In silico analysis of polymorphisms in microRNAs that target genes affecting aerobic glycolysis
Introduction
Cancer cells preferentially utilize glucose to generate ATP via glycolysis despite the availability of oxygen, and this phenomenon is called the Warburg effect (1). This metabolic reprogramming favors the proliferative and invasive phenotypes of many cancers (2). Increased aerobic glycolysis in cancer cells provides key carbon precursors for the synthesis of nucleic acids, phospholipids, cholesterol, and porphyrins in addition to energy (3). Moreover, glycolytic intermediates such as glucose-6-phosphate, phosphoenolpyruvate, and dihydroxyacetone act as the precursors for nucleotide, amino acid, and lipid biosynthesis, respectively (4-6). A plethora of mechanisms have been proposed by various investigators to explain the Warburg effect in cancer cells including oncogene activation, loss of tumor suppressors, mitochondrial dysfunction, epigenetic changes, posttranslational modifications, and microRNA (miRNA) regulation. Interestingly, several genes involved in aerobic glycolysis are regulated by miRNAs (7,8).
miRNAs are gene regulators that enhance translational repression by binding, with imperfect base pairing, to complementary sequences in the 3'-untranslated regions (3'-UTRs) of their target mRNAs (9,10). Aberrant expression of miRNAs has been associated with breast cancer, prostate cancer, colorectal cancer, and several other human proliferative diseases (11-13). Interestingly, many cancer genes, including RAS family members, have target sequences that bind and are regulated by miRNAs (13). Several studies have suggested that examination of miRNA profiles may facilitate identifying tumor origin and predicting cancer relapse; hence, miRNAs can serve as reliable and superior prognostic indicators (14,15).
Single nucleotide polymorphisms (SNPs) have been studied widely to identify those that play major roles in influencing cancer, treatment prognosis, and survival (16-18). SNPs in miRNA binding sites add another layer of complexity to understanding cancer (19-21). In this regard, SNPs located in miRNA genes were studied and associated with cancer susceptibility (22). Recently, a polymorphism in the 3'-UTR of IFNRA1 was shown to influence hepatocellular carcinoma risk, likely through miRNA (miR)-1231-mediated regulation (23). Moreover, miRNA-disrupting polymorphisms in the 3'-UTR of BRCA1 were investigated by Pelletier et al. (24) to identify new genetic markers in breast cancer, and Landi et al. (13) reported an association between 3'-UTR polymorphisms and colorectal cancer risk. An increased risk for non-small cell lung cancer (NSCLC) was associated with an SNP in the MIRLET7 binding site in v-Ki-ras 2 Kirsten rat sarcoma viral oncogene homolog (KRAS) (21). Furthermore, cataloguing polymorphisms in miRNAs is crucial, and Iwai and Naraba (25) conducted a large comprehensive study analyzing 173 different miRNAs in 96 individuals. Polymorphisms were identified in various regions of ten different miRNAs (25).
miRNA-target interactions might also be influenced by the mutations affecting the miRNA as well (26). Mutations in pri- or pre-miRNA may influence stability or processing. Mutations in the promoter of pri-mRNA or cis or trans may influence the transcription rate of mature miRNAs (26), and mutations in the seed region of the miRNAs affect target recognition (27). Finally, copy number variation might affect copies of the miRNA (26). miRNA variations in human cancer cell lines were previously demonstrated (28). Hence, many studies suggest that SNPs in miRNAs themselves provide another additional layer of complexity in carcinogenesis, and systems biology analyses of miRNA polymorphisms may be useful in the near future (22). Recently, targeting glucose metabolism in cancer cells undergoing aerobic glycolysis was suggested as a promising therapeutic strategy. Therefore, cataloguing polymorphisms in miRNAs that target genes controlling aerobic glycolysis is crucial to understanding metabolic adaptation in cancer cells. The purpose of the present study is to computationally predict the polymorphisms in miRNAs that control genes involved in aerobic glycolysis and presumably affect metabolic survival of cancer cells. To this end, we computationally analyzed polymorphisms in miRNAs that control aerobic glycolysis. The resulting catalogue may be helpful for forming hypotheses and performing experiments to develop anti-cancer therapeutics targeting aerobic glycolysis.
Materials and methods
Selection of miRNAs that control aerobic glycolysis
miRNAs predicted to target genes directly involved in aerobic glycolysis were chosen from a recent review article and are known to be deregulated in cancer cells that undergo metabolic reprogramming for survival (7) (Table 1). We chose miR-143, miR-223, miR-320a, and miR-375 targeting hexokinase 2 (HKII), solute carrier family 2 (facilitated glucose transporter), member 4 (GLUT4), phosphofructokinase, muscle (PFKM), and pyruvate dehydrogenase kinase, isozyme 1 (PDK1), respectively. Additionally, we chose miR-133b, miR-137, miR-326, and miR-340 targeting pyruvate kinase, muscle (PKM2) and included miR-29a, which targets monocarboxylate transporter (MCT), phosphoglycerate kinase 1 (PGK1), and Enolase1, and miR-29b that targets MCT. Oncogenes and tumor suppressor genes also modulate glucose metabolism indirectly, so we also included miRNAs that control these genes: miR-96, miR-18a, miR-217 (targeting KRAS); miR-21 (targeting p53); and miR-100, miR-126, miR-155, miR-200b/c miR-210, miR-424, miR-451 and miR-34a targeting mechanistic target of rapamycin (serine/threonine kinase) (mTOR), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), hypoxia inducible factor 1, alpha subunit (HIF-1α), prolyl hydroxylase 2 (PHD2), iron-sulfur cluster assembly proteins (ISCU1/2), Cullin 2, liver kinase B1 (LKB1), and sirtuin 1 (SIRT1), respectively. In total, 26 miRNAs were included that regulate transporter genes, glycolytic enzymes, and oncogenes or tumor suppressor genes (Table 1). At least one miRNA controlling each gene is conserved according to miRNA viewer (http://people.csail.mit.edu/akiezun/microRNAviewer/) (29).
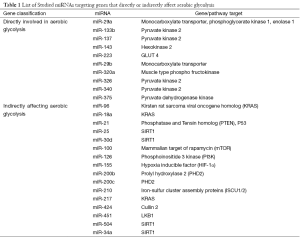
Full table
In silico prediction of SNPs occurring in miRNA genes
SNPs for the selected human miRNAs were retrieved from publically available databases: miRNASNP (http://www.bioguo.org/miRNASNP/) (30), dragon database of polymorphic regulation of miRNA genes (dPORE-miRNA) (http://cbrc.kaust.edu.sa/dpore and http://apps.sanbi.ac.za/dpore) (31), and miRNA SNiPer (http://integratomics-time.com/miRNA-SNiPer/) (27). miRNASNP provides a complete list of SNPs, including those in human pre-miRNAs and miRNA flanking sequences (30). It also provides information regarding SNPs in other species and target gain and loss by SNPs in miRNA seed regions or the 3'-UTR of target mRNAs (30). Furthermore, information about transcriptional regulation of miRNAs by SNPs was gathered from dPORE-miRNA, which focuses on SNPs that affect transcription factor binding sites (TFBS) and transcriptional regulation of miRNAs (31). miRNA SNiPer 3.0 was used to detect polymorphisms within miRNA genes (pre-miRNA, mature and seed regions) (27).
Results
We computationally analyzed the SNPs in miRNA genes that regulate aerobic glycolysis using publically available databases. Mining the miRNA SNP2.0 database revealed several SNPs in pre-miRNA flanking regions (−1 to +1 kb) and pre-miRNA regions (Table 2). MiR-126 and miR-25 had the most polymorphisms in the upstream and downstream pre-miRNA flanking regions, respectively, whereas miR-504 and miR-451 had the fewest. These miRNAs target genes that control aerobic glycolysis indirectly. MiR-504 showed the fewest polymorphisms (Table 2) and is reported to negatively regulate p53, which highlights the need for investigation of polymorphisms in pre-miRNA flanking regions and the transcriptional regulation of miRNAs.
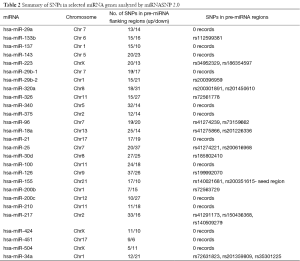
Full table
The results of our analysis for SNPs (nucleotide change and position) within miRNA genes (pre-miRNA, seed, and mature regions) by miRNA SNiPer are shown in Table 3. Both miRNASNP and miRNA SNiPer corroborate the SNPs in miRNAs with few exceptions. Additional SNPs in pre-miRNA genes were found in miR-96, miR-155, miR-25, and miR-34a by miRNASNP. We further investigated the impact of SNPs in promoter regions of miRNAs that target genes directly involved in aerobic glycolysis. RNA polymerase II is involved in the transcription of pri-miRNAs similar to protein-coding genes. The transcriptional machinery controlling miRNA biogenesis is not well understood. dPORE-miRNA provides information about the impact of SNPs in the promoter regions of human miRNA genes (intergenic as well as intragenic) and consequent transcriptional control of miRNA genes. Each miRNA gene has multiple promoters, and the promoter regions validated by the UCSC Genome browser were analyzed for SNPs. SNPs that modify TFBS or creating new TFBS in promoter regions of selected miRNA genes as analyzed by dPORE-miRNA are shown in Table 4.

Full table
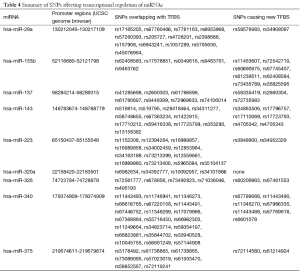
Full table
Discussion
The genetic variation that occurs within miRNA gene sequences has profound and broad biological effects beyond those of SNPs in miRNA binding sites (27). Micro RNAs have multiple targets, and SNPs can enhance, diminish, generate, or abolish binding to target sequences (27). SNPs in miRNA-binding regions of cancer genes were intensely catalogued previously (32). However, polymorphisms in miRNA genes in relation to cancer are less well investigated. Recently, several studies attempted to elucidate the Warburg effect in relation to miRNA-linked metabolic pathways including aerobic glycolysis. Genes that directly or indirectly control aerobic glycolysis and the associated miRNAs have been reviewed elsewhere (7). Interestingly, ubiquitous loss of miR-126 and increased PI3K signaling is reported in colon cancer cell lines and primary colon tumors (33). It can be observed from Table 2. miR-126 had the most polymorphisms in the pre-miRNA flanking regions. Whether these polymorphisms in pre-miRNA flanking region influence the transcriptional control of miR-126 will require further investigation.
Several studies recently investigated the impact of polymorphisms in pre-miRNA flanking regions and pre-miRNA regions. Bensen et al. (34) identified germline variation in the 5'-region proximal to pre-miRNA gene sequences and suggested the need for further studies to validate the association with breast cancer risk among African Americans and breast cancer-specific survival. Hu et al. (35) studied genetic polymorphisms in pre-miRNA flanking regions in patients with NSCLC and suggested rs928508 as a prognostic biomarker of NSCLC. Xu et al. (36) identified the rs895819 polymorphism within pre-miR-27a as influential in NSCLC patients; this SNP may also serve as a risk factor for breast cancer in younger Chinese populations (37). A study in 240 gastric cancer patients found an association between the pre-miR-30c A/G polymorphism altering mature miR-30c expression and increased risk of gastric cancer in a Chinese population (38). SNPs in coding regions may be synonymous or non-synonymous, and utilization of several databases for predicting deleterious effects of SNPs are reported (39,40). Catalogue of SNPs (nucleotide change and position) within miRNA genes (pre-miRNA, seed and mature regions and in the promoter regions is provided in the Tables 3,4.
Conclusions
In summary, the data presented herein emphasize the utility of publicly available databases with information regarding SNPs in miRNA genes. Using these databases, we comprehensively catalogued the SNPs in selected miRNA genes directly or indirectly related to the Warburg effect. These SNPs can affect regulation of miRNA biogenesis and alter miRNA levels, thereby affecting genes that directly or indirectly control aerobic glycolysis. These data may inform hypotheses and experimental design for studying the Warburg effect and miRNA SNPs in cancer. Although we did not study polymorphisms in all the miRNAs currently known to affect genes controlling aerobic glycolysis, this study provides useful information for application by the scientific community and a basis for further study.
Acknowledgements
The authors express their gratitude towards the study institutes. PS Suresh would like to thank Faculty recharge programme of UGC for their support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [PubMed]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891-9. [PubMed]
- Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 2007;39:211-22. [PubMed]
- Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell, 4th edition. New York: Garland Science; 2002.
- Berg JM, Tymoczko JL, Stryer L. Biochemistry, 5th edition. New York: W H Freeman, 2002.
- Cooper GM. The cell: a molecular approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000.
- Upadhyay M, Samal J, Kandpal M, et al. The Warburg effect: insights from the past decade. Pharmacol Ther 2013;137:318-30. [PubMed]
- Gao P, Sun L, He X, et al. MicroRNAs and the Warburg Effect: new players in an old arena. Curr Gene Ther 2012;12:285-91. [PubMed]
- Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466:835-40. [PubMed]
- Gennarino VA, D'Angelo G, Dharmalingam G, et al. Identification of microRNA-regulated gene networks by expression analysis of target genes. Genome Res 2012;22:1163-72. [PubMed]
- Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005;65:7065-70. [PubMed]
- Hulf T, Sibbritt T, Wiklund ED, et al. Discovery pipeline for epigenetically deregulated miRNAs in cancer: integration of primary miRNA transcription. BMC Genomics 2011;12:54. [PubMed]
- Landi D, Gemignani F, Barale R, et al. A catalog of polymorphisms falling in microRNA-binding regions of cancer genes. DNA Cell Biol 2008;27:35-43. [PubMed]
- Chen PS, Su JL, Hung MC. Dysregulation of microRNAs in cancer. J Biomed Sci 2012;19:90. [PubMed]
- Parpart S, Wang XW. microRNA Regulation and Its Consequences in Cancer. Curr Pathobiol Rep 2013;1:71-9. [PubMed]
- Schmidt MK, Tommiska J, Broeks A, et al. Combined effects of single nucleotide polymorphisms TP53 R72P and MDM2 SNP309, and p53 expression on survival of breast cancer patients. Breast Cancer Res 2009;11:R89. [PubMed]
- Mates IN, Jinga V, Csiki IE, et al. Single nucleotide polymorphisms in colorectal cancer: associations with tumor site and TNM stage. J Gastrointestin Liver Dis 2012;21:45-52. [PubMed]
- Nahon P, Zucman-Rossi J. Single nucleotide polymorphisms and risk of hepatocellular carcinoma in cirrhosis. J Hepatol 2012;57:663-74. [PubMed]
- Pelletier C, Weidhaas JB. MicroRNA binding site polymorphisms as biomarkers of cancer risk. Expert Rev Mol Diagn 2010;10:817-29. [PubMed]
- Slaby O, Bienertova-Vasku J, Svoboda M, et al. Genetic polymorphisms and microRNAs: new direction in molecular epidemiology of solid cancer. J Cell Mol Med 2012;16:8-21. [PubMed]
- Nicoloso MS, Sun H, Spizzo R, et al. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res 2010;70:2789-98. [PubMed]
- Kunej T, Godnic I, Horvat S, et al. Cross talk between microRNA and coding cancer genes. Cancer J 2012;18:223-31. [PubMed]
- Zhou C, Yu Q, Chen L, et al. A miR-1231 binding site polymorphism in the 3'UTR of IFNAR1 is associated with hepatocellular carcinoma susceptibility. Gene 2012;507:95-8. [PubMed]
- Pelletier C, Speed WC, Paranjape T, et al. Rare BRCA1 haplotypes including 3'UTR SNPs associated with breast cancer risk. Cell Cycle 2011;10:90-9. [PubMed]
- Iwai N, Naraba H. Polymorphisms in human pre-miRNAs. Biochem Biophys Res Commun 2005;331:1439-44. [PubMed]
- Georges M, Coppieters W, Charlier C. Polymorphic miRNA-mediated gene regulation: contribution to phenotypic variation and disease. Curr Opin Genet Dev 2007;17:166-76. [PubMed]
- Zorc M, Skok DJ, Godnic I, et al. Catalog of microRNA seed polymorphisms in vertebrates. PLoS One 2012;7:e30737. [PubMed]
- Diederichs S, Haber DA. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res 2006;66:6097-104. [PubMed]
- Kiezun A, Artzi S, Modai S, et al. miRviewer: a multispecies microRNA homologous viewer. BMC Res Notes 2012;5:92. [PubMed]
- Gong J, Tong Y, Zhang HM, et al. Genome-wide identification of SNPs in microRNA genes and the SNP effects on microRNA target binding and biogenesis. Hum Mutat 2012;33:254-63. [PubMed]
- Schmeier S, Schaefer U, MacPherson CR, et al. dPORE-miRNA: polymorphic regulation of microRNA genes. PLoS One 2011;6:e16657. [PubMed]
- Landi S, Bottari F, Gemignani F, et al. Interleukin-4 and interleukin-4 receptor polymorphisms and colorectal cancer risk. Eur J Cancer 2007;43:762-8. [PubMed]
- Guo C, Sah JF, Beard L, et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer 2008;47:939-46. [PubMed]
- Bensen JT, Tse CK, Nyante SJ, et al. Association of germline microRNA SNPs in pre-miRNA flanking region and breast cancer risk and survival: the Carolina Breast Cancer Study. Cancer Causes Control 2013;24:1099-109. [PubMed]
- Hu Z, Shu Y, Chen Y, et al. Genetic polymorphisms in the precursor MicroRNA flanking region and non-small cell lung cancer survival. Am J Respir Crit Care Med 2011;183:641-8. [PubMed]
- Xu Q, He CY, Liu JW, et al. Pre-miR-27a rs895819A/G polymorphisms in cancer: a meta-analysis. PLoS One 2013;8:e65208. [PubMed]
- Zhang N, Huo Q, Wang X, et al. A genetic variant in pre-miR-27a is associated with a reduced breast cancer risk in younger Chinese population. Gene 2013;529:125-30. [PubMed]
- Mu YP, Su XL. Polymorphism in pre-miR-30c contributes to gastric cancer risk in a Chinese population. Med Oncol 2012;29:1723-32. [PubMed]
- Suresh PS, Venkatesh T, Rajan T. Single nucleotide polymorphisms in genes that are common targets of luteotropin and luteolysin in primate corpus luteum: computational exploration. Gene 2012;511:353-7. [PubMed]
- Venkatesh T, Suresh PS. Exploration of deleterious single nucleotide polymorphisms in the components of human P bodies: an in silico approach. Gene 2013;528:360-3. [PubMed]


