
Comparison of magnetic resonance imaging and computed tomography in the diagnosis of acute pancreatitis: a systematic review and meta-analysis of diagnostic test accuracy studies
Introduction
Acute pancreatitis (AP) is a rapid attack of pancreatitis, in which patients experience persistent epigastric pain, accompanied by nausea and vomiting symptoms. The etiology of AP is abnormal activation of pancreatic enzymes due to a variety of causes, causing self-digestion of pancreatic tissue, which causes inflammation (1). It is more common in adult patients, with an incidence of 5–30/100,000, which is increasing annually (2). Heavy alcohol consumption and overeating are the most common causes of AP, and surgical trauma, autoimmune diseases, endocrine disorders, and special drug use may also provoke the disease (3,4). It is characterized by acute onset, rapid development, and poor prognosis, and timely diagnosis and identification of the cause are the key to effectively guide clinical protocols and improve prognosis. At present, the commonly used diagnostic modality for this disease is biochemical index examination combined with hematuria amylase expression, but it has a certain rate of missed diagnosis (5). CT and MRI are commonly used in clinical imaging diagnosis, both of which can clearly show the changes of pancreatic morphology, surrounding tissue and peritoneal effusion in patients with acute pancreatitis (6). A study by Bieliuniene et al. (7) showed that MRI has higher recognition of pancreatic enlargement, especially in the display of peripancreatic effusion and pancreatic contour, due to the special composition of acini and glandular ducts, soft texture and pancreatic juice in pancreas. However, the study by Stimac et al. (8) showed that there was no difference in sensitivity, specificity, positive predictive value, negative predictive value and accuracy between the two methods in the diagnosis of acute pancreatitis. In order to understand clearly the diagnostic effectiveness of the two and to provide more precise evidence, our study included the diagnostic comparison literatures in recent years and performed a meta-analysis. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-812/rc).
Methods
Inclusion criteria
We defined the inclusion of studies according to PICOS (Participants, Intervention, Control, Outcome, Study): (I) study types: all included studies were diagnostic research, with no restriction imposed on whether there was only one study center, the articles could be prospective or retrospective research, and they were in Chinese and English; (II) participants: the participants were human and all patients had suspected AP symptoms within 2 days, and the patients had clinical symptoms such as epigastric pain, nausea and vomiting, and jaundice; (III) interventions & control: all the studies should compare magnetic resonance imaging (MRI) and CT for the AP diagnosis; the reference standard of the included studies was different, but we confirmed the diagnosis based on the clinical manifestations of AP (acute upper abdominal pain with or without back radiation and/or vomiting) and elevated serum total amylase (200 UI/L) and/or lipase levels (more than 3 times the upper limit of normal) (9). (IV) Out comes: the included study should contain diagnostic data [number of true positive cases (TP), false positive cases (FP), false negative cases (FN), true negative cases (TN)].
Exclusion criteria
(I) Literature types such as review, experience sharing, and case analysis; (II) study subjects were animals (dogs, mice, and so on), patients without symptoms of AP, such as chronic pancreatitis, autoimmune pancreatitis, pancreatic adenocarcinoma; (III) reference standard for diagnosis was not described; (IV) data required for diagnostic meta-analysis could not be provided.
Literature search
(I) Search strategy: we performed an electronic search using keyword combinations between Jan–Feb, 2022, and the search keywords used were: “computed tomography”, “CT”, “magnetic resonance imaging”, “MRI”, “magnetic resonance cholangiopancreatography” or “MRCP”, “magnetic resonance cholangiography”, “MRC”, and “pancreatitis”; (II) the database searched included PubMed, Web of Science, The Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang Data.
Literature selection
The search was completed independently by 2 researchers. Repeat documents were removed using the deduplication function of the software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014). The investigators then read the title, abstract, and full text of the articles, and excluded any that did not qualify. In case of any dispute in this process, and where a consensus could not be reached through discussion, a third person was invited to arbitrate.
Data extraction and conversion
After completing the literature screening, 2 researchers re-read the full texts, and characteristic information of articles (author, publication time, study site), participant information (gender, age), and diagnostic information (reference standard, sample size, diagnostic tools and process, diagnostic interval time), diagnostic data [number of true positive cases (TP), false positive cases (FP), false negative cases (FN), true negative cases (TN)] were extracted. If the diagnostic data could not be obtained from an article, we tried to obtain the TP, FP, FN, and TN data using the total number of participants, number of positive diagnoses, number of negative diagnoses, sensitivity, and specificity provided in the article.
Risk assessment of literature bias
Literature risk of bias was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies checklist (QUADAS tool) which was built in the RevMan 5.4 software containing the following 4 aspects for the included studies: patient selection, index test, reference standard, and flow and timing. Evaluation was based on 3 grades: A, B, and C. Among them, grade A had no bias risk in 4 aspects, grade B had one or more unclear risk of bias, and grade C had one or more high bias risks.
Statistical methods
RevMan 5.4 software and Stata 16.0 software (released by Stata Corp LLC, College Station, TX, USA) was used for statistical analysis to calculate the pooled sensitivity (Sen), specificity (Spe), and 95% confidence interval (CI) with forest plot. Heterogeneity was analyzed with Q test, and if P<0.1 or I2>50%, significant heterogeneity was indicated, and the random-effects model was selected. A summary receiver operating characteristic curve (SROC) was drawn and area under the curve (AUC) was calculated, Z test was applied to compare the two methods. When there was a threshold effect, the SROC curve plan showed a “shoulder-arm” distribution, the absence of which indicated that there was no threshold effect.
Results
Literature screening process and results
The document retrieval flow chart is shown in Figure 1. Initially, 388 articles were retrieved, and after screening, a total of 8 articles were included and selected. A total of 566 patients participated in diagnosis.
Basic characteristics of articles
The basic characteristics and participant characteristics of the included articles are shown in Tables 1,2, including 4 retrospective studies and 5 prospective studies. The minimum number of patients participating in the diagnosis was 21 and the maximum was 124.
Table 1
| First author | Year | Country | Study type (P/R) | Gender ratio (M/F) | Age (years) | Number of cases | Quality level |
|---|---|---|---|---|---|---|---|
| Arvanitakis et al. (10) | 2004 | Belgium | R | 23/16 | 47 [15–86] | 39 | C |
| Costache et al. (11) | 2017 | USA | R | 61/39 | 64 | 100 | B |
| Zhang et al. (12) | 2021 | China | P | 78/46 | 51.47±9.24 | 124 | A |
| Arvanitakis et al. (13) | 2007 | Belgium | R | 26/9 | 64 [27–89] | 35 | A |
| Kim et al. (14) | 2006 | Korea | P | 36/4 | 30–70 | 40 | A |
| Xu et al. (15) | 2021 | China | P | 47/42 | 21–70 | 89 | A |
| Jiang et al. (16) | 2021 | China | P | 37/23 | 51.76±5.48 | 60 | A |
| Lai et al. (17) | 2015 | China | P | 32/26 | 57.43±10.55 | 58 | A |
| Islim et al. (18) | 2014 | Turkey | R | 13/8 | 58.1±16.32 | 21 | A |
P/R, prospective/retrospective; M/F, male/female.
Table 2
| First author | MRI | CT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | TP | FP | FN | TN | ||
| Arvanitakis et al. (10) | 15 | 17 | 3 | 4 | 14 | 18 | 4 | 3 | |
| Costache et al. (11) | 55 | 14 | 6 | 25 | 50 | 22 | 11 | 17 | |
| Zhang et al. (12) | 92 | 6 | 4 | 22 | 81 | 7 | 15 | 21 | |
| Arvanitakis et al. (13) | 20 | 11 | 2 | 2 | 19 | 10 | 3 | 3 | |
| Kim et al. (14) | 12 | 5 | 8 | 15 | 1 | 6 | 19 | 14 | |
| Xu et al. (15) | 67 | 1 | 5 | 16 | 57 | 3 | 15 | 14 | |
| Jiang et al. (16) | 41 | 2 | 1 | 16 | 32 | 5 | 10 | 13 | |
| Lai et al. (17) | 42 | 1 | 3 | 12 | 35 | 3 | 10 | 10 | |
| Islim et al. (18) | 10 | 1 | 0 | 10 | 6 | 0 | 4 | 11 | |
MRI, magnetic resonance imaging; CT, computed tomography; TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Literature bias assessment
In this study, all articles outlined that the participants were selected by continuous or random sample selection. There was no significant bias in the index test, there was a descriptive reference standard, and the reference standard error was significantly biased. However, the diagnostic purpose of an article (10) was mainly the severity of AP, rather than the confirmed diagnosis of AP, which may have involved some case selection bias and process bias, and another article (11) did not describe the necessary time interval between the reference standard, MRI test, and CT test (Figures 2,3).
Meta-analysis results
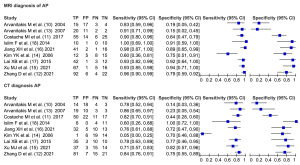
Forest plot of diagnosis
A total of 9 articles included MRI diagnosis, and there was heterogeneity between the articles (I2=81%, df=2.00, P=0.003). The random-effects model analysis resulted in a diagnostic sensitivity of 92%, 95% CI: 85% to 96%, a specificity of 74%, 95% CI: 50% to 89%, a positive likelihood ratio of 3.5, 95% CI: 1.6 to 8.0, a negative likelihood ratio of 0.11, 95% CI: 0.05 to 0.24, and a diagnostic odds ratio (DOR) of 32, 95% CI: 7 to 136.
A total of 9 articles included CT diagnosis, and there was heterogeneity between the articles (I2=95%, df=2.00, P<0.0001). The random-effects model analysis resulted in a diagnostic sensitivity of 73%, 95% CI: 55% to 85%, a specificity of 64%, 95% CI: 42% to 82%, a positive likelihood ratio of 2.0, 95% CI: 1.1 to 3.6, a negative likelihood ratio of 0.43, 95% CI: 0.24 to 0.76, and a DOR of 5, 95% CI: 2 to 14 (Figure 4).
SROC curve
An SROC curve was drawn (Figure 5), and the AUC values of MRI and CT diagnosis were 0.93, 95% CI: 0.90 to 0.95 and 0.74, 95% CI: 0.70 to 0.78, respectively. The AUC value of MRI was significantly greater than CT (Z=3.684, P=0.023).

Fagan plot
Figures 6,7 are Fagan plots of diagnosis by MRI and CT, and the positive posterior probability after MRI diagnosis is 47%, which is higher than that of CT diagnosis (34%).


Source of heterogeneity
As shown by the SROC curve, the curve plan did not present a “shoulder-arm”-like distribution, suggesting that there was no threshold effect.
Discussion
There are 2 types of AP: edema type and gangrene type. After effective intervention, the edema type has a predominantly good prognosis, but the gangrenous type progresses rapidly and easily causes critical complications, which can be life-threatening for the patient (19). Early diagnosis of the disease has guiding significance for subsequent treatment. A variety of imaging techniques have the characteristics of high resolution and clear imaging, which can directly show the shape of the pancreas, reflect the degree of pancreatic necrosis, and facilitate the judgment of the condition (20). As a commonly used imaging technique, CT examination has the advantages of clear imaging, fast examination speed, wide scanning range, and simple operation; pancreatic lesions can be clearly visualized by sweeping and contrast-enhanced scanning; its density resolution is high, and it is not affected by intestinal gases and fat during the examination, which has promoted its popularity in clinical practice (21). The latest international consensus in Atlanta, USA (22) included CT examination in the diagnosis of AP, and AP can now be diagnosed by meeting 2 of the following 3 characteristics: (I) acute attack with typical abdominal pain symptoms of AP (persistent, severe, and epigastric pain); (II) serum lipase or amylase activity >3 times the upper limit of normal; (III) characteristic features of AP found on CT, MRI, or ultrasound. The use of MRI is another diagnostic option, as the use of iodinated contrast medium in CT in patients with severe AP may aggravate their condition, and CT should thus be used with caution in clinical practice.
In this study, 9 articles on MRI and CT in the diagnosis of AP were summarized. Compared to CT, MRI had higher sensitivity, specificity, and DOR, suggesting that MRI has higher accuracy. The AUC of SROC plot in the study showed that the AUC of MRI was higher than that of CT (0.93 vs. 0.74). The Fagan plot showed that the positive posterior probability after MRI diagnosis was 47%, which was higher than that of CT diagnosis (34%), suggesting that the diagnostic efficiency of MRI was higher. The reason for this is that the tissue resolution of MRI can clearly reflect the vascular proliferation at the edge of the lesion and the entry of components in the blood into the tissue, which has certain advantages in the diagnosis of pancreatic injury (23). The MRI technology has been continuously improved and matured, and multi-directional and multi-sequence imaging can be performed. The combination of different scanning sequences can improve diagnostic accuracy and reduce the occurrence of missed diagnosis and misdiagnosis; in addition, MRI can reflect blood flow changes, facilitate the assessment of lesions according to tissue density, and distinguish the soft tissue morphological changes of lesions (24). The contrast agent injected during the enhanced MRI scan is gadoterate dextran. Compared with the iohexol contrast agent used in CT, it is safe, does not aggravate the patient’s condition, has no radiation risk, can be repeatedly examined, and has more prominent advantages in clinical application (25).
In this study, we did not distinguish MRI and CT techniques in detail. With the development of imaging, a variety of technical improvements have emerged for MRI and CT. Shinya et al. (26) reported that the application of the MRI technique of diffusion-weighted imaging (DWI) in the diagnosis of AP was a more advanced MRI technique, which had clearer imaging than CT and could detect pancreatic cancer causing AP, while common MRI could only detect choledocholithiasis and pancreatic division causing AP.
This study had some limitations, including that the number of articles included was small, the number of participants was still small, and there was a lack of multi-center diagnostic testing with large sample size; the main diagnostic purpose of departmental study was to distinguish the severity of AP, rather than confirm the diagnosis of AP; and the quality of the 4 included retrospective studies was not as good as that of those with prospective design, thus the reliability of the results is questionable.
Conclusions
This study showed that MRI has higher accuracy and sensitivity than CT in the diagnosis of AP. Although no study has demonstrated that MRI can reduce the mortality of AP or improve its prognosis, MRI provides a valuable imaging examination to differentiate the population with suspected AP and can be used as the first choice of imaging examination for clinical diagnosis of AP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-812/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-812/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol 2016;22:2256-70. [Crossref] [PubMed]
- Aghdassi A, Simon P, Pickartz T, et al. Endoscopic management of complications of acute pancreatitis: an update on the field. Expert Rev Gastroenterol Hepatol 2018;12:1207-18. [Crossref] [PubMed]
- DiMaio CJ. Management of complications of acute pancreatitis. Curr Opin Gastroenterol 2018;34:336-42. [Crossref] [PubMed]
- Baron TH, DiMaio CJ, Wang AY, et al. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020;158:67-75.e1. [Crossref] [PubMed]
- Ogawa H, Takehara Y, Naganawa S. Imaging diagnosis of autoimmune pancreatitis: computed tomography and magnetic resonance imaging. J Med Ultrason (2001) 2021;48:565-71. [Crossref] [PubMed]
- İlhan M, Üçüncü M, Gök AFK, et al. Comparison of contrast-enhanced CT with diffusion -weighted MRI in the Evaluation of patients with acute biliary pancreatitis. Turk J Surg 2017;33:153-7. [Crossref] [PubMed]
- Bieliuniene E, Brøndum Frøkjær J, Pockevicius A, et al. CT- and MRI-Based Assessment of Body Composition and Pancreatic Fibrosis Reveals High Incidence of Clinically Significant Metabolic Changes That Affect the Quality of Life and Treatment Outcomes of Patients with Chronic Pancreatitis and Pancreatic Cancer. Medicina (Kaunas) 2019;55:649. [Crossref] [PubMed]
- Stimac D, Miletić D, Radić M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007;102:997-1004. [Crossref] [PubMed]
- Italian Association for the Study of the Pancreas (AISP). Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532-43. [Crossref] [PubMed]
- Arvanitakis M, Delhaye M, De Maertelaere V, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004;126:715-23. [Crossref] [PubMed]
- Costache MI, Costache CA, Dumitrescu CI, et al. Which is the Best Imaging Method in Pancreatic Adenocarcinoma Diagnosis and Staging - CT, MRI or EUS? Curr Health Sci J 2017;43:132-6. [PubMed]
- Zhang D, Guo J, Wang T, et al. Comparative study on CT and MRI findings and diagnostic value in patients with acute pancreatitis. Advances in Modern Biomedicine 2021;21:1687-90.
- Arvanitakis M, Koustiani G, Gantzarou A, et al. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis 2007;39:473-82. [Crossref] [PubMed]
- Kim YK, Ko SW, Kim CS, et al. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging 2006;24:1342-9. [Crossref] [PubMed]
- Xu MJ. Comparison of diagnostic efficacy of magnetic resonance imaging and multi-slice spiral CT in acute pancreatitis. Journal of Practical Medical Technology 2021;28:1445-7.
- Jiang XH. Clinical value of CT and MRI in the diagnosis of acute pancreatitis. Medical Equipment 2021;34:15-6.
- Lai XB, Hong JH. Clinical value of multi-slice spiral CT and MRI in the diagnosis of acute pancreatitis. Journal of Medical Imaging 2015;25:1125-7.
- Islim F, Salik AE, Bayramoglu S, et al. Non-invasive detection of infection in acute pancreatic and acute necrotic collections with diffusion-weighted magnetic resonance imaging: preliminary findings. Abdom Imaging 2014;39:472-81. [Crossref] [PubMed]
- Inoue C, Nishihama K, Hayasaki A, et al. Case Report: A Difficult-to-Diagnose Case of Hyperinsulinemic Hypoglycemia Surgically Treated After Developing Acute Pancreatitis. Front Endocrinol (Lausanne) 2021;12:731071. [Crossref] [PubMed]
- Dhaka N, Samanta J, Kochhar S, et al. Pancreatic fluid collections: What is the ideal imaging technique? World J Gastroenterol 2015;21:13403-10. [Crossref] [PubMed]
- Kothari S, Kalinowski M, Kobeszko M, et al. Computed tomography scan imaging in diagnosing acute uncomplicated pancreatitis: Usefulness vs cost. World J Gastroenterol 2019;25:1080-7. [Crossref] [PubMed]
- Zhao K, Adam SZ, Keswani RN, et al. Acute Pancreatitis: Revised Atlanta Classification and the Role of Cross-Sectional Imaging. AJR Am J Roentgenol 2015;205:W32-41. [Crossref] [PubMed]
- Sun H, Zuo HD, Lin Q, et al. MR imaging for acute pancreatitis: the current status of clinical applications. Ann Transl Med 2019;7:269. [Crossref] [PubMed]
- Hirota M, Kimura Y, Ishiko T, et al. Visualization of the heterogeneous internal structure of so-called "pancreatic necrosis" by magnetic resonance imaging in acute necrotizing pancreatitis. Pancreas 2002;25:63-7. [Crossref] [PubMed]
- Kim DB, Paik CN, Song DS, et al. The Role of Endoscopic Ultrasonography and Magnetic Resonance Cholangiopancreatography in Patients With Acute Pancreatitis After Negative Computed Tomography Findings of the Etiology. Pancreas 2018;47:1165-71. [Crossref] [PubMed]
- Shinya S, Sasaki T, Nakagawa Y, et al. The efficacy of diffusion-weighted imaging for the detection and evaluation of acute pancreatitis. Hepatogastroenterology 2009;56:1407-10. [PubMed]
(English Language Editor: J. Jones)





