Toxicity and early outcomes of regorafenib in multiply pre-treated metastatic colorectal adenocarcinoma-experience from a tertiary cancer centre in India
Background
Colorectal cancers are the fourth most common cancers diagnosed in India according to the latest WHO estimates (1). Even as effective treatment strategies have steadily improved the outcomes over past few decades, the high incidence rate coupled with a predilection for higher upfront metastatic disease in a younger population in India makes it an important health problem. At our centre, we see around 400 cases of metastatic colorectal cancers (mCRC) per year. Regorafenib is a multikinase inhibitor (MKI) with potent inhibitory activity against vascular endothelial growth factor receptors 1-3 (VEGFR1, VEGFR2, and VEGFR3), PDGFRB, FGFR1, RAF and the mutant oncogenic kinases KIT, RET, and BRAF (2). The phase III CORRECT trial had shown overall survival benefit of 1.4 months with regorafenib compared to placebo in pre-treated mCRC. There were only 15% Asian patients in the trial (3). It is known that some drugs can differ in toxicity and efficacy profiles according to ethnicity (4,5). For this very reason, the phase III CONCUR trial was designed to assess the results of regorafenib in a broader Asian population with metastatic CRCs from mainland China, Hong Kong, South Korea, Taiwan, and Vietnam. This study confirmed that the overall survival advantage with regorafenib compared to placebo was seen in Asian population as well (6). However, neither of these studies had enrolled patients from India and to the best of our knowledge, there is no data about the toxicity and efficacy of regorafenib in Indian population. This audit aims to report the side effect profile and early outcomes with the use of regorafenib for mCRC treated at our centre in the past two years.
Materials and methods
Selection of cases: the records of twenty three cases of metastatic colorectal adenocarcinomas treated with regorafenib at our centre between June 2013 till September 2015 were reviewed. Twelve of these patients received regorafenib as a part of a patient named program. Patients had a performance status ranging from ECOG 0-2 at the time of starting regorafenib. Patients who had been previously treated with fluoropyrimidine-based chemotherapy, oxaliplatin and irinotecan were considered for treatment with regorafenib. When indicated and feasible, anti-VEGF therapy with bevacizumab, and, in KRAS wild type, anti-EGFR therapy with cetuximab had also been used prior to regorafenib. All patients had received at least two non-cross resistant lines of therapy prior to regorafenib.
Targeted therapy: regorafenib was used in a starting dose of 160 mg given once a day continuously for three weeks followed by one week break. However, poor tolerance to this dose in the initial few treated patients prompted for lowering of the starting dose in subsequent patients. The starting dose was 120 mg in the remaining patients with dose escalation or de-escalation based on patient’s tolerance as decided by the treating physician. The minimum dose used was 80 mg and maximum was 160 mg once a day. Regorafenib was given once in the morning half an hour after a light breakfast. In case of grade II hand foot syndrome (HFS), the drug was withheld for one atleast week or till the time the toxicity disappeared followed by restarting at the same dose. In case of a recurrent grade II HFS or single episode of grade III HFS, dose was reduced by 40 mg. For all grade III toxicities except drug related fever and back pain, dose reduction was done by 40 mg. If conservative and symptomatic treatment failed to control fever and back pain for seven days then dose reduction was considered.
Toxicity and response assessment: the toxicity was recorded by the treating physician and reported using CTCAE version 4.03 (7). Fever and back pain were considered to be regorafenib induced when clinical assessment and relevant investigations revealed no other cause of fever or back pain and there was a temporal association of the same with the start of regorafenib. Initial follow-up was weekly for first cycle followed by three weekly or earlier if indicated. Response assessment was done using RECIST 1.1 criteria (8). Response evaluation was done with an axial imaging modality every three monthly or earlier if clinically indicated.
Statistical analysis: the data was analysed retrospectively. Progression free survival was calculated from date of start of treatment with regorafenib till radiologically proven disease progression. Median follow-up time was calculated from start of regorafenib till the date of last follow-up. Five patients were still on therapy at the time of this report. Data was censored at 15th September 2015 for calculating progression free survival (PFS) and median follow-up time.
Results
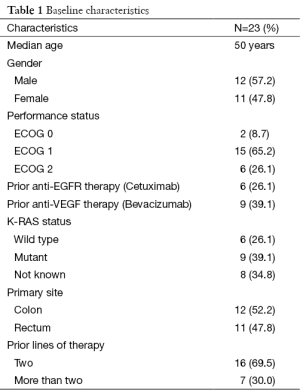
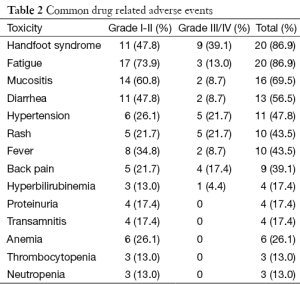
The median age of patients in this study was 50 years. The sex distribution was almost equal with 12 male and 11 female patients. Almost forty percent patients (9/23) had presented with upfront metastatic disease whereas rest had relapsed after initial curative intent treatment. Nine out of twenty three patients (39.1%) had one or more co-morbid illness with diabetes mellitus and hypertension being the most common co-morbidities observed, seen in five patients each. Coronary artery disease was present in two patients. These patients received a median of two lines of chemotherapy prior to treatment with regorafenib. Twenty six percent (6/23) and thirty nine percent (9/23) patients had received prior treatment with cetuximab and bevacizumab respectively. Baseline characteristics of the patients have been enumerated in Table 1. At least one grade III or higher non hematologic toxicity was seen in 65.2% (15/23) cases. Most common grade III adverse events were HFS in nine patients (39.1%), rash 5 (21.7%), hypertension in 5 (21.7%), back pain in 4 (17.4%), fatigue in 3 (13%), mucositis in 2 (8.7%), fever in 2 (8.7%) and hyperbilirubinemia in 1 patient (4.3%). Overall, the most common adverse events were HFS and fatigue seen in 82.6% (19/23) patients. Grade II and above HFS mandating drug interruption was seen in 65.2% (15/23) patients. There was no grade III or higher hematologic toxicity in any of the patients. One patient required stoppage of treatment due to grade III hepatotoxicity in form of hyperbilirubinemia. The common drug related adverse events have been shown in Table 2. Mean duration on drug was 3.8 months. Dose reduction was required for 86.9% (20/23) patients. Thirteen patients were started at a lower dose of 120 mg to begin with due to poor tolerance to 160 mg dose as observed in the first ten patients. Only three patients could tolerate the full dose of 160 mg till progression. An unusual side effect which was observed was low back pain. A distinct temporal association was noted between the start of regorafenib and onset of back pain. Four patients had grade III back pain attributable to regorafenib. All four patients had grade II or III fever and three of these patients also had grade III fatigue. The pain and fever resolved after stopping regorafenib and they were restarted on same dose after a gap of seven days. Two patients had recurrent grade III fatigue and required dose reduction and the other two could be successfully continued on the same dose. Five patients were still on regorafenib at the time of report of this study. All patients had at least one response evaluation done while on regorafenib. Response assessment showed best response as progressive disease in 56.5% (13/23), stable disease in 34.8% (8/23) and partial response in 8.7% (2/23) patients. One patient developed grade III hyperbilirubinemia while on regorafenib and had to be stopped after 1.5 months. Response CT for this patient also showed progression at this point. The median PFS was 3 months in the study population with a median follow-up time of 4.5 months at the time of censoring.
Full table
Full table
Discussion
The past decade and a half has seen a lot of new therapeutic strategies coming up in mCRC in form of anti-EGFR and anti-VEGF therapies contributing to improvement in outcomes of these tumours (9-11). This has but naturally led to increase in the number of patients presenting for treatment with progression post multiple lines of therapies. Regorafenib has been approved for use in such patients based on a modest, albeit statistically significant survival advantage shown in two phase III randomised controlled trials. Till recently, regorafenib was not available for marketing in India and had to be procured from abroad or was being provided under a patient named program making it difficult to obtain good amount data for applicability in Indian setting. To the best of our knowledge, neither has this drug been studied as a part of any clinical trial with patients of Indian ethnicity nor has any clinical practice based experience been reported previously from India. In this study we looked to analyse our initial experience with regorafenib in mCRC treated at our centre.
Regorafenib started at a standard dose of 160 mg in initial few patients was poorly tolerated with high incidence of grade III/IV drug related adverse events. This lead to frequent drug interruption and dose reduction prompting the starting dose to be decreased to 120 mg in the subsequent patients with an intention of escalating the dose to 160 mg in patients with no grade III/IV toxicities. However none of the patients started on this lower dose could be escalated to the standard dose of 160 mg. Almost two-thirds of the patients had at least one grade III/IV toxicity. In a report from a non clinical trial setting from Korea, grade III/IV drug related adverse event with regorafenib in mCRC was seen in 37.5% patients, which seems to be much lower than what has been seen in our study (65.2%) (12). Also, in both the CORRECT and CONCUR trials, grade III/IV drug related adverse events was 54%, which is again lower compared to our report (3,6). HFS and fatigue was seen in 86.9% patients. This is particularly high compared to the incidence in previous reports, ranging from 17% to 57%. A similar high incidence of HFS in Indian population as compared to the western literature was noted with the use of another MKI, sunitinib, in renal cell carcinoma (13). Low back pain, a side effect not classically described with regorafenib, was seen in nine patients with grade III severity in four of them. As described earlier, fever and fatigue were frequent accompaniments with this low back pain suggesting a possible immune mediated mechanism for the same. The mean duration on drug in our study is 3.8 months which is slightly longer in our study. This finding, despite the higher toxicity rate, could be attributed to the fact that response imaging was routinely performed every three monthly as a part of standard practice at our centre and not earlier unless clinically indicated.
Despite the dose reductions and modifications in majority of our patients, the PFS was 3 months which is comparable to the PFS of 3.2 months in seen the CONCUR trial (6). Overall survival (OS) has not been reported as five patients were still ongoing treatment. The major purpose of this study was to highlight the higher toxicity even with a lower starting dose of regorafenib. In a setting where no other therapeutic strategy has shown an overall survival advantage, regorafenib, a costly affair, becomes the automatic choice in a fit patient. However, one needs to weigh the risks and benefits of this therapy given that the somewhat modest advantage comes with significant amount of side effects.
The most important caveat of this study is its retrospective nature. Also, a formal quality of life (QOL) analysis has not been performed in this cohort. A QOL analysis can be an important tool in decision making especially when explaining the risks and benefits to the patient. Another very important issue not addressed in our study is the cost benefit ratio of this therapy. Regorafenib has only recently been approved for marketing in India. The heavy cost incurred with this therapy is likely to be one of the major deterrents for a more wide applicability of regorafenib in Indian setting.
Conclusions
Regorafenib, although an effective treatment strategy in multiply pre-treated mCRC, is associated with significant side effects and a prohibitive cost.
Acknowledgements
Bayer had provided Regorafenib for few of our patients through patient named program.
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol 2010;11:75-84. [PubMed]
- Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740-50. [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 28, 2009 (v.403: June 14, 2010).
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [PubMed]
- Kim ST, Kim TW, Kim KP, et al. Regorafenib as Salvage Treatment in Korean Patients with Refractory Metastatic Colorectal Cancer. Cancer Res Treat 2015;47:790-5. [PubMed]
- Krishna VM, Noronha V, Prabhash K, et al. Sunitinib in metastatic renal cell carcimoma: a single-center experience. Indian J Cancer 2013;50:268-73. [PubMed]


