Recent advances in pharmacogenomics research of anti-asthmatic drugs: a narrative review
Introduction
Bronchial asthma, a common respiratory disease in children and young adults, is characterized by hyperresponsiveness and reversible narrowing of the airways which manifest clinically as shortness of breath, cough, and/or wheezing. Airway inflammation is the pathological features of asthma, which is closely related to immune response cells, inflammation mediators, cytokines and adhesion molecules. The past 3 decades have witnessed a rapid increase in the prevalence of bronchial asthma. It is projected that approximately 400 million people worldwide will experience asthma by 2025 (1). An epidemiological study in the United States revealed that there were more than 20 million people (or 8% of the overall population) with bronchial asthma nationwide in 2016, and the cost of diagnosing and treating the disease was estimated to be 20 billion USD (2). Asthma is mainly treated medically; however, as with most medications used in disease treatment, anti-asthmatic drugs have notably different responsiveness among individual patients, which limits the clinical efficacy of the medications. A lot of elements may affect individual response to medications, including gender, age, diet, smoking, disease status, and drug interactions (3). A large proportion of such interindividual variability in drug responsiveness can be explained by genetic factors, and genetic variation across an array of genes has been revealed as associated with differences in patients’ response to anti-asthmatic drugs. Genomics has been used to study the effects of genetic variation of many genes, at the levels of both DNA and RNA. Genomics in the field of drug therapy has focused on how individual genetic differences affect interindividual variability in drug responsiveness. Pharmacogenomics is a new discipline, which offers the possibility of personalized drug selection with genetic information to improve effectiveness or avoid adverse reactions. Here, we have summarized the recent advances in the application of genomics in anti-asthmatic medications. Specifically, several genes associated with common asthma drugs were elaborated. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-291/rc).
Methods
Information used to write this paper was collected from the sources listed in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 1/1/2021 |
| Databases and other sources searched | PubMed, Index to Chiropractic Index to Chiropractic, MANTIS, ERIC (Educational Resources Information Center), AMED (Allied and Complementary Medicine Database), CINAHL (Cumulative Index to Nursing and Allied Health Literature), EMBASE/Excerpta Medica, Cochrane Database of Systematic Reviews |
| Search terms used (including MeSH and free text search terms and filters) | Bronchial asthma, genomics, single nucleotide polymorphisms, genetic variants |
| Timeframe | 1987–2020 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | None used |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Jie Li conducted the selection, consensus was obtained by all researchers discussion |
Any additional considerations, if applicable
None used
Genomics: overview and analytic methods
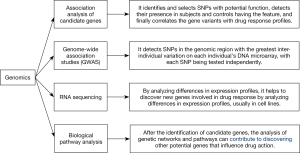
Genomics focuses on the effects of genetic variations in a group of genes. Such changes include single nucleotide polymorphism (SNP), base insertion or deletion, copy number variation (CNV), and variable number of tandem repeats (VNTR). Several of these variants influence the number, timing, and function of encoded proteins, thus affecting certain physiological and pathological processes of the organism and its response to the outside world (4). As shown in Figure 1, the commonly used analytical methods in genomics include candidate gene association, genome-wide association studies (GWAS), RNA sequencing, and biological pathway analysis. Candidate gene association analysis is the study of associations between variants in genes of interest and disease phenotypes and is commonly used to analyze alleles in patients with different drug responses. Building on the existing knowledge of the function of a specific gene, it identifies and selects SNPs with potential function, detects their presence in patients and controls having the feature, and finally correlates the gene variants with drug response profiles. The genetic association is large when the minor allele frequencies (MAF) of the SNPs are greater than 10%. The strength of candidate gene association analysis is that it needs a relatively small sample size and is simple and economical to conduct; however, it requires prior knowledge of the function of genes associated with drug response, and selection of genes can be difficult if only limited information is available (5). Meanwhile, GWAS allows the analysis of thousands of SNPs, associating them with specific phenotypes or drug responses. It typically detects SNPs in the set of genomic regions with the greatest inter-individual variation on each individual’s DNA microarray, with each SNP being tested independently. The GWAS method is characterized by its powerful statistical ability as it can process large sample sizes and detect and analyze entire genomes (6). In contrast, RNA sequencing helps to discover new genes involved in drug response by analyzing differences in expression profiles, usually in cell lines (7). Biological pathway analysis means that after the identification of candidate genes, other potential genes that influence drug action can be discovered by analyzing genetic networks and pathways (8).
Genomic association of commonly used anti-asthmatic drugs
β2-adrenergic receptor agonists, inhaled glucocorticoids (ICS), leukotriene modulators, and anticholinergics are the most commonly used medications for asthma. These drugs can be divided into 2 groups: (I) anti-inflammatory drugs, which include ICS and long-acting beta agonists (LABA); and (II) drugs for rapid relief of symptoms such as acute bronchial stenosis, chest tightness, and wheezing, including short-acting beta agonists (SABA) (9). To date, most genomic studies on asthma pharmacotherapy have focused on 3 drug classes: beta agonists, ICS, and leukotriene modulators.
β2-agonist-related gene variations
Relying on the duration of action, β2-agonists are fallen into 3 classes: SABA (e.g., fenoterol, isoprenaline, levoproterenol, and salbutamol), LABA (e.g., salmeterol and formoterol), and ultra-long-acting agonists (e.g., vilanterol and indacaterol) (10).
Discovered by Kobilka et al. in 1987, the adrenoreceptor beta 2 (ADRB2) gene and is localized to chromosome 5q31-q32, an area linked with asthma- associated phenotypes (11). More than 80 SNPs have been identified for ADRB2, the most common being Arg16Gly (rs1042713) and Glu27Gln (rs1042714). The approximated frequency of the Arg16 variant is 39.3% in whites, 49.2% in blacks, and 51.0% in Han Chinese (12). It has been shown that homozygotes of Arg16 have a greater bronchodilating effect of salbutamol compared to homozygotes of Gly16, with a significant increase in forced expiratory volume 1 (FEV1) after drug administration. However, the decline in FEV1 was also faster in individuals with Arg16 genotype after LABA use, and several patients who received the treatment of salmeterol even suffered from severe asthma exacerbations (13). Children who are homozygous for Arg16 have poor outcomes while receiving the treatment with LABA and ICS, and therefore montelukast has been recommended as an alternative to salmeterol as customized second-line asthma controller therapy in asthmatic kids. Other uncommon nonsynonymous coding variants of ADRB2 have been disclosed. For instance, the SNP rs1800888 encodes a threonine on Thr164Ile; compared with carriers of wild-type Thr164, individuals homozygous for Ile164 are 3- to 4-fold less responsive to LABA (14).
The adenylyl cyclase type 9 (ADCY9) gene is part of the signaling pathway of the β2-adrenergic receptor (β2-AR). Slob et al. found that the SNP Ile772Met (rs2230739 in ADCY9) was related to acute bronchodilation to SABA in asthmatic patients and also with changes in lung function in response to ICS (15). Arginases, which are encoded by ARG1 and ARG2, are metabolized in vivo into L-arginine, which in turn generates nitric oxide (NO) in the presence of nitric oxide synthase (NOS), and NO is an endogenous bronchodilator. Ziyab et al. found that ARG1 polymorphisms (rs2781659 and rs2781667) were related to acute SABA-induced bronchodilation in asthmatic patients (16). A Dutch asthma population-based cohort study demonstrated that 2 polymorphisms in ARG2 (rs17249437 and rs3742879) were related to asthma and more serious airway obstruction (17). The bioactivity of NO is mediated through the formation of S-nitrosothiols (SNOs), whereas S-nitrosoglutathione reductase (GSNOR) metabolizes SNO. A recent sequencing study of the GSNOR gene in the United States identified 13 SNPs, with an allele frequency of >5%. The authors demonstrated an interaction between GSNOR and ADRB2 in Mexicans, which was believed to be associated with decreased bronchial responsiveness to bronchodilators (18). Through GWAS, Kabesch et al. identified 4 asthma-associated SNPs (rs350729, rs1840321, rs1384918, and rs1319797) in the spermatogenesis associated serine rich 2 like (SPATS2L) gene on chromosome 2, which may be associated with β2-adrenergic receptor downregulation (19).
ICS-related gene variations
The earliest studies on glucocorticoid responsiveness were focused on the glucocorticoid receptor gene nuclear receptor subfamily 3, group C, member 1 (NR3C1), which is located on chromosome 5q31. It has been shown that 2 SNPs of this gene have a potential impact on glucocorticoid responsiveness, one of which, Asn363Ser (rs56149945 in NR3C1), has been recognized in some populations, and lymphocytes of individuals carrying this genetic variant have a higher sensitivity to dexamethasone compared to non-carriers (20).
The corticotropin releasing hormone receptor 1 (CRHR1) gene encodes the main receptor for corticotropin-releasing hormone and is a core regulator of corticosteroid synthesis and catecholamine generation. Rijavec et al. observed a great association correlation between improved pulmonary function after ICS treatment and CRHR1 SNPs (rs1876828, rs242939, and rs242941), and individuals homozygous for this polymorphism had significantly higher mean FEV1 than other patients (21). A low-affinity receptor for immunoglobulin E (IgE), a core molecule for B-cell stimulation is encoded by the Fc epsilon receptor 2 (FCER2) gene. It was observed that the SNP rs28364072 of FCER2 is related to a growing risk of re-exacerbation after ICS treatment in asthmatic children, who also had significantly higher serum IgE levels, possibly by a mechanism in which FCER2 variants adversely affect the normal negative feedback mechanism on IgE synthesis (22). The stress stimulated phosphoprotein 1 (STIP1) gene encodes a heat shock protein, which is essential for assembling and activating of the glucocorticoid receptor. It was shown that SNPs (rs6591838, rs2236647, and rs1011219) in STIP1 are greatly related to improved FEV1 responses in asthmatic patients with reduced lung function after 4 weeks of glucocorticoid treatment (23). Weitzel et al. performed RNAseq analysis of the transcriptome of 4 classes of human airway smooth muscle (ASM) cells and identified cysteine rich secretory protein LCCL domain with 2 (CRISPLD2), encoding a secreted protein associated with lung growth and endotoxin control (24). The CRISPLD2 gene was found to have an SNP associated with ICS resistance in asthmatic patients. Reverse transcription polymerase chain reaction (RT-PCR) and western blotting further displayed that dexamethasone treatment grew the expression of CRISPLD2 messenger RNA (mRNA) and protein levels in ASM cells, and functional researches confirmed that CRISPLD2 could regulate the anti-inflammatory roles of glucocorticoids in ASM (25). Another candidate gene associated with ICS treatment response is T-box transcription factor 21 (TBX21). Mice with a targeted deletion of the TBX21 gene rapidly exhibited airway hyperresponsiveness, increased airway eosinophilia, and accelerated airway remodeling processes. The SNP rs2240017 of TBX21 was related to improved bronchoprotection (26). Hernandez-Pacheco et al. conducted a cohort study and found that patients heterozygous for rs2240017 had significantly lower airway hyperresponsiveness during ICS treatment compared to those homozygous for this SNP (27).
Leukotriene modulator-related gene variations
Leukotriene modulators have potent anti-inflammatory activity and can improve the clinical course of asthma with minimal side effects. Depending on their mechanism of action, they are divided into 2 classes: cysteinyl leukotriene receptor antagonists (e.g., montelukast, zafirlukast, pranlukast, and tomelukast) and 5-lipoxygenase inhibiting agents (e.g., zileuton).
To date, the vast majority of pharmacogenetic studies on leukotriene modulators have focused on the variants of 5-LOX gene (ALOX5) and LTC4 synthase (LTC4S). Located on chromosome 10q11.12, the ALOX5 gene has 14 exons. Its activity is related to many repetitive sequences in the promoter area Sp1/Erg1. Mutant ALOX5 repeat polymorphism has been related to declined exacerbation rates in montelukast-treated asthma patients. Another study in Spain showed a reduced number of acute asthma exacerbations and increased FEV1 in patients with wild-type alleles or heterozygotes; in addition, these patients had increased urinary leukotriene E4 concentrations, reflecting increased leukotriene biosynthesis (28). Candidate gene analysis suggested that other ALOX5 SNPs (rs2115819, rs4987105, and rs4986832) might also affect the response to montelukast (29). The leukotriene C4 synthase gene (LTC4S) is one of to the S-glutathione synthase family, catalyzing the transformation of LTA4 to LTC4. The most significant SNP identified so far is rs730012, which is associated with increased generation of LTC4 in eosinophils (30). Pham et al. found a 73% reduction in the risk of acute asthma exacerbations in montelukast-treated patients homozygous for rs730012 (31). The ATP binding cassette C1 (ABCC1) gene, which encodes multi-drug resistance protein 1 (MRP1) and exerts a significant effect on the transmembrane transport of LTC4, has also been studied. A polymorphism of this gene (rs119774 in LTC4) was associated with the montelukast treatment response, and individuals heterozygous for rs119774 had 24% elevated FEV1 compared to those homozygous for this polymorphism (32). Meanwhile, LTA4 hydrolase acts to convert LTA4 to LTB4, and the gene encoding it is located on chromosome 12q22. A polymorphism of this gene (rs2660845 in LTA4) is related to with the risk of acute asthma exacerbations during montelukast treatment. Individuals heterozygous for rs2660845 have a 4-fold higher risk of acute asthma exacerbations than the homozygous individuals (33). The mechanism may be that this SNP lowers LTA4 hydrolase activity, leading to a decrease in LTB4 synthesis, which stimulates the LTC4-synthesis pathway to promote the synthesis of cysteinyl leukotriene. The solute carrier organic anion transporter family member 2B1 (SLCO2B1) gene encodes protein 2B1, which exerts a significant effect on the active transport of organic anions by the intestinal wall. rs12422149 is associated with the transport and serum level of montelukast, and individuals with rs12422149 had 39% lower serum level of montelukast than controls (34). A summary of the asthma drug treatment response-related genes is shown in Table 2.
Table 2
| Class | Gene name |
|---|---|
| β2-receptor agonists | ADRB2, Arg16, ADCY9, ARG1, ARG2, GSNOR, and SPATS2L |
| Inhaled glucocorticoids (ICS) | NR3C1, CRHR1, FCER2, STIP1, CRISPLD2, and TBX21 |
| Leukotriene modifiers | ALOX5, LTC4S, ABCC1, and SLCO2B1 |
Future prospects
Many pharmacogenomic studies conducted so far have had limitations including small sample scale, inaccurate phenotype definition, unreasonable population stratification, and shortage of reproducibility, which need to be addressed in future studies. High-throughput techniques have made large-size genotyping and expression studies possible in recent years. In addition, gene-environment interactions, mutual effects between variants in various genes and genetic pathways, epigenetic regulation, and transcriptional regulation of small interfering RNAs (siRNAs) and long-stranded non-coding RNAs (lncRNAs) are also topics for future pharmacogenomics studies. For instance, DNA methylation is an epigenetic alternation in which the addition of methyl to the cytosine residues of cytosine- and guanine-rich (CpG islands) DNA fragments within gene promoters stops the binding of transcription elements, which leads to downregulation of gene expression and may affect disease susceptibility (35). Interferon (IFN) gene promoter hypermethylation and interleukin-4 (IL-4) promoter hypomethylation have been revealed as related to elevated airway IgE levels in asthmatic patients, and DNA methylation of the 5-LO promoter regulates the expressions of key genes in the leukotriene pathway (36).
Most current studies have focused on the effect of single gene polymorphisms on drug efficacy, but the pharmacogenomics of asthma is inherently complex, with each factor having a small effect on drug responsiveness, and no single locus has yet been able to predict the variability in drug responsiveness. Therefore, developing statistical models to predict treatment responsiveness based on multiple genetic loci is warranted. Integrative genomics approaches that combine genome-wide SNP data with gene expression profiles will also be useful tools for recognizing new genes or mechanisms that leading to inter-individual modifiability in drug reaction. In recent years, great strides have been made in human genome analysis technologies and international information sharing networks. Large whole-genome sequencing projects, such as the NHLBI Exome Sequencing Project, 1000 Genomes, and gene sequencing projects in African ancestral populations, have achieved excellent results and created databases of rare genetic variants that could serve pharmacogenetic studies in different racial and ethnic groups in the future.
Summary
Although the etiology of asthma is still not fully elucidated, genetic factors have been demonstrated to play key important roles. Response to anti-asthmatic drug therapy varies widely among patients, and some patients may even experience life-threatening adverse drug reactions. Genomic approaches can screen for genetic variants associated with drug response. Stratifying patients prior to treatment helps to optimize drug selection, maximize the effectiveness of individual treatment, and minimize the risk of adverse reactions. Genomics can also offer new visions to the mechanisms of drug action and facilitate the growth of novel therapeutic options in the future.
Acknowledgments
Funding: This work was supported by the Project of Shenzhen Basic Research Plan (No. JCYJ20210324114205014) and the Key Laboratory of Shenzhen Respiratory Disease (No. ZDSYS201504301616234).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-291/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-291/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol 2020;42:5-15. [Crossref] [PubMed]
- Leong AB, Ramsey CD, Celedón JC. The challenge of asthma in minority populations. Clin Rev Allergy Immunol 2012;43:156-83. [Crossref] [PubMed]
- Russell RJ, Brightling C. Pathogenesis of asthma: implications for precision medicine. Clin Sci (Lond) 2017;131:1723-35. [Crossref] [PubMed]
- Davis JS, Weiss ST, Tantisira KG. Asthma Pharmacogenomics: 2015 Update. Curr Allergy Asthma Rep 2015;15:42. [Crossref] [PubMed]
- Isidoro-García M, Sánchez-Martín A, García-Sánchez A, et al. Pharmacogenetics and the treatment of asthma. Pharmacogenomics 2017;18:1271-80. [Crossref] [PubMed]
- Kim KW, Ober C. Lessons Learned From GWAS of Asthma. Allergy Asthma Immunol Res 2019;11:170-87. [Crossref] [PubMed]
- Martin A, Downing J, Maden M, et al. An assessment of the impact of pharmacogenomics on health disparities: a systematic literature review. Pharmacogenomics 2017;18:1541-50. [Crossref] [PubMed]
- Kalinin AA, Higgins GA, Reamaroon N, et al. Deep learning in pharmacogenomics: from gene regulation to patient stratification. Pharmacogenomics 2018;19:629-50. [Crossref] [PubMed]
- Boulet LP, O'Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med 2015;372:641-8. [Crossref] [PubMed]
- Wade A, Chang C. Evaluation and treatment of critical asthma syndrome in children. Clin Rev Allergy Immunol 2015;48:66-83. [Crossref] [PubMed]
- Kobilka BK, Frielle T, Dohlman HG, et al. Delineation of the intronless nature of the genes for the human and hamster beta 2-adrenergic receptor and their putative promoter regions. J Biol Chem 1987;15:7321-7. [Crossref] [PubMed]
- Sood N, Connolly JJ, Mentch FD, et al. Leveraging electronic health records to assess the role of ADRB2 single nucleotide polymorphisms in predicting exacerbation frequency in asthma patients. Pharmacogenet Genomics 2018;28:256-9. [Crossref] [PubMed]
- Turner S, Francis B, Vijverberg S, et al. Childhood asthma exacerbations and the Arg16 beta2-receptor polymorphism: A meta-analysis stratified by treatment. J Allergy Clin Immunol 2016;138:107-113.e5. [Crossref] [PubMed]
- Shah NJ, Vinod Kumar S, Gurusamy U, et al. Effect of ADRB2 (adrenergic receptor beta2) gene polymorphisms on the occurrence of asthma and on the response to nebulized salbutamol in South Indian patients with bronchial asthma. J Asthma 2015;52:755-62. [Crossref] [PubMed]
- Slob EMA, Vijverberg SJH, Palmer CNA, et al. Pharmacogenetics of inhaled long-acting beta2-agonists in asthma: A systematic review. Pediatr Allergy Immunol 2018;29:705-14. [Crossref] [PubMed]
- Ziyab AH, Mukherjee N, Kurukulaaratchy RJ, et al. Leptin receptor gene polymorphisms and sex modify the association between acetaminophen use and asthma among young adults: results from two observational studies. Respir Res 2018;19:179. [Crossref] [PubMed]
- Mohamed-Hussein AAR, Sayed SS, Eldien HMS, et al. Beta 2 Adrenergic Receptor Genetic Polymorphisms in Bronchial Asthma: Relationship to Disease Risk, Severity, and Treatment Response. Lung 2018;196:673-80. [Crossref] [PubMed]
- Chiang CH, Chuang CH, Liu SL, et al. Genetic polymorphism of transforming growth factor beta1 and tumor necrosis factor alpha is associated with asthma and modulates the severity of asthma. Respir Care 2013;58:1343-50. [Crossref] [PubMed]
- Kabesch M, Tost J. Recent findings in the genetics and epigenetics of asthma and allergy. Semin Immunopathol 2020;42:43-60. [Crossref] [PubMed]
- Farzan N, Vijverberg SJ, Arets HG, et al. Pharmacogenomics of inhaled corticosteroids and leukotriene modifiers: a systematic review. Clin Exp Allergy 2017;47:271-93. [Crossref] [PubMed]
- Rijavec M, Žavbi M, Lopert A, et al. GLCCI1 Polymorphism rs37973 and Response to Treatment of Asthma With Inhaled Corticosteroids. J Investig Allergol Clin Immunol 2018;28:165-71. [Crossref] [PubMed]
- Keskin O, Uluca Ü, Birben E, et al. Genetic associations of the response to inhaled corticosteroids in children during an asthma exacerbation. Pediatr Allergy Immunol 2016;27:507-13. [Crossref] [PubMed]
- Fang Y, Ren X, Feng Z. Genetic correlation of SOCS3 polymorphisms with infantile asthma: an evidence based on a case-control study. Int J Clin Exp Pathol 2015;8:9586-91. [PubMed]
- Weitzel KW, Aquilante CL, Johnson S, et al. Educational strategies to enable expansion of pharmacogenomics-based care. Am J Health Syst Pharm 2016;73:1986-98. [Crossref] [PubMed]
- Xie X, Shi X, Chen P, et al. Associations of TIM-1 Genetic Polymorphisms with Asthma: A Meta-analysis. Lung 2017;195:353-60. [Crossref] [PubMed]
- Albertson TE, Schivo M, Gidwani N, et al. Pharmacotherapy of critical asthma syndrome: current and emerging therapies. Clin Rev Allergy Immunol 2015;48:7-30. [Crossref] [PubMed]
- Hernandez-Pacheco N, Farzan N, Francis B, et al. Genome-wide association study of inhaled corticosteroid response in admixed children with asthma. Clin Exp Allergy 2019;49:789-98. [Crossref] [PubMed]
- Thompson MD, Capra V, Clunes MT, et al. Cysteinyl Leukotrienes Pathway Genes, Atopic Asthma and Drug Response: From Population Isolates to Large Genome-Wide Association Studies. Front Pharmacol 2016;7:299. [Crossref] [PubMed]
- Grzeskowiak LE, Grieger JA, Clifton VL. Strategies towards improving pharmacological management of asthma during pregnancy. Pharmacol Res 2018;130:85-92. [Crossref] [PubMed]
- Zheng Y, Wang H, Luo L, et al. A meta-analysis of the association between CTLA-4 genetic polymorphism and susceptibility of asthma. Medicine (Baltimore) 2018;97:e11380. [Crossref] [PubMed]
- Pham DL, Kim SH, Losol P, et al. Association of autophagy related gene polymorphisms with neutrophilic airway inflammation in adult asthma. Korean J Intern Med 2016;31:375-85. [Crossref] [PubMed]
- Berenguer AG, Fernandes AT, Oliveira S, et al. Genetic polymorphisms and asthma: findings from a case-control study in the Madeira island population. Biol Res 2014;47:40. [Crossref] [PubMed]
- Alghobashy AA, Elsharawy SA, Alkholy UM, et al. B2 adrenergic receptor gene polymorphism effect on childhood asthma severity and response to treatment. Pediatr Res 2018;83:597-605. [Crossref] [PubMed]
- Tizaoui K, Berraies A, Hamdi B, et al. Association of vitamin D receptor gene polymorphisms with asthma risk: systematic review and updated meta-analysis of case-control studies. Lung 2014;192:955-65. [Crossref] [PubMed]
- Park HW, Weiss ST. Understanding the Molecular Mechanisms of Asthma through Transcriptomics. Allergy Asthma Immunol Res 2020;12:399-411. [Crossref] [PubMed]
- Hur GY, Broide DH. Genes and Pathways Regulating Decline in Lung Function and Airway Remodeling in Asthma. Allergy Asthma Immunol Res 2019;11:604-21. [Crossref] [PubMed]