
Efficacy and safety of hepatic artery infusion chemotherapy with mFOLFOX in primary liver cancer patients with hyperbilirubinemia and ineffective drainage: a retrospective cohort study
Introduction
Primary liver cancer (PLC), predominantly hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), is the sixth most common cancer worldwide and the third most common cause of cancer mortality (1). Unfortunately, most patients are already in the advanced stages at diagnosis (2,3), and some will suffer from hyperbilirubinemia. Due to the poor liver function associated with hyperbilirubinemia, there is currently no proven therapeutic strategy for patients with end-stage PLC and hyperbilirubinemia, and supportive care is the only available option (4,5).
Percutaneous transhepatic biliary drainage (PTBD) and endoscopic nasobiliary drainage (ENBD) are effective palliative procedures to treat obstructive jaundice (6). However, some patients may have malignant biliary obstruction due to primary ICC or compression by an intraductal periductal tumor (7). Drainage treatments, such as PTBD and ENBD, cannot relieve extrabiliary tumor compression radically. Therefore, the total bilirubin (TBIL) of such patients tends to remain high even after drainage treatment (>51 µmol/L, which is 3 times the upper limit), making them unsuitable for surgery, chemotherapy, transhepatic arterial chemotherapy embolization (TACE), as well as tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) treatment. Moreover, jaundice becomes more serious with tumor progression and invasion, and increased compression of more branches of the bile duct (8,9). Long-term jaundice increases the risk of infections, and the quality of life and immunity status continue to deteriorate (10). High levels of TBIL may still persist even after adequate drainage in patients with PLC. Thus, an appropriate localized tumor-targeting therapy combined with drainage is urgently needed to alleviate bile duct compression and reduce tumor burden.
Recently, hepatic artery infusion chemotherapy (HAIC) combined with oxaliplatin, fluorouracil, and leucovorin (FOLFOX) was shown to be effective in treating liver cancer (11), and has been adopted for clinical use in China. HAIC combined with the FOLFOX regimen increased perfusion time and drug concentrations in tumors, and achieved obvious clinical efficacy in patients with unresectable HCC and ICC (12-14). The toxicities associated with HAIC were also found to be acceptable (15). Thus, it has become a standard treatment for liver cancer in China (16). Drugs are often selectively distributed to tumors due to the “siphon effect” of rich arterial blood supply to the tumor area. Combined with the “first-pass effect” of the liver, most chemotherapeutic agents are metabolized and spread to the whole body in only small amounts. Therefore, we speculate that HAIC can be tolerated by patients with severe PLC. Although ICC and HCC are both major pathological types of liver cancer, there are significant differences in their pathogenesis, biological behavior, and prognosis (17). HAIC together with FOLFOX regimen has been reported to be effective in both HCC and ICC patients (18,19). Therefore, we believe that HAIC has the potential to treat PLC patients with hyperbilirubinemia, unfortunately, there is no relevant study focused on this point. This current study was designed to evaluate the safety and efficacy of HAIC with a modified oxaliplatin, fluorouracil, and leucovorin (mFOLFOX) regimen in patients with end-stage liver cancer who are not suitable for other anti-tumor treatments due to the development of jaundice. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-978/rc).
Methods
Patient characteristics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Tsinghua Changgung Hospital Affiliated to Tsinghua University (No. 21333-6-01). All patients signed informed consent documents. From June 2017 to January 2021, our center admitted 1,478 patients with unresectable PLC. Of these, 34 patients were enrolled based on the following inclusion criteria: (I) PLC was confirmed by clinical evidence; (II) TBIL was more than three times the upper limit of normal (>51 µmol/L); (III) patients were treated with drainage, but the drainage did not function effectively or the TBIL remained high after a period of decline; (IV) patients not suitable for any other treatment because of a high level of TBIL after multidisciplinary team (MDT) discussions; (V) patients aged 18 years or older; (VI) patients with complete follow-up data; and (VII) patients who provided informed consent voluntarily. Patients were excluded based on the following exclusion criteria: (I) patients complicated with serious infections or cardiovascular diseases; (II) patients with severe ascites; (III) the prothrombin time (PT) was >17 seconds; (IV) the major laboratory indicators were in line with one of the following: white blood cell count <2.0×109/L, platelet count <50.0×109/L, hemoglobin <80 g/L, K+ <3.0 mmol/L, or Na+ <125 mmol/L; (V) patients with other tumors or distant organ metastasis; and (VI) patients aged more than 75 years.
Drug doses and schedule
Patients whose TBIL remained greater than 51 µmol/L for at least 5 days after biliary drainage tube placement were included in the study based on the decision of the MDT. HAIC therapy was administered 5–60 days after PTBD. A microcatheter was selectively placed into the artery supplying the tumor, using digital subtraction hepatic arterial angiography and computed tomography hepatic arterial angiography. Magnesium isoglycyrrhizinate (0.2 g) and polyene phosphatidylcholine (930 mg) were infused into the hepatic artery slowly (20,21). The pipe orifice of the microcatheter was connected to the artery infusion pump to administer the mFOLFOX chemotherapy agents: oxaliplatin 85 mg/m2 in total, 50 mg or less in 1 day for 2–3 days; and 400 mg/m2 5-fluorouracil plus 400 mg/m2 leucovorin per day for 3 days (22-24).
Assessments
The data collection concluded on June 2021. Patient visits were scheduled during 3, 4–7, 8–14 and 14–30 days in the first month after HAIC. Then, before the treatment was discontinued, patient visits were scheduled every 3 months to monitor the safety and efficacy. The following data were analyzed: age, gender, white blood cell (WBC) count, hemoglobin (HGB), platelet (PLT) count, and serum levels of albumin (ALB), alanine transaminase, alkaline phosphatase, aspartate aminotransferase, glutamyl transpeptidase, indirect bilirubin, total bilirubin (TBIL), alpha fetoprotein (AFP), carcinoembryonic antigen, carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), and protein induced by Vitamin K absence-II (PIVKA-II). Quality of life (QOL) was assessed using the World Health Organization Quality-of-Life assessment instrument (WHOQOL-100) (25). Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.5 (CTCAE5.5). In addition, enhanced computed tomographic scans were performed every 3 months during follow-up period to evaluate anatomical tumor response.
The primary endpoints included the proportion of patients with a decline in bilirubin to levels below three times the upper limit, the quality-of-life score (WHOQOL-100 score), and the incidence of adverse events. The secondary endpoints were overall survival (OS), disease control rate (DCR), overall response rate (ORR) as per the investigator assessment (RECIST1.1) (26), and the proportion of patients receiving systemic anti-tumor therapy after HAIC.
Statistical analysis
The statistical significance of the difference between the continuous variables was determined using the independent-sample t-test or Wilcoxon rank-sum test. Pre- and post-treatment data were analyzed by paired t-test for normally distributed data, such as the differences of TBIL and tumor-markers before and after HAIC. Wilcoxon rank-sum test was used for variables of skewed distribution, such as the comparison of AFP change before and after HAIC in HCC subgroup. A 2-sided P value ≤0.05 indicated a statistically significant difference. The chi-square test was used to compare categorical variables. The Kaplan-Meier method was used to estimate OS. All statistical analyses were performed using the Statistical Package for Social Sciences version 21.0.
Results
Patient characteristics
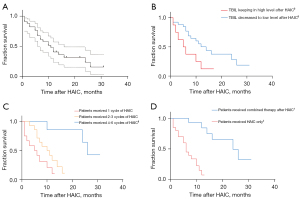
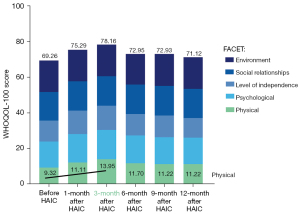
A total of 34 patients, involving 81 cycles of HAIC, were included in this study (Figure 1). As of June 2021, there were 22 deceased patients and the median OS (mOS) was 9.5 months. A total of 12 patients were still being followed up and the median follow-up time was 10.8 months. The ORR was 14.7% and the DCR was 61.8% (Figures 2,3). Furthermore, 26 patients achieved a better QOL 1–3 months after HAIC, and the average WHOQOL-100 score increased from 69.26 to 78.16 within 3 months after treatment (Figure 4; P<0.01). Among all enrolled patients, 3 died within 1 month during the HAIC perioperative period. Serious adverse events (SAEs) occurred in 5 patients, and the toxicity of 29 patients was within grade 3.
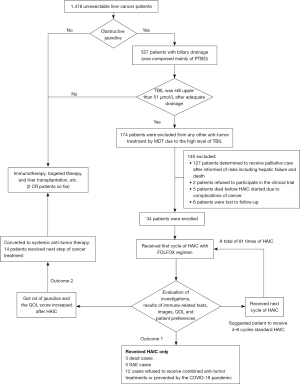


All 34 patients underwent adequate drainage before study initiation; 15 received drainage for right hepatic obstruction and 4 for left hepatic obstruction, while 15 patients received multiple drainage tubes for bilateral hemihepatic obstruction. After adequate biliary drainage, the TBIL decreased from 260.2 to 127.8 µmol/L, which was still considerably higher than the normal range (Table 1). The TBIL dropped to 68.3 µmol/L 1 month after HAIC. Overall, the TBIL was significantly lower than that before treatment (Table 2; P<0.01). Prior to treatment, the TBIL of all 34 patients was higher than 51 µmol/L, and in 26 (76.47%) patients, this reduced to less than 51 µmol/L after treatment.
Table 1
| Characteristics | Values (total n=34) |
|---|---|
| Age (years) | 57.1±12.3 |
| Gender (male) | 19 (52.6%) |
| Total bilirubin (before PTBD) | 260.2±157.6 |
| Total bilirubin (before HAIC) | 127.75±123.95 |
| Complete blood count | |
| RBC | 3.77±0.57 |
| HGB | 114.79±17.26 |
| WBC | 7.42±2.83 |
| PLT | 212.21±97.84 |
| Tumor markers | |
| CA-199 | 4,206.434±6,275.34 |
| AFP | 347.14±1,105.41 |
| PIVKA-II | 693.23±2,211.66 |
| HCC/ICC | 9/25 |
| Reason for jaundice | |
| HCC: bile duct tumor thrombus/tumor compress | 4/5 |
| ICC: intrahepatic tumor compress/bile duct wall thickening | 13/12 |
| AJCC [I/II/III (A/B/C)/IV] | 3/13/16 (13/2/1)/2 |
PTBD, percutaneous transhepatic biliary drainage; HAIC, hepatic artery infusion chemotherapy; RBC, red blood cell; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; CA-199, carbohydrate antigen 199; AFP, alpha fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; AJCC, American Joint Committee on Cancer.
Table 2
| Indicators | Time after HAIC treatment (day) | t-test | |||||
|---|---|---|---|---|---|---|---|
| Pre | 3 | 4–7 | 8–14 | 15–30 | 30+ | ||
| Average TBIL (μmol/L) | 127.75 | 117.33 | 111.08 | 98.27 | 95.24 | 81.06 | P<0.01 |
| Blood routine analysis | P>0.05 | ||||||
| PLT (×109/L) | 213.04 | 182.38 | 166.74 | 177.47 | 187.60 | 203.51 | |
| WBC (×109/L) | 6.81 | 6.59 | 5.93 | 6.45 | 6.69 | 6.90 | |
| RBC (×1012/L) | 3.68 | 3.65 | 3.63 | 3.61 | 3.69 | 3.80 | |
| HGB (g/L) | 112.65 | 111.78 | 110.32 | 110.19 | 113.15 | 114.23 | |
TBIL, total bilirubin; HAIC, hepatic artery infusion chemotherapy; PLT, platelet; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin.
Toxicity and other adverse reactions
All patients were evaluated for complications. There were 3 cases of complication-related mortality (Figure 2) and SAEs occurred in 5 patients. The main causes of AEs or SAEs were hepatic decompensation, hepatic hemorrhage, and myelosuppression. Therefore, this study focused on the changes in peripheral blood cell counts and liver functions. Before HAIC treatment, the average WBC count was 6.88×109/L, the PLT count was 213.04×109/L, the red blood cell (RBC) count was 3.68×1012/L, and the HGB was 112.65 g/L. The RBC count and HGB declined to 3.61×1012/L and 110.19 g/L at about 10 days after HAIC, respectively (P>0.05). However, 1 month after HAIC, the RBC and HGB levels increased to levels that were comparable to the levels before HAIC (Table 2; RBC 3.68 vs. 3.80×1012/L, P>0.05; HGB 112.65 vs. 114.23 g/L; P>0.05). The PLT count decreased rapidly to the lowest point of 166.74×109/L at 5 days after HAIC, which was significantly lower than that before surgery (P<0.05). These results suggested that it may be beneficial to increase the leukocyte count and perform blood transfusions prior to HAIC treatment. Partial splenic embolization was also performed at the same time as HAIC in patients with grade 2 or above adverse events of thrombocytopenia. The average PLT after 1 month of HAIC increased to 203.51×109/L, which was not significantly different from the preoperative values (P>0.05; Table 2). To evaluate other adverse events, PRO-CTCAE and CTCAE5.0 were adopted to integrate the perspectives of both patients and doctors (27). The main AEs below grade 3 included vomiting, fever, and injection site reactions (Table 3).
Table 3
| Adverse reactions | Grade 0/1/2 | Grade 3/4 | Grade 5 (death) |
|---|---|---|---|
| Investigations† | |||
| Activated partial thromboplastin time prolonged | 76/4/2 | 0/– | – |
| Blood bilirubin level increased | 63/12/0 | 2/1 | – |
| Platelet count decreased | 47/17/13 | 1/0 | – |
| White blood cell count decreased | –/8/2/0 | 0/0 | – |
| Gastrointestinal disorders | |||
| Diarrhea | 57/7/4 | ||
| Vomiting | 41/7/29 | 1/0 | 0 |
| General disorders and administration site conditions | |||
| Infusion site extravasation‡ | 74/3/1 | 0/0 | 0 |
| Injection site reaction‡ | 71/5/1 | 1/0 | 0 |
| Malaise | 54/23/1 | 0/– | – |
| Fever | 61/12/5 | 0/0 | 0 |
| Hepatobiliary disorders* | |||
| Hepatic failure | –/–/– | –/3 | 2 |
| Hepatic hemorrhage | –/–/– | –/2 | 1 |
| Hepatic pain | 21/47/8 | 2/– | – |
| Immune system disorders | |||
| Allergic reaction | 71/2/5 | 0/0 | 0 |
†, adverse reaction grade was calculated after the baseline was corrected for multiples above the normal range, if the baseline was abnormal. ‡, injection site reactions included the pain, lipodystrophy, edema, phlebitis of the skin around the surgical site of transhepatic implanted duct, and skin around biliary drainage pipe. *, items under this category includes reactions that was clinically evaluated as liver dysfunction within one month after HAIC, despite some of patients had the tendency of liver disorder before HAIC. HAIC, hepatic artery infusion chemotherapy.
Treatment efficacy
The average level of TBIL was on a downward trajectory following HAIC treatment and was significantly decreased after 1 month of therapy (127.8 vs. 68.3 µmol/L; P<0.01). Furthermore, 29 patients achieved bilirubin reduction after HAIC treatment. Among the patients whose TBIL reduced, 21 achieved disease control by the end of the study. In addition, 26 patients with TBIL levels less than 51 µmol/L after HAIC achieved a longer mOS compared to the other 8 patients (11.9±7.9 vs. 7.0±5.2 months; Figure 3B). In addition, 14 patients could tolerate long-term anti-tumor combination therapy after the HAIC phase due to the decreased bilirubin levels and improved QOL achieved with HAIC. These 14 patients had a longer mOS compared with patients who received fewer than 4–6 cycles of treatment (17.2±7.2 vs. 6.3±3.7 months, Figure 3C). Not all patients received the standard 2–4 cycles of HAIC with the mFOLFOX regimen due to the disruption caused by the COVID-19 outbreak and the patients’ choice of conservative treatment. Among the 34 patients enrolled in this study, 13 received only 1 cycle of HAIC, 11 received 2–3 cycles of HAIC, and 10 patients received 4–6 cycles or more. Improved tumor control and decreased TBIL levels were achieved in patients who received 2–4 cycles of HAIC compared with those who received only a single cycle of HAIC (Figure 4).
Patients were followed up with 96 questions covering 24 facets from 5 domains based on the WHOQOL (Table 4). The QOL improved continually from 1 month to 3 months after HAIC, and the improvement was most obvious in the physical domain. Among the 24 facets, “pain and discomfort”, “energy and fatigue”, and “sleep and rest” showed the biggest improvement (P<0.01; Table 4). The follow-up results showed that the WHOQOL score, especially in the physical and psychological domains, increased significantly 3 months after HAIC compared with scores before treatment (78.16 vs. 69.26; P<0.05; Figure 5). Thereafter, the WHOQOL score decreased gradually at 6–12 months after HAIC with progression of the tumors (71.12 vs. 69.26; P>0.05; Figure 5).
Table 4
| Indicators | Follow-up time after HAIC (month) | t-test (pre vs. 3 months) | |||||
|---|---|---|---|---|---|---|---|
| Pre | 1 | 3 | 6 | 9 | 12 | ||
| Domain | |||||||
| Physical* | 9.32 | 12.11 | 13.95 | 11.70 | 11.22 | 11.22 | P<0.01 |
| Pain and discomfort | 9.24 | 12.52 | 14.07 | 11.44 | 11.25 | 11.56 | – |
| Energy and fatigue | 9.29 | 11.97 | 14.07 | 11.83 | 10.91 | 10.11 | – |
| Sleep and rest | 9.44 | 11.87 | 13.71 | 11.83 | 11.50 | 12.00 | – |
| Psychological | 14.54 | 15.94 | 16.44 | 15.56 | 15.03 | 14.78 | P>0.05 |
| Level of independence | 11.76 | 13.11 | 13.59 | 12.07 | 12.21 | 11.08 | P>0.05 |
| Social relationships | 16.03 | 16.44 | 16.46 | 16.46 | 16.61 | 16.30 | P>0.05 |
| Environment | 17.61 | 17.69 | 17.72 | 17.16 | 17.85 | 17.74 | P>0.05 |
| WHOQOL score | 69.26 | 75.29 | 78.16 | 72.95 | 72.93 | 71.12 | P<0.05 |
*, “pain and discomfort”, “energy and fatigue”, and “sleep and rest” were three facets belonging to the “physical” domain. WHOQOL, World Health Organization Quality of Life; HAIC, hepatic artery infusion chemotherapy.
Discussion
In addition to symptomatic drainage measures such as PTBD, patients with liver cancer and end-stage hyperbilirubinemia require loco-regional therapy to relieve biliary tract compression by reducing the tumor load. In the clinical setting, TACE and other vascular embolization therapies are unavailable due the poor associated liver function, in which case, HAIC is the only option.
Safety
Limited toxicity to the hematopoietic system
Blood transfusion is often received by end-stage patients, and it severely interferes with the result of routine blood tests. In addition, biliary obstruction can greatly influence the WBC count (28). Therefore, in this study, short-cycle metabolic parameters from routine blood tests, such as platelet (PLT) count, WBC and RBC count, and hemoglobin (HGB) levels, were used as the main outcome measurements.
Inevitably, the high concentration of chemotherapeutic drugs distributed to a local tumor can lead to low blood cell counts (29). This was observed in our study cohort as demonstrated by the reduced RBC, WBC, and PLT counts, as well as HGB, between days 5–11. However, the average blood cell count at 30 days post-HAIC reached or exceeded the preoperative levels before HAIC, owing to the metabolism of the drugs and the application of management strategies such as transfusion and recombinant human granulocyte-stimulating factor (G-CSF) injection. The PLT data in this study suggested that the lowest levels were reached within 5–7 days after initiation of HAIC (Table 2). Therefore, patient vital signs should be monitored continuously, and blood routine examinations should be conducted regularly during this latter timeframe. The aforementioned management strategy should be applied in a timely manner if an obvious change in blood cell count is observed. Interestingly, declining platelet count over several weeks may indicate a disorder of the hematopoietic system. In this situation, a partial splenic embolism may be considered and combined with the next cycle of HAIC. During the 81 times HAIC was administered in this study, a partial splenic embolism was considered and combined with HAIC on 5 occasions. Meanwhile, no serious adverse events occurred due to hematopoietic system disorders.
In general, the hepatotoxicity of the PTBD and HAIC combination regimen is relatively low, and it is acceptable.
Acceptable hepatotoxicity
In this study cohort, HAIC administration after PTBD was safe for the vast majority of patients, despite total serum bilirubin levels greater than 51 µmol/L. Therefore, high TBIL levels were not a contraindication to HAIC. While the TBIL values were 3–8 times higher than the normal limit before HAIC, the TBIL of most patients declined consistently and slowly after HAIC. Interestingly, the TBIL of some patients increased within 3–7 days of HAIC administration. However, this phenomenon appeared transient and did not influence the safety at the end of the treatment. The participants of this study were end-stage patients who were unresponsive to other treatments. Without HAIC, their TBIL will continue to increase and symptoms such as cholangitis will persist (10,30,31). Furthermore, the liver function of these patients was poor prior to treatment. The dissociation of bilirubin and transaminase even had appeared on two enrolled patients before any treatment. Severe liver dysfunction followed by hepatorrhagia and severe infection-related death occurred in 3 patients. These small number of deaths suggested that not all liver failure could be reversed by HAIC. Indeed, the effects of HAIC are muti-faceted and complicated, and the advantages and disadvantages of receiving HAIC must be considered carefully in each individual patient suffering from hyperbilirubinemia.
Other moderate adverse reactions
Gastrointestinal disorders and administration site conditions are other adverse reactions observed in our study cohort. None of the adverse events exceeded grade 3, and most were managed successfully by local or noninvasive intervention.
Notably, our adverse reaction management plan achieved much success in preventing gastrointestinal disorders during HAIC intervention. As described in a previous study, the gastroduodenal artery can be occluded with a microcoil when the catheter is placed near the distal part of the common hepatic artery (15-17). This intravascular intervention can reduce the incidence of gastrointestinal adverse events from the source. Therefore, antiemetics can be administered as a symptomatic treatment to most patients with mild digestive tract reactions. In addition, the infusion of oxaliplatin can be reduced to 2 days if the patient’s condition deteriorates.
Overall, with multi-disciplinary support, including interventional radiologists, internists, and intensivists, HAIC is relatively safe for patients who are not suitable nor responsive other anti-tumor therapies.
Efficacy
Overcoming obstructive jaundice
Liver function continues to deteriorate in patients with obstructive jaundice due to bile duct constriction caused by the tumor. The Child-Pugh score must be more than 7 when the TBIL is higher than 51 µmol/L (32). Moreover, if obstructive jaundice cannot be treated, the ability of the liver to produce coagulation factors and albumin deteriorates. As a consequence, most anti-tumor therapies, such as ICI or TKI drugs, are not suitable (33-35). In HCC patients, HAIC may help in eliminating bile duct tumor thrombus and in ICC patients, it may help to reduce the tumor burden. Herein, we report two typical cases to support this point of view (Figures S1,S2). The TBIL decreased to less than 51 µmol/L in 26 patients after HAIC therapy, and were significantly lower than the values before HAIC.
A total of 34 patients with PLC were enrolled in this study, including 25 cases of HCC and 9 cases of ICC. The average AFP of the 25 patients with HCC decreased from 4,814.3±6,434.1 to 2,422.1±4,044.6 ng/mL, while the average CA199 of the 9 patients with ICC also decreased from 1,166.7±1,666.9 to 411.6±921.6 U/mL after 1–6 cycles of HAIC, but the aforementioned values showed no statistical significance (Figure 4A). However, if patients were grouped based on whether they received standard cycles of HAIC, patients with 4–6 cycles of HAIC showed a statistically significant decrease in the levels of the main tumor markers during the last cycle of HAIC and achieved a longer mOS compared with patients who received fewer than 4–6 cycles of treatment (Figures 3C,4B). Furthermore, grouping based on whether patients converted to systemic anti-tumor therapy after HAIC showed that such patients had a statistically significant decrease in the levels of main tumor markers at the end of therapy and gained a longer mOS compared with patients who did not convert to systemic anti-tumor therapy (Figures 3D,4C). This result also suggested that as a local treatment, HAIC may have its own limitations. The tumor control effect might be limited if patients receive only 1 cycle of HAIC. However, patients who are able to receive 4–6 cycles of HAIC might achieve a temporary reduction in tumor load and a decrease in bilirubin levels after HAIC. A good clinical effect was eventually achieved if a sequential systemic treatment was administered in time. From the perspective of tumor therapy, a mOS of 9.5 months and 61.8% DCR were achieved through HAIC and subsequent anti-tumor therapy in this study. This was much longer than the mOS of 4.2 months in patients with end-stage jaundice who received only supportive care in other study (36) or in our center. Therefore, HAIC provided the opportunity to administer other anti-tumor therapies in certain patients, by reducing tumor load and relieving jaundice.
Improving the quality of life
When the tumor burden was reduced and patients were relieved of jaundice by HAIC, pain relief and diet recovery were the most obvious improvements in their daily lives. Therefore, the domains of “pain and discomfort”, “energy and fatigue”, and “sleep and rest” in the WHOQOL-100 assessment showed an obvious improvement. This led to an increase in the scores in the physical and psychological domains at 3–6 months after HAIC, making it possible for patients to receive systemic anti-tumor treatment. However, it is difficult for such patients to achieve partial response (PR) or complete response (CR) using the only the symptomatic treatment of PTBD and the local control treatment of HAIC. Once the HAIC with the mFOLFOX regimen experiences drug resistance, jaundice reoccurs due to biliary tract obstruction or constriction by the tumors. This explains the decrease in QOL scores after 6–12 months of follow-up. An individualized anti-tumor treatment strategy, including TKI drugs, ICI drugs, radiotherapy, and even liver transplant, must be established as soon as possible when obstruction-related symptoms are relieved in the short term.
Limitations
There were some limitations to this study. First, hyper-bilirubinemia (TBIL >3 times the upper limit) was an inclusion criterion and often cannot be resolved by drainage in end-stage liver cancer patients. Therefore, adverse reactions above grade 3 or even death were inevitable in the some of the included patients. However, it should be noted that supportive care was the only option for these patients in the current clinical setting. Second, the sample size was small and bias may have occurred in some subgroup analyses. Despite this, a very short OS was observed in the subgroup of patients who suffered jaundice even after HAIC. In addition, failure to reduce TBIL after HAIC treatment was mainly noted in patients with mass-forming cholangiocarcinoma. In such patients, future studies should examine the ability of local drug injections, such as oncolytic viruses, in combination with HAIC to improve treatment efficacy.
HAIC should be considered as an effective method when patients with advanced-stage liver cancer experience malignant obstructive jaundice that cannot be relieved by drainage. The current study established the significance of HAIC in the treatment of advanced-stage liver cancer patients. Indeed, by overcoming jaundice, HAIC may enable patients suffering from malignant biliary obstruction to receive other drug therapies, such as TKI and ICI.
Acknowledgments
Funding: This work was supported by the CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-056) and the National Natural Science Foundation of China (No. 81930119).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-978/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-978/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-978/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Tsinghua Changgung Hospital Affiliated to Tsinghua University (No. 21333-6-01). All patients signed informed consent documents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yoshida H, Taniai N, Yoshioka M, et al. Current Status of Laparoscopic Hepatectomy. J Nippon Med Sch 2019;86:201-6. [Crossref] [PubMed]
- Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010;51:1274-83. [Crossref] [PubMed]
- Sinn DH, Choi GS, Park HC, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One 2019;14:e0210730. [Crossref] [PubMed]
- Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends 2021;15:155-60. [Crossref] [PubMed]
- Qian XJ, Zhai RY, Dai DK, et al. Treatment of malignant biliary obstruction by combined percutaneous transhepatic biliary drainage with local tumor treatment. World J Gastroenterol 2006;12:331-5. [Crossref] [PubMed]
- Garcea G, Ong SL, Dennison AR, et al. Palliation of malignant obstructive jaundice. Dig Dis Sci 2009;54:1184-98. [Crossref] [PubMed]
- Sha J, Dong Y, Niu H. A prospective study of risk factors for in-hospital mortality in patients with malignant obstructive jaundice undergoing percutaneous biliary drainage. Medicine (Baltimore) 2019;98:e15131. [Crossref] [PubMed]
- Björnsson E, Gustafsson J, Borkman J, et al. Fate of patients with obstructive jaundice. J Hosp Med 2008;3:117-23. [Crossref] [PubMed]
- Ljungdahl M, Osterberg J, Ransjö U, et al. Inflammatory response in patients with malignant obstructive jaundice. Scand J Gastroenterol 2007;42:94-102. [Crossref] [PubMed]
- Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer 2012;1:62-70. [Crossref] [PubMed]
- He M, Li Q, Zou R, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol 2019;5:953-60. [Crossref] [PubMed]
- Liang RB, Zhao Y, He MK, et al. Hepatic Arterial Infusion Chemotherapy of Oxaliplatin, Fluorouracil, and Leucovorin With or Without Sorafenib as Initial Treatment for Advanced Hepatocellular Carcinoma. Front Oncol 2021;11:619461. [Crossref] [PubMed]
- Cai Z, He C, Zhao C, et al. Survival Comparisons of Hepatic Arterial Infusion Chemotherapy With mFOLFOX and Transarterial Chemoembolization in Patients With Unresectable Intrahepatic Cholangiocarcinoma. Front Oncol 2021;11:611118. [Crossref] [PubMed]
- Wang X, Hu J, Cao G, et al. Phase II Study of Hepatic Arterial Infusion Chemotherapy with Oxaliplatin and 5-Fluorouracil for Advanced Perihilar Cholangiocarcinoma. Radiology 2017;283:580-9. [Crossref] [PubMed]
- Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. [Crossref] [PubMed]
- Kumagai S, Togashi Y, Sakai C, et al. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity 2020;53:187-203.e8. [Crossref] [PubMed]
- Zhou Q, Cai H, Xu MH, et al. Do the existing staging systems for primary liver cancer apply to combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma? Hepatobiliary Pancreat Dis Int 2021;20:13-20. [Crossref] [PubMed]
- Shirono T, Niizeki T, Iwamoto H, et al. Therapeutic Outcomes and Prognostic Factors of Unresectable Intrahepatic Cholangiocarcinoma: A Data Mining Analysis. J Clin Med 2021;10:987. [Crossref] [PubMed]
- Sui M, Jiang X, Chen J, et al. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother 2018;106:125-33. [Crossref] [PubMed]
- Hsu SJ, Xu X, Chen MP, et al. Hepatic Arterial Infusion Chemotherapy with Modified FOLFOX as an Alternative Treatment Option in Advanced Hepatocellular Carcinoma Patients with Failed or Unsuitability for Transarterial Chemoembolization. Acad Radiol 2021;28:S157-66. [Crossref] [PubMed]
- He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer 2017;36:83. [Crossref] [PubMed]
- Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018;69:60-9. [Crossref] [PubMed]
- Goyal L, Zheng H, Abrams TA, et al. A Phase II and Biomarker Study of Sorafenib Combined with Modified FOLFOX in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res 2019;25:80-9. [Crossref] [PubMed]
- The World Health Organization Quality of Life Assessment (WHOQOL). development and general psychometric properties. Soc Sci Med 1998;46:1569-85. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051-9. [Crossref] [PubMed]
- Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol 2009;6:533-41. [Crossref] [PubMed]
- Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit Rev Oncol Hematol 2014;90:190-9. [Crossref] [PubMed]
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29. [Crossref] [PubMed]
- Duan F, Cui L, Bai Y, et al. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: a systematic review and meta-analysis. Cancer Imaging 2017;17:27. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Ng KYY, Wong LWJ, Ang AJS, et al. Real-world efficacy and safety of immune checkpoint inhibitors in advanced hepatocellular carcinoma: Experience of a tertiary Asian Center. Asia Pac J Clin Oncol 2021;17:e249-61. [Crossref] [PubMed]
- Spahn S, Roessler D, Pompilia R, et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers (Basel) 2020;12:3830. [Crossref] [PubMed]
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol 2016;65:1140-7. [Crossref] [PubMed]
- Connor S, Barron E, Redhead DN, et al. Palliation for suspected unresectable hilar cholangiocarcinoma. Eur J Surg Oncol 2007;33:341-5. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


