|---|---|
| Vehicle | – | 0.150±0.020 | 0.188±0.036 | 0.227±0.038 |
| MTX | 2 mg/kg | 0.153±0.023 | 0.167±0.025 | 0.201±0.024 |
| JWJXY | ||||
| Low dose | 8 g/kg | 0.150±0.026 | 0.163±0.021 | 0.201±0.027 |
| Medium dose | 16 g/kg | 0.133±0.011 | 0.155±0.023 | 0.183±0.021* |
| High dose | 32 g/kg | 0.174±0.048 | 0.170±0.020 | 0.201±0.019 |
*, P<0.05 vs. the vehicle group. IMQ, imiquimod; JWJXY, Jia Wei Jing Xie Yin; MTX, methotrexate-positive control group; SD, standard deviation.
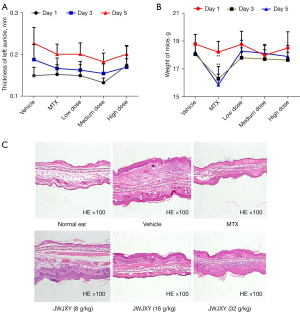
Table 3
| Group | Dose | Day 1 weight (g) | Day 3 weight (g) | Day 5 weight (g) |
|---|---|---|---|---|
| Vehicle | – | 18.780±0.696 | 18.060±0.648 | 18.210±0.666 |
| MTX | 2 mg/kg | 18.210±0.772 | 16.310±0.870** | 15.878±0.758** |
| JWJXY | ||||
| Low dose | 8 g/kg | 18.760±0.944 | 17.800±0.720 | 18.290±0.770 |
| Medium dose | 16 g/kg | 17.990±0.788 | 17.720±0.660 | 18.120±0.564 |
| High dose | 32 g/kg | 18.500±1.160 | 17.640±0.564 | 17.900±0.800 |
**, P<0.01 vs. the vehicle group. IMQ, imiquimod; JWJXY, Jia Wei Jing Xie Yin; MTX, methotrexate-positive control group; SD, standard deviation.
The severity of psoriatic skin lesions in IMQ-induced mice was improved by JWJXY. A mouse model of psoriasis induced by IMQ was established. Each group was administered either a saline vehicle, MTX (2 mg/kg), or a low, medium, or high dose of JWJXY (8, 16, and 32 g/kg, respectively) for 6 days after the day of modeling. The ear thickness (Figure 1A) and body weight (Figure 1B) were measured on day 1, day 3, and day 5. The mice were sacrificed for histopathological examination of the skin lesions (Figure 1C). Results are expressed as mean ± SD (n=10). Ear thickness compared with that of the vehicle group: P<0.05. Body weight compared with that of the vehicle group: P<0.01.
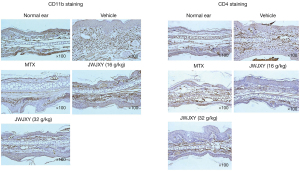
Effect of JWJXY on CD11b and CD4 cell infiltration in psoriatic skin induced by IMQ
Despite the complex pathogenesis of psoriasis, recent basic and clinical studies have indicated that psoriasis is an immune-mediated inflammatory disease. Adaptive and innate immune cells play an important role in the expansion and progression of psoriasis (1). We performed an immunohistochemical examination of the skin lesions in our psoriasis mouse model, and the results showed that medium and high doses of JWJXY could ameliorate the CD11b and CD4 cell infiltration in psoriatic skin induced by IMQ. Compared with that in the vehicle group, the skin infiltration of CD11b and CD4 cells was significantly reduced in the medium and high dose JWJXY groups (Figure 2).
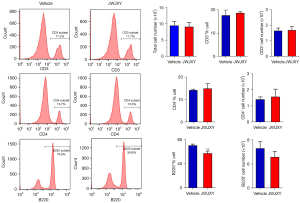
Effect of JWJXY on IMQ-induced subsets of psoriatic lymphocytes
To investigate the effect of JWJXY on spleen cell surface markers in IMQ-induced mice, the quantity of CD3, CD4, and B220 cells within spleen subsets of the mice in the JWJXY group and the vehicle group were analyzed by flow cytometry. Surface marker staining showed a noteworthy decline in the quantity of B220 cells. There was no apparent effect on the quantity of CD3+ T and CD4+ T cells in spleen leukocytes (Figure 3).
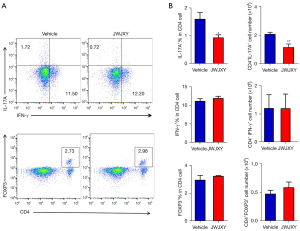
Effect of JWJXY on Th17 cell expression in IMQ-induced psoriasis
T lymphocytes play a key role in the pathogenesis of psoriasis. Naive T cells can be differentiated into pathogenic effector cells, such as Th1 and Th17, and the immunosuppressive regulatory T cells (Tregs). To further clarify whether JWJXY can regulate Th cell subsets in psoriasis, flow cytometry was used to examine the response of Th17 (IL-17A+), Th1 (IFN-γ+), and Treg (CD4+FOXP3+) in psoriatic mice. The results showed that JWJXY significantly inhibited the expression of IL-17A+ in CD4+ T cells but did not modify the proportion of IFN-γ+ or FOXP3+ in CD4+ T cells (Figure 4). Therefore, JWJXY mainly affected the expression of Th17 cells but did not affect Th1 or Treg cells.
Effect of JWJXY on Th17 cell-related gene expression in IMQ-induced psoriasis
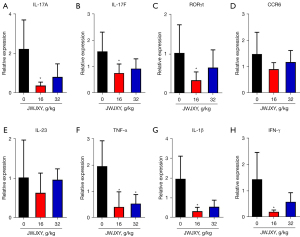
Th17 cells are Th cell subsets that secrete IL-17 and are derived from primary CD4+ T cells, IL-17 cytokines play an important role in organ-specific immune response in a variety of physiologic functions and disorders (12). Th17 cell differentiation is regulated via RORγt, the master regulator of Th17 cells (13). CCR6 is the only receptor for the chemokine CCL20, which combines to form a CCL20/CCR6 axis that induces chemotaxis of Th17 cells and is involved in the development of psoriatic lesions (14). Our study showed that the Th17 response and IL-17A production in the psoriasis mouse model were significantly inhibited by JWJXY. To further explore the effect of JWJXY on IL-17A production, we used q-PCR to determine whether JWJXY may influence the responses of Th17-linked genes within the skin cells of mice with IMQ-induced psoriasis (Figure 5). The results revealed that the transcription of IL-17A, IL-17F, RORγt, TNF-α, IL-1β, and IFN-γ was notably decreased in the JWJXY group, while the CCR6 and IL-23 response was uninfluenced.
Discussion
JWJXY has its origins in Wu Jun’s Jing Xie Yin, which has been used in clinic to treat psoriasis for many years with good effect. Modern pharmacological study has shown that buffalo horn has antipyretic, sedative, anti-inflammatory, and hemostatic effects (15). Lithospermum has anti-inflammatory, antibacterial, anti-viral, anti-tumor, anti-allergic, and anti-thrombotic pharmacological effects (16). Alkannin inhibits the proliferation of HaCaT cells mediated by IL-22 and IL-17, inhibits the secretion of inflammatory factors such as S100A7/A8 induced by IL-22 and IL-17 and chemokines such as CXCL1/2 and CCL20, and inhibits IL-17-induced vascular endothelial growth factor (VEGF) expression by blocking the Janus kinase 2/signal transducers and activators of transcription 3 (JAK2/STAT3) pathway (17-19). Oldenlandia diffusa has anti-tumor, anti-inflammatory, and antioxidant activity. In addition, it can modulate immunity and has pharmacological effects of reducing cardiac contractions (20). Tripterygium has good therapeutic effect on autoimmune diseases such as psoriasis, as it can suppress inflammation, antagonize inflammatory mediators, and influence cellular and humoral immunity (21).
IMQ-induced psoriatic lesions in BALB/c mice are mediated by the IL-23/RORγt/IL-17 axis. The lesions are characterized by increased epidermal hyperplasia, aberrant distinction, neutrophilic epidermal aggregation in microabscesses, neovascularization, and infiltration with CD4+ T cells, CD11c+ dendritic cells, and serous dendritic cells. IMQ is widely used to model psoriasis, as it induces IL-23, IL-17A, and IL-17F expression and increases Th17 cells in the spleen in a similar way to the pathogenesis of psoriasis in humans (22). In previous clinical study, we found that JWJXY had an obvious therapeutic effect on psoriasis (8). Previous experimental study has shown that JWJXY downregulated serum IL-17 expression (9). However, its specific mechanism of action needs to be elucidated.
Psoriasis has been shown to be a T-cell mediated autoimmune disease. CD4+ T cells can be divided into 4 subsets based on the cytokines produced and their role: Th1, Th2, Th17, and CD4+CD25+ Treg (23). Th cells are key factors in the diversity of the mammalian immune response. In adaptive immunity, Th cells clear infected cells by secreting unique cytokine subsets. For example, Th1 cells produce large amounts of IFN-γ to eliminate intracellular pathogens, Th17 cells produce IL-17A and IL-17F and defend the host against fungi and extracellular bacteria (24). The Th17 specific transcription factor RORγt is a Th17 marker that promotes IL-17A gene expression and induces Th17 cell differentiation (25). Th17 cells and their effector factors such as IL-17A and IL-17F mediate the progress of psoriatic lesions. The results of our animal experiment showed that JWJXY reduced the transcription of the Th17 cell-related genes IL-17A, IL-17F, RORγt, TNF-α, IL-1β, and IFN-γ in mouse spleen, which is consistent with the results of previous clinical blood tests. Immunohistochemistry showed that JWJXY decreased the infiltration of CD11b and CD4 cells in the skin of mice with IMQ-induced psoriasis. JWJXY significantly inhibited the secretion of IL-17A and the expression of IL-17A+ in CD4+ T cells in the specific immune response of the psoriasis model but did not affect the proportion of IFN-γ+ or FOXP3+ in CD4+ T cells. Therefore, JWJXY represses the response of Th17 cells in psoriasis but does not influence the responses of Th1 cells or Treg cells. These results suggest that JWJXY may downregulate the expression of IL-17A via constraining the Th17-mediated inflammatory response and limiting the activation of pro-inflammatory response and neutrophils, which may be the mechanism of its therapeutic effect.
In conclusion, this study showed that JWJXY alleviates the damage of psoriasis by inhibiting the differentiation of Th17 cells, which provides an experimental basis for continued research on the clinical application of JWJXY in the treatment of psoriasis. However, this study has some limitations. The Chinese herbal compound used in our study has a complex composition, and its functional components were not studied. In addition, the specific molecular mechanism of the treatment of psoriasis was not further studied and will be the subject of our future research.
Acknowledgments
Funding: This research was funded by a grant from the Applied Basic Research Program of Yunnan Province (No. 202001AZ070001-067). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-967/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-967/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-967/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiment was approved by Medical Ethics Committee of Yunnan Provincial Hospital of Traditional Chinese Medicine (No. S2020-010), in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sato Y, Ogawa E, Okuyama R. Role of Innate Immune Cells in Psoriasis. Int J Mol Sci 2020;21:6604. [Crossref] [PubMed]
- Gisondi P, Geat D, Pizzolato M, et al. State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis. Curr Opin Pharmacol 2019;46:90-9. [Crossref] [PubMed]
- Li B, Huang L, Lv P, et al. The role of Th17 cells in psoriasis. Immunol Res 2020;68:296-309. [Crossref] [PubMed]
- Chang HW, Yan D, Singh R, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018;6:154. [Crossref] [PubMed]
- Rizzo HL, Kagami S, Phillips KG, et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol 2011;186:1495-502. [Crossref] [PubMed]
- Yang DK, Zhang QH, Wu J. Wu Shi Jing Xie Yin treat psoriasis. New Chinese Medicine 2006;38:58.
- Guo YY, Wu J. Treatment of 30 cases of psoriasis vulgaris with Jing Xie Yin. Guangxi Journal of Traditional Chinese Medicine 2006;29:25-6.
- Yang DK, Guo YY, Wu J, et al. Curative effect observation on 60 cases of psoriasis vulgaris with blood-heat syndrome treated by Jia Wei Jing Xie Yin. Dermatology and Venereology 2019;41:631-3.
- Yang DK, Guo YY, Qin JP, et al. Therapeutic effect of Jia Wei Jing Xie Yin on imiquimod-induced psoriasis mice and the effect of IL-17. Chinese Journal of Ethnomedicine and Ethnopharmacy 2020;29(5):6-8+26.
- Sakai K, Sanders KM, Youssef MR, et al. Mouse model of imiquimod-induced psoriatic itch. Pain 2016;157:2536-43. [Crossref] [PubMed]
- Huang SW, Chen YJ, Wang ST, et al. Azithromycin impairs TLR7 signaling in dendritic cells and improves the severity of imiquimod-induced psoriasis-like skin inflammation in mice. J Dermatol Sci 2016;84:59-70. [Crossref] [PubMed]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123-32. [Crossref] [PubMed]
- Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121-33. [Crossref] [PubMed]
- Furue K, Ito T, Tsuji G, et al. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol 2020;91:e12846. [Crossref] [PubMed]
- Wu XY. Study on Antipyretic Effect and Its Components of Antelope Horn and Cornu Bubali[D]. Beijing: Peking Union Medical College, 2019.
- Zhang F, Wang WJ, Jing HL. Modern Pharmacological Research and Application in Dermatology of Purple Grass. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2020;26:168-72.
- Zhao S, Lin XF, Pan M, et al. Effects of shikonin on the proliferation of HaCaT induced by IL-22 and the expression of S100A7 and S100A8. Journal of Yangzhou University (Agricultural and Life Science Edition) 2014;35:20-3.
- Xie XR, Zhang L, Liu X, et al. Effects of shikonin on IL-17-induced proliferation of keratinocytes and expression of chemokines. Zhongguo Zhong Yao Za Zhi 2015;40:946-9. [PubMed]
- Xu Y, Xu X, Gao X, et al. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int Immunopharmacol 2014;19:327-33. [Crossref] [PubMed]
- Wei YH. Research Progress of Oldenlandia Diffusa. Shanxi Journal of Traditional Chinese Medicine 2018;34:53-6.
- Han JT. Research Progress on Pharmacological Action and Clinical Application of Tripterygium Hypoglaucum Hutch. Modern Medicine & Health 2009;25:2459-60.
- van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009;182:5836-45. [Crossref] [PubMed]
- Brzustewicz E, Bryl E. The role of cytokines in the pathogenesis of rheumatoid arthritis--Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015;76:527-36. [Crossref] [PubMed]
- Hirahara K, Aoki A, Nakayama T. Pathogenic helper T cells. Allergol Int 2021;70:169-73. [Crossref] [PubMed]
- Tang L, Yang X, Liang Y, et al. Transcription Factor Retinoid-Related Orphan Receptor γt: A Promising Target for the Treatment of Psoriasis. Front Immunol 2018;9:1210. [Crossref] [PubMed]
(English Language Editor: C. Gourlay)


