
A new visual quantitative assessment of ultrasound attenuation parameters for the mild liver steatosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is becoming a worldwide epidemic, with an increasing number of obese people and patients with associated metabolic syndrome due to the prevalence of high-fat and high-calorie diets (1,2). NAFLD patients with chronic hepatitis B (CHB) may have a higher hepatocellular carcinoma (HCC) risk (3,4). The prevalence of CHB combined with fatty liver in China is also gradually increasing. Invasive liver biopsy remains the gold standard for evaluating liver steatosis. However, due to sampling errors, a relatively high cost, poor repeatability, and complications of bleeding and pain, it is not suitable for large-scale screening and regular follow-up evaluation of NAFLD. Although conventional ultrasound, as an economic method free of radiation-damage, is widely used for the qualitative diagnosis of NAFLD, it cannot identify liver steatosis less than 30% (5,6). Determining a convenient method for the early detection and quantitative follow-up of liver steatosis was the focus of this research. Controlled attenuation parameter (CAP) has been applied in the quantitative assessment of hepatic steatosis using attenuation imaging, but it was performed without the guidance of the grey scale sonogram, and it was difficult to complete the measurement free of focal liver lesions (FLLs) in real time, and it may take more time to become an experienced sonographer, and maybe it will take a longer time for the measurement. In the present study, we used a new visual method with the real-time guidance of the gray scale sonogram for the hepatic steatosis quantitative analysis, namely LiSA, which is based on similar physical principles to CAP. This is the first study comparing the diagnostic efficiency of LiSA versus CAP in the quantitative assessment of liver steatosis over 5% in CHB patients. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-989/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ruijin Hospital (2017-72). Written informed consent was obtained from all patients.
General information
A total of 304 CHB patients who undertook liver biopsy in our hospital between November 2018 and December 2019 were reviewed retrospectively. Finally, 151 patients were included in the analysis according to the exclusion criteria. The exclusion criteria were as follows: (I) patients did not undergo both measurement of CAP and LiSA; (II) patients whose serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were 5 times the upper limit of the normal value; (III) patients with hepatitis C, hepatitis E, drug-induced liver injury, hepatolenticular degeneration, and autoimmune liver disease; (IV) patients who consumed more than 140 g/week alcohol for men and >70 g/week for women in the previous 12 months; (V) patients with distance between the skin and the liver membrane >25 mm or body mass index (BMI) ≥28 kg/m2; and (VI) patients on total parenteral nutrition (TPN) and those with liver transplantation, other end-stage diseases, or malignant tumors. The 151 patients were divided into 2 groups according to the degree of liver steatosis confirmed by histopathology: an S0 group (n=93) with fat content <5% and an S1 group (n=58) with fat content ≥5%. All 151 patients (89 males and 62 females; mean age 43.5±12.3 years) in both groups underwent LiSA and CAP examinations simultaneously.
Instruments
LiSA was measured quantitatively by the Hepatus platform using the Mindray ultrasound system (Mindray, probe LFP5-1U/s, China), and CAP was measured using FibroScan502 (Echosens, probe M, France). The obtained values were both expressed as dB/m.
Patient position and preparation before examination
According to the recommendation of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) (7) for FibroScan, the patient was advised to fast for more than 2 h before examination, lie on the examination table calmly, raise the right arm over the head to fully expose the intercostal space, lay down the left arm naturally, and breathe calmly during examination.
The process of quantitative measurement
(I) CAP was performed as follows: coupling gel was applied to the probe, which was kept perpendicular to the skin surface of the intercostal space with light pressure, keeping the probe indicator green. The distributed intensity of the M waveform on the display map was uniform, and the A waveform was linear. A success rate ≥80% (the number of successful detection times/total detection times) was defined as effective measurement. Each patient underwent at least 10 measurements, and the interquartile range (IQR) of 10 times was less than 40 dB/m. The median of 10 valid measurements was used as the final result and recorded as the CAP value. (II) For LiSA measurement: LiSA was performed by selecting an appropriate intercostal space avoiding the biliary tract, large blood vessels, and focal lesions in the liver. Other detection requirements were similar to those for CAP. A success rate ≥80% was defined as effective measurement. Each patient underwent at least 10 successful measurements, and the median of 10 valid measurements was used as the final result and recorded as the LiSA value (keeping IQR <40 dB/m). During the examination, the patient was advised to breathe calmly and hold his/her breath for 5–6 s, keeping the probe indicator green (Figure 1).
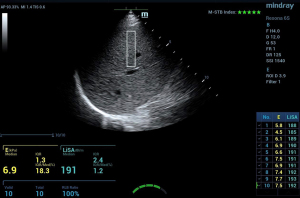
Requirements of the operators
CAP was conducted by technicians with more than 5-year experience and who had performed more than 500 FibroScan measurements. LiSA measurements were completed by a fixed technician with 2-year experience who had performed Hepatus measurements more than 300 times.
Test for the repeatability
Two operators using LiSA evaluated 45 patients randomly: one senior doctor (operator A: with 15-year experience in abdominal ultrasound, including using the Hepatus platform performed at least 300 measurements) and one junior doctor (operator B: with 2 years abdominal ultrasound experience, including using the Hepatus platform performed at least 25 measurements). We assessed the intraoperator variability, for the operator A, to evaluated intraoperator variability according to the differences between twice measurements obtained each patient.
Ultrasound-guided percutaneous liver biopsy
The Bard Magnum b2017-iopsy gun and the Magnum MN1620 biopsy needle (16G, Bard, USA) were used for ultrasound-guided liver biopsy. The samples were more than 15 mm in length and contained at least 10 complete structures of the hepatic portal area, devoid of broken liver tissues samples. The histological specimens were fixed with 10% neutral formaldehyde, paraffin embedded, and sectioned continuously, then stained with hematoxylin and eosin (HE) and Masson’s trichrome. The NAFLD activity score (NAS) and fibrosis stage were determined according to the guidelines of the Clinical Research Network Pathological Working Group on Nonalcoholic Steatohepatitis of the National Institutes of Health (8). Steatosis is pathologically defined as a condition in which the fat content in the liver is more than 5%. Liver fibrosis was graded by the Scheuer scoring system. The final pathological report was made by 2 senior pathologists according to the relevant pathological diagnosis criteria.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 and Delong statistical software. Measurement data conforming to the normal distribution are expressed as the mean ± standard deviation (SD), while data not conforming to the normal distribution are expressed as median (IQR). Uniformity of continuous variables within a group was determined by the intraclass correlation coefficient (ICC). Correlation analysis between the LiSA value and continuous variables or rank variables was performed with Spearman’s correlation coefficient. Multivariate linear regression analysis was used to analyze multiple factors, and the 95% confidence interval (CI) was calculated. In the diagnostic value analysis, the receiver operating characteristic (ROC) curve was constructed, and the area under the ROC curve (AUC) was calculated. According to the highest Youden index (sensitivity + specificity − 1), the diagnostic optimal cutoff point and the corresponding diagnostic sensitivity and specificity were determined. The statistical software Delong was used to determine whether there were any statistical differences between the 2 methods, with P<0.05 indicating significant differences.
Results
Patient clinical and pathological characteristics
Comparisons and descriptions of the general clinical background data of the enrolled patients are listed in Table 1. A total of 203 patients who met the inclusion criteria were included in this study. The patients were divided into the S0 group (liver fat content <5%) or S1 group (liver fat content ≥5%) according to liver fat content validated by liver biopsy. A larger waistline was found in the S1 group (P=0.049). There were no significant differences in the other clinical, serum, and histological indicators between the 2 groups of patients. Comparisons of the pathological fibrosis stage and inflammation grade between the S0 and S1 groups were shown in Table 2. Among the 203 patients, 108 were in the early fibrosis stage (<2) and 115 had mild inflammation grade (<2). The fibrosis and inflammation degree were similar between the S0 and S1 groups.
Table 1
| Characteristics | S0 group (N=93) | S1 group (N=58), mean ± SD | P |
|---|---|---|---|
| Age (years), mean ± SD | 44.4±12.9 | 41.2±11.6 | 0.085 |
| Gender (female), n (%) | 13 (13.9) | 10 (17.2) | 0.067 |
| Body mass index (kg/m2), mean ± SD | 20.97±4.10 | 22.38±2.53 | 0.058 |
| Waistline (cm), mean ± SD | 79.46±13.21 | 83.89±11.13 | 0.049* |
| Fasting plasma glucose (μmol/L), mean ± SD | 4.86±1.21 | 5.46±2.21 | 0.071 |
| Alanine aminotransferase (IU/L), mean ± SD | 35.46±16.20 | 40.15±20.05 | 0.142 |
| Aspartate aminotransferase (IU/L), mean ± SD | 40.86±20.15 | 41.76±12.17 | 0.098 |
| Total bilirubin (μmol/L), mean ± SD | 11.76±12.05 | 12.68±10.14 | 0.056 |
| Total cholesterol (mmol/L), mean ± SD | 3.86±2.18 | 4.48±3.03 | 0.090 |
| Triglycerides (mmol/L), mean ± SD | 1.03±0.75 | 1.20±1.51 | 0.076 |
| High-density lipoprotein, mean ± SD | 1.08±0.76 | 1.13±0.68 | 0.068 |
| Low-density lipoprotein, mean ± SD | 2.80±0.87 | 2.29±0.96 | 0.059 |
*, P<0.05.
Table 2
| Pathology | S0 group (N=93) | S1 group (N=58) | P |
|---|---|---|---|
| Fibrosis stage | |||
| ≥2 | 28 | 15 | 0.064 |
| <2 | 65 | 43 | 0.078 |
| Inflammation grade | |||
| ≥2 | 22 | 14 | 0.107 |
| <2 | 71 | 44 | 0.056 |
Intraoperator and interoperator agreement of LiSA
In both cases, agreement was very good: regarding interoperator variability, LiSA had an ICC of 0.88 (95% CI: 0.82–0.94; n=45), and the ICC for intraobserver agreement was 0.91 (95% CI: 0.83–0.95). There were no significant differences between the 2 measurements obtained per patient (P>0.05).
Performance of LiSA and CAP in terms of identifying hepatic steatosis
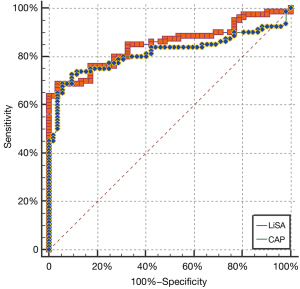
There were statistically significant differences in the mean values of CAP and LiSA between the S0 (<5%) and S1 (≥5%) groups (P=0.032 and P=0.029; Table 3). There was no significant difference for the performance of LiSA and CAP in assessing liver steatosis over 5% (AUC 0.825 vs. 0.798, P=0.068; Figure 2). Using the optimal cutoff point (230 dB/m) for LiSA, the diagnostic specificity and sensitivity were 89.18% and 79.16%, respectively. Using the optimal cutoff point (228 dB/m) for CAP, the diagnostic specificity and sensitivity were 87.20% and 76.31%, respectively.
Table 3
| Values | S0 group (N=93) | S1 group (N=58) | P |
|---|---|---|---|
| LiSA (dB/m) | 209±30 | 245±39 | 0.032* |
| CAP (dB/m) | 210±36 | 239±40 | 0.029* |
*, P<0.05. LiSA, liver steatosis analysis; CAP, controlled attenuation parameter.
Single factor correlation analysis of the LiSA value
It was found that LiSA was positively correlated with AST (P=0.015), triglyceride (TG; P<0.001), total cholesterol (TCH; P<0.01), fasting blood glucose (FBG; P<0.05), waist circumference (WC; P<0.001), BMI (P<0.001), and fatty change of the liver (P<0.001), but was not correlated with ALT (P>0.05), high-density lipoprotein (P>0.05), and hepatic fibrosis (P>0.05).
Discussion
Patients with CHB or chronic hepatitis C (CHC) are at risk for developing liver cirrhosis, hepatic dysfunction, and HCC. Hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is a well-known risk factor for HCC. An increasing incidence of HCC has aroused high concern all over the world, along with its association with NAFLD. NAFLD is becoming a worldwide epidemic. As we know, NAFLD is primarily associated with obesity and insulin resistance, and is regarded as a hepatic manifestation of metabolic syndrome. NAFLD may develop into nonalcoholic steatohepatitis (NASH), showing more than 5% steatosis with intralobular inflammation and ballooning degeneration. Concomitant NAFLD was found to be associated with a higher HCC risk in CHB patients (1-4). The prevalence of CHB combined with fatty liver in China is also gradually increasing. Therefore, this study mainly included CHB patients with fatty liver or suspected fatty liver for analysis.
Liver biopsy remains the gold standard for the assessment of fatty liver or fibrosis. However, it is invasive and is associated with a high risk of bleeding, pain, and bile leakage, and is therefore not suitable for general survey, screening, and dynamic follow-up observation. Developing a more convenient and noninvasive method for the early detection of liver steatosis and for the effective and dynamic monitoring of fatty liver has become a research hotspot.
Conventional ultrasound is one of the most commonly used imaging methods for the screening and surveillance of fatty liver. It is a noninvasive, economic, convenient, and radiation-free method, and is especially suitable for children, the elderly, and other special groups. However, conventional ultrasound is always operator-dependent or instrument-dependent, with no quantitative diagnosis, and it cannot diagnose hepatic steatosis less than 30% (5,6). Although CT and MRI can also be used for the diagnosis of fatty liver, radiation damage limits the application of CT, especially for children and pregnant women. Though, magnetic resonance imaging hepatic proton density fat fraction (MRI-PDFF) have a higher diagnostic accuracy for the assessment of liver fat content, MRI requires special equipment with higher requirement, it is expensive and time-consuming for patients. In addition, the MRI scanner is an enclosed space and therefore claustrophobia is occasionally a problem for some patients.
Therefore, developing a method for early-stage fatty liver detection and quantitative assessment of follow-up has become a hot topic of research. CAP is one of the most commonly used quantitative methods of liver steatosis, based on the FibroScan platform. In the present study, we used a new method of fatty liver quantitative analysis, LiSA, based on the ultrasound signal attenuation principle. It is able to measure the attenuation degree of ultrasonic echo signals of the liver tissues and produce a value related to fat content quantitatively.
Knowing that CAP and LiSA share the same physical principle, we excluded patients with a distance from the skin surface to the liver capsule >25 mm and BMI ≥28 kg/m2, which may lead to difficult detection with CAP (9). Because we only had the M probe of FibroScan, theoretical measurement depth more than 25–65 mm under the skin may overestimate the attenuation degree of the liver tissues. Previous studies have shown that the measurement value of CAP is limited in patients with an excessively high BMI. When BMI is ≥28 kg/m2, accurate detection becomes difficult, or measurement errors are likely to occur. The CAP value measured by the liver fat attenuation coefficient was not affected by hepatic inflammation, fibrosis, different etiologies, and other factors. BMI is an independent risk factor for CAP measurement (9-12). It was found in our study that the LiSA value was correlated with AST, TG, TCH, FBG, WC, BMI, and the degree of liver steatosis, which is similar to the findings of previous clinical studies using CAP (11-13). The possible reason is that metabolic disorder syndrome and fatty liver often interact, as both cause and interact with each other. Insulin resistance is the core physiological mechanism of the 2 conditions. Fatty liver increases the output of glucose and low-density lipoprotein cholesterol (LDL-C), while LDL-C is the main transport form of endogenous TG synthesized by the liver. Patients with metabolic disorder syndrome often have abdominal obesity, dyslipidemia, and abnormal blood glucose. These adverse effects will lead to the deposition of fatty tissue in the liver and hepatic insulin resistance. Some previous studies have demonstrated that abdominal obesity and dyslipidemia are independent risk factors for fat deposition and insulin resistance in patients with metabolic disorder syndrome (14,15).
In the present study, we used ROC curves to compare the diagnostic efficiency of CAP vs. LiSA in detecting hepatic steatosis over 5%. We found that both methods can be effectively applied to the diagnosis of hepatic steatosis, with AUCs of 0.812 and 0.778 for LiSA and CAP, respectively. Owing to the visual gray-scale image guidance, LiSA measurement is an easy-to-perform ultrasound technique. It can easily avoid intrahepatic masses and control the validity of data measurement during the process in real-time so as to improve the reliability of the procedure. It is especially advantageous for patients with atrophic cirrhosis or pneumonectasis that may prevent liver detection from the routine intercostal site. The CAP method is used to evaluate the mean attenuation degree of the ultrasonic signal in A mode generated by transient force low-frequency impulses induced by external vibration. It is a single channel signal system composed of multiple signals. The LiSA method uses a multi-array element probe to measure the mean attenuation of the liver tissue with an increased region of interest (ROI). It can provide higher resolution and more information to distinguish the local positions in more detail within a certain space. The echo signal detected by LiSA is more representative and more advantageous in methodology. In summary, both LiSA and CAP can diagnose fatty liver over 5%. They are also applicable for early general survey and screening of fatty liver, dynamic follow-up, and treatment efficacy evaluation of fatty liver patients.
Although previous studies have shown that different etiologies and the degree of liver fibrosis cannot significantly change the quantitative CAP value of liver fat, BMI and the different instrument platforms used by the 2 methods may affect the test value and the optimal cutoff point (6,16-19). As the number of samples included in this study is relatively small, further studies are required for detailed statistics with different BMIs or liver fat content.
There were several limitations of this study. Firstly, the sample size was relatively small in this single-center study. A larger sample size or a multicenter study may help minimize selection bias. Secondly, the biopsy samples were smaller than the area that measured by LiSA and CAP. Thus, it might not represent the whole disease status of the liver. Future studies with a comprehensive study design should be carried out.
In conclusion, LiSA and CAP are noninvasive, inexpensive methods for diagnosing and liver steatosis. They can be easily applied to obtain immediate results with high sensitivity.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-989/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-989/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-989/coif). ZC is from Shenzhen Mindray Biomedical Electronic Co., Ltd. As a doctor of clinical application of technology, ZC provided a lot of help to the use and adjustment of the machine during the development of the project. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ruijin Hospital (2017-72) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Endocrinology branch of Chinese Medical Association. Consensus on diagnosis and treatment of non-alcoholic fatty liver disease and related metabolic disorders (Second Edition). Journal of Clinical Hepatobiliary Disease 2018;34:2103-8.
- Tong W, Ju L, Qiu M, et al. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res 2016;46:933-43. [Crossref] [PubMed]
- Fan J, Zhuang H. Guidelines for the prevention and treatment of fatty liver in China (Popular Science Edition). Shanghai: Shanghai Science and Technology Press 2015:18-20.
- Lee YB, Ha Y, Chon YE, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol 2019;25:52-64. [Crossref] [PubMed]
- Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082-90. [Crossref] [PubMed]
- Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 2007;47:239-44. [Crossref] [PubMed]
- Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Short Version). Ultraschall Med 2017;38:377-94. [Crossref] [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Shen F, Zheng RD, Mi YQ, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol 2014;20:4702-11. [Crossref] [PubMed]
- Karlas T, Petroff D, Garnov N, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One 2014;9:e91987. [Crossref] [PubMed]
- Shen F, Xu Z, Pan Q. Analysis of influencing factors and repeatability of controlled attenuation parameters of FibroScan in evaluating fatty liver. Journal of Practical Liver Diseases 2013;16:59-62.
- Shen F, Zheng R, Mi Y, et al. A multi-center clinical study of a novel controlled attenuation parameter for assessment of fatty liver. Zhonghua Gan Zang Bing Za Zhi 2014;22:926-31. [PubMed]
- Sasso M, Tengher-Barna I, Ziol M, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan®: validation in chronic hepatitis C. J Viral Hepat 2012;19:244-53. [Crossref] [PubMed]
- Smits MM, Ioannou GN, Boyko EJ, et al. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol 2013;28:664-70. [Crossref] [PubMed]
- Ryoo JH, Choi JM, Moon SY, et al. The clinical availability of non alcoholic fatty liver disease as an early predictor of the metabolic syndrome in Korean men: 5-year's prospective cohort study. Atherosclerosis 2013;227:398-403. [Crossref] [PubMed]
- Masaki K, Takaki S, Hyogo H, et al. Utility of controlled attenuation parameter measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol Res 2013;43:1182-9. [Crossref] [PubMed]
- Kumar M, Rastogi A, Singh T, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol 2013;28:1194-201. [Crossref] [PubMed]
- Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902-10. [Crossref] [PubMed]
- Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022-30. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


