
Evaluation of the effects of four types of tea on the activity of cytochrome P450 enzymes with a probe cocktail and HPLC-MS/MS
Introduction
Tea, which is native to China, is the second most popular drink worldwide after plain water (1). Globally, more than two billion cups of tea are consumed daily, and tea is an essential part of some people’s lives (1). It has multiple associated health benefits, including anti-inflammatory (2,3), antitumor (4,5), and cardiovascular disease preventive (6,7) properties, as well as glucose and lipid metabolism regulating (8,9), effects. Among the main bioactive substances in tea, the most noteworthy include catechins, polyphenols, caffeine, and polysaccharides. According to their degree of fermentation, teas are categorized as green, yellow, white, oolong, black, or dark, with green tea being non-fermented, oolong tea being semi-fermented, and black tea being fully fermented (10).
Cytochrome P450 (CYP450) enzymes are responsible for the phase I metabolism of xenobiotics (food, drugs, chemical toxins, and environmental carcinogens) and endogenous substances (such as vitamins and steroid hormones) (11-14). The CYP450 superfamily includes numerous isoforms, such as CYP3A4, CYP2C9, CYP1A2, CYP2C19, and CYP2D6, which are involved in the metabolism of over 90% of marketed drugs (15). The pathway and speed of drug metabolism are dependent on the activity of these CYP450 isoforms, which can be induced or inhibited by drugs or other xenobiotic substances, and thereby contribute to drug-drug interactions (DDIs) (16-18). In general, cytochrome P (CYP) inhibition is more common and more serious than CYP induction. When CYP inhibition occurs, the concentration of CYP-mediated drugs in plasma increases; for drugs with narrow therapeutic windows, this leads to an increase in the incidence of unwanted side effects. Some components in food can also influence the activity of CYP450 enzymes, resulting in unwanted side effects or therapeutic failure (19).
Previous reports have mainly focused on the effects of tea polyphenols and catechins on CYP450 isoforms (20-22), and most of these studies have used animal models. Therefore, the aim of this research was to establish a probe cocktail approach for evaluating the inhibitory effects of aqueous extracts from four types of tea (green tea, Ti Kuan Yin tea, black tea, and Pu’er tea) on five CYP450 isoforms (CYP1A2, CYP3A1/2, CYP2C6, CYP2C19, and CYP2D6). Preliminary and screening investigations of tea-drug interactions were conducted employing an in vitro approach to minimize the use of animals. This study also aimed to explore potential tea-drug interactions and provide information about the pharmacology, toxicology, and adverse reactions of tea consumption, with a view to promoting the rational administration of drugs. We present the following article in accordance with the Materials Design Analysis Reporting (MDAR) checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5490/rc).
Methods
Chemicals and materials
Metoprolol tartrate (MT, purity >99%), omeprazole (OMP, purity >98%), phenacetin (PNT, purity >98%), tolbutamide (TOL, purity >98%), gliclazide (GLZ, purity >95%), testosterone (T, purity >98%), and nicotinamide adenine dinucleotide phosphate (NADPH, purity >98%) were obtained from Meilunbio (Dalian, China). Male rat liver microsomes (RLMs, 20 mg protein·mL−1) were purchased from Encyclopedia Bio. Co. Ltd. (Chengdu, China). The chromatographic column was an Intersil ODS-2 column (5 µm, 4.6 mm ×150 mm) (Phenomenex, USA). High-performance liquid chromatography (HPLC)-grade methanol, formic acid, ammonium acetate, ethyl acetate, and other reagents were supplied by Sigma-Aldrich Co. (Alcobendas, Madrid, Spain). The green, Ti Kuan Yin (oolong), and Pu’er (dark) teas were provided by Yi Jiangnan Tea Industry Co. Ltd. (Hangzhou, China). The black tea was procured from Ming Tea Industry Co. Ltd. (Wuhan, China). Ultrapure water was obtained using a Millipore Milli-Q20 purification system (Millipore, Bedford, MA, USA).
Preparation of standards and quality control samples
Stock solutions of MT, OMP, PNT, TOL, and T were prepared independently in methanol at 4.058, 4.012, 8.125, 24.12, and 16.01 mg·mL−1, respectively. A mixed working solution containing 81.16 µg·mL−1 MT, 80.24 µg·mL−1 OMP, 162.5 µg·mL−1 PNT, 964.8 µg·mL−1 TOL, and 320.2 µg·mL−1 T was prepared by appropriately diluting the individual stock solutions with 10 mM phosphate-buffered saline (PBS) (pH 7.4) on the day of analysis. Eight calibration standards were prepared using the mixed working solution by spiking in blank RLMs (5 mg·mL−1) and PBS (10 mM, pH 7.4). The concentrations of the calibrators used in the assay were as follows: 25.36, 50.72, 101.4, 253.6, 507.2, 1,014, 2,029, and 4,058 ng·mL−1 MT; 25.07, 50.15, 100.3, 250.7, 501.5, 1,003, 2,006, and 4,012 ng·mL−1 OMP; 50.78, 101.6, 203.1, 507.8, 1,016, 2,031, 4,062, and 8,125 ng·mL−1 PNT; 301.5, 603.0, 1,206, 3,015, 6,030, 12,060, 24,120, and 48,240 ng·mL−1 TOL; and 100.1, 200.1, 400.2, 1,001, 2,001, 4,002, 8,005, and 16,010 ng·mL−1 T. The following quality control (QC) samples were prepared in the same manner: 50.72, 507.2 and 2,029 ng·mL−1 MT; 50.15, 501.5 and 2,006 ng·mL−1 OMP; 101.6, 1,016 and 4,062 ng·mL−1 PNT; 603.0, 6,030 and 24,120 ng·mL−1 TOL; and 200.1, 2,001 and 8,005 ng·mL−1 T. GLZ (5.115 µg·mL−1 in methanol) was used as the internal standard (IS). All stock solutions were stored at −20 °C.
Microsomal incubations
Incubation studies were performed under two sets of conditions. Firstly, the enzyme kinetics of a probe cocktail containing five drugs were investigated. Secondly, the incubation conditions were tested and optimized in terms of the microsomal protein concentration and incubation time. The probe substrates were incubated for 30 min at 37 °C in a final volume of 200 µL containing RLMs (0.5 mg protein·mL−1), PBS (100 mM, pH 7.4), MgCl2 (10 mM), NADPH (1 mM), and probe substrates. The concentrations of the specific CYP substrates were set to their tested values of the Michaelis constant (Km) as follows: MT (CYP2D6), 4.921 µM; OMP (CYP2C19), 9.662 µM; PNT (CYP1A2), 33.02 µM; TOL (CYP2C6), 157.1 µM; and T (CYP3A1/2), 44.05 µM. The incubation systems were initiated through the addition of NADPH, and all incubations were terminated by the addition of 200 µL of ice-cold ethyl acetate.
Enzyme kinetic study
Kinetic studies were performed for the five CYP-relevant probe reactions for each drug in each incubation system at the following concentrations: 1, 2, 10, 20, 40, and 80 µM for MT and OMP; 5, 10, 25, 50, 100, and 200 µM for PNT and T; and 10, 20, 40, 80, 160, and 320 µM for TOL. Triplicate samples of each group were analyzed. For each probe drug, the maximum rate of an enzyme catalysed reaction (Vmax) and Km were obtained by fitting the velocity data to the Michaelis-Menten kinetic model using GraphPad Prism 7.0 software (La Jolla, CA).
The Michaelis-Menten equation is as follows:
where CLv is the rate of drug elimination, Vmax represents the maximum reaction rate achieved by the system at saturation of the substrate concentration, Km equals the concentration of the substrate when the value of the rate of reaction is half of Vmax, and [S] is the substrate concentration.
Optimization of incubation conditions
The incubation conditions were optimized using the single factor method to select the optimal incubation time and protein concentration. Incubation times of 5, 10, 20, 30, 45, and 60 min were tested individually. Triplicate analyses were conducted for each incubation system. Linear regression analysis was conducted by plotting the variation in the probe drug concentration after incubation (ΔC) against the incubation time. A series of protein concentrations (0.1, 0.2, 0.4, 0.5, 0.6, and 0.8 mg·mL−1) of RLMs in the incubation systems were investigated and evaluated by linear regression analysis (by plotting ΔC against the protein concentration). The optimal values for the incubation time and RLM protein concentration were optimized found to be 30 minutes and 0.5 mg microsomal protein·mL−1, respectively.
Sample pretreatment
Microsomal sample preparation
A dual liquid-liquid extraction (LLE) method was established to preprocess the RLM samples. Prior to analysis, the routine daily calibration standards and QC were performed, and the subject samples were thawed at room temperature. A total of 200 µL of an RLM sample was combined with 0.5 mL of ethyl acetate and 10 µL of IS working solution (5.115 µg·mL−1) in a 2 mL centrifuge tube. The tube was vigorously vortexed for 5 min and then centrifuged at 12,000 rpm for 5 min. Subsequently, 450 µL of the organic layer (upper layer) was separated and transferred to another tube. The aqueous (lower) layer was adjusted to a basic pH by the addition of 50 µL of ammonium hydroxide, vortexed for 2 min, and then extracted with 0.5 mL of ethyl acetate. All extractions from the organic layer were pooled and evaporated to dryness under a nitrogen stream. The residue was redissolved in 1 mL of the initial mobile phase (A:B =45:55) for analysis by HPLC-tandem mass spectrometry (HPLC-MS/MS). To mitigate the photolysis of OMP, the entire process was conducted in the dark.
Aqueous tea extracts
Teas obtained from a supermarket were ground with a mortar and pestle. The crushed samples (20±1 g) were weighed and ultrasonically extracted at a ratio of 10:100 with boiling water for 15 min. After filtration, the residue was extracted twice with water, and the extract was pooled, concentrated, and dried under vacuum. The resulting tea powder was collected and stored at −20 °C before dissolution in 100 mL of PBS to obtain aqueous extracts (equivalent to 200 mg of tea·mL−1). Next, a series of concentrations of aqueous extracts was acquired by diluting the original aqueous extracts of tea with PBS.
Chromatographic and mass spectrometric conditions
HPLC-MS/MS analysis was performed using an Agilent HPLC instrument (Agilent, USA) comprising an autosampler, binary gradient pump, and thermostatted column oven, coupled with a triple quadrupole mass spectrometer (AB Sciex-API3000, USA). An Intersil ODS-2 column (5 µm, 4.6×150 mm) and matching guard column (4×3 mm) (Phenomenex, USA) were employed for separation at 30 °C. The HPLC instrument was operated under gradient separation with 0.1% aqueous formic acid (mobile phase A) and methanol (mobile phase B) at a flow rate of 0.8 mL/min with a split ratio of 1:3. The gradient started at 45% B, before increasing linearly to 90% B in 8 min, and subsequently reverting back to the initial condition of 45% B in 2 min. The final composition of the mobile phase was 45% B (held for 2 minutes for re-equilibration). For analysis, the injection volume was set at 10 µL.
MS determination was performed with positive electrospray ionization (ESI+) and multiple reaction monitoring (MRM). Nitrogen gas was used as the auxiliary, nebulizer, collision, and curtain gas. The main source parameters were optimized as follows: nebulizer gas, 10 L/min; curtain gas, 8 L/min; collision-activated dissociation gas, 3 L/min; source temperature, 450 °C; and turbo ion spray voltage, 5,000 V. The Analyst 1.4.1 software (MDS Inc., Concord, Ontario, Canada) was used to monitor the LC-MS/MS system as well as for data acquisition and processing.
Method validation
Validation of the analytical method was performed by measuring the specificity, linearity, sensitivity, matrix effects, recovery, precision, accuracy, and stability against the currently accepted US Food and Drug Administration’s bioanalytical method validation guidelines (23).
Selectivity and specificity
To investigate whether endogenous matrix constituents would interfere with the assay, six individual blank RLM samples, blank samples spiked with drugs at the lower limits of quantification (LLOQs) and the IS, and a microsomal sample incubated with the substrate cocktail and active RLMs were individually analyzed and evaluated for interference.
Calibration curves and lower limits of quantitation
The linearity of the detector response of the analytes was evaluated by injecting a total of eight working calibration standard solutions into the LC-MS/MS instrument on three consecutive days. The response was obtained for each sample by dividing the peak area of the analyte by the peak area of the corresponding IS. Calibration curves were constructed by plotting the ratio of the analyte to IS response versus the theoretical concentration (x) with a 1/x weighting factor. The sensitivity of the method was evaluated by determining the LLOQs, which needed to meet the requirement of a signal-to-noise (S/N) ratio of at least 10:1, with the acceptable deviations of the accuracy and precision set to ±20%. The LLOQs were measured using six replicates on the same day.
Precision and accuracy
The precision and accuracy of the method were assessed by analyzing six replicate samples at low, intermediate, and high concentration levels (QC-L, QC-M, and QC-H, respectively). The intraday precision and accuracy assays were performed on the same day, whereas the interday precision and accuracy assays were conducted on three consecutive days. Precision was expressed as the relative standard deviation (RSD%), and the accuracy was calculated as the relative error (RE%) between the measured and nominal concentrations. A value of less than ±15% was needed for the intra/interday precision and accuracy.
Matrix effect and extraction recovery
The extraction recovery of analytes was assayed by comparing the response of the analytes in postextraction samples to that in preextraction spiked samples at the three QC levels. The matrix effect was estimated by comparing the peak area of the matrix-matched sample and that of a standard solution at an equivalent low, intermediate, or high concentration levels. The matrix effect was estimated by a comparison between the peak area of the matrix-matched sample and that of a standard solution at an equivalent concentration for low, intermediate and high concentration levels. The experiments were performed using six replicates at three concentrations (QC-L, QC-M, and QC-H).
Stability
The stability experiments were conducted at three QC levels (n=5). The benchtop stability of the analytes was determined after 6 hours at ambient temperature, and the short-term stability was determined after 1 week at −20 °C. For evaluation of the autosampler stability, the microsomal samples were stored in the autosampler at 25 °C for 24 h prior to analysis. For the assessment of extract stability, the samples were redissolved after 12 h at ambient temperature prior to LC-MS/MS analysis.
CYP inhibition assay
The inhibitory effects of the four teas on the activity of CYP450 isoforms were evaluated in a probe cocktail incubation system (as described above). Ten microliters of aqueous tea extract was added to the incubation system before NADPH was added. After incubation, all microsomal samples were prepared following the steps for microsomal sample preparation described above. The microsomal samples were prepared and then subjected to the cocktail-based LC-MS/MS analysis to determine the probe drug concentrations. The control groups were treated with an equivalent amount of PBS instead of a tea extract. Triplicate samples of each group were analyzed.
Screening tea with CYP450 inhibitory properties
Teas at three concentration levels (1,000, 600, and 100 µg·mL−1) were co-incubated with the probe substrates under optimized conditions. The control group consisted of the probe substrates without tea. The activity of the enzymes was represented by the CLv of each CYP450 isoform. The enzymes in the control group were regarded as exhibiting 100% activity, and the residual activity (ReA%) of the enzymes was calculated by the average CLv value in the experimental group versus that in the control group. The inhibitory rate of enzyme activity was expressed as follows: . SPSS16.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing.
Determination of half-maximal inhibitory concentration
The tea concentrations were set to determine the half-maximal inhibitory concentration (IC50) as follows: 100, 200, 400, 800, 1,000, 1,200, and 1,400 µg·mL−1 for green tea to investigate CYP1A2 and CYP2C6 inhibition; 600, 800, 1,000, 1,200, 1,400, 1,600, and 1,800 µg·mL−1 for Ti Kuan Yin tea to study CYP1A2 inhibition; 300, 400, 500, 600, 700, 800, and 900 µg·mL−1 for black tea to analyze CYP1A2 inhibition; and 100, 200, 400, 600, 800, 1,000, and 1,200 µg·mL−1 for Pu’er tea to research CYP2C6 inhibition. The remaining substrates after incubation were analyzed and the data were analyzed using GraphPad Prism 7.0 software (La Jolla, CA, USA).
Statistical analysis
SPSS16.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze the inhibitory rate of enzyme activity. GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA, USA) was used to determine the kinetic parameters and IC50. P<0.05 was considered statistically significant.
Results
Establishment of the analytical method
Chromatographic separation of the targeted analytes was conducted using a single 12-min LC-MS/MS run. All of the analytes in this study yielded prominent protonated precursor molecular ions [M+H]+ in the positive ionization mode. The most abundant fragment ions were chosen for MRM fragmentation. The precursor and product ions selected for each analyte, as well as their collision-induced dissociation conditions and retention time, are shown in Table 1 and Figures S1-S6.
Table 1
| Analyte | Precursor ion mass (m/z) | Product ion mass (m/z) | Retention time (min) | Declustering potential (V) | Entrance potential (V) | Collision energy (eV) | Collision cell exit potential (V) |
|---|---|---|---|---|---|---|---|
| Metoprolol tartrate | 268.1 | 116.2 | 1.95 | 60 | 10 | 38 | 20 |
| Meprazole | 346.2 | 198.1 | 5.02 | 54 | 8 | 15 | 20 |
| Phenacetin | 180.1 | 110.2 | 6.11 | 54 | 9 | 30 | 20 |
| Tolbutamide | 271.3 | 155.2 | 7.98 | 54 | 10 | 21 | 13 |
| Testosterone | 289.2 | 109.1 | 9.90 | 54 | 8 | 35 | 11 |
| Gliclazide | 324.4 | 127.2 | 8.79 | 63 | 9 | 30 | 13 |
Validation of the analytical method
Typical chromatograms of a blank RLM sample (A), a blank RLM sample spiked with drugs at the LLOQs and the IS (B), and an RLM sample incubated with the substrate cocktail and active RLMs (C) are shown in Figure 1. Endogenous substances had no significant influence on the detection of analytes. The calibration curves for all the drugs were linear over the determined concentration ranges, with the correlation coefficient (R2) values all exceeding 0.996. The standard curve, correlation coefficient, calibration range, and LLOQs are presented in Table 2 and Figure S7. The obtained intra/interday accuracy and precision data of all analytes were within the acceptable range, and are summarized in Table 3. The intraday accuracy ranged from −3.98% to 10.42%, and the precision ranged from 3.53% to 12.91%. The interday accuracy ranged from −3.49% to 11.64%, and the precision ranged from 4.39% to 11.30%. These results suggested that the method had suitable accuracy and precision. As shown in Table 4, the extraction recoveries of all the analytes were in the range of 71.31% to 95.17%. The matrix effects of most analytes at the low, intermediate, and high concentration levels were in the range of 96.46% to 113.62%. The stability of the probes was also satisfactory, because the QC concentrations at the end of each condition remained within ±15% of the theoretical values (MT: −2.60–3.62%; OM: 1.61–5.06%; PNT: 0.27–12.76%; TOL −10.00–4.13%; and T: 1.09–9.94%).
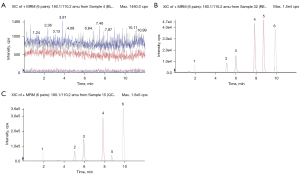
Table 2
| Analyte | Standard curve | R2 | Calibration range (ng·mL−1) | LLOQ (ng·mL−1) |
|---|---|---|---|---|
| Metoprolol tartrate | y=0.00264x + 0.0545 | 0.9976 | 25.36–4,058 | 25.36 |
| Omeprazole | y=0.0157x + 0.277 | 0.9986 | 25.07–4,012 | 25.07 |
| Phenacetin | y=0.0153x + 0.155 | 0.9975 | 50.78–8,125 | 50.78 |
| Tolbutamide | y=0.0018x + 0.375 | 0.9981 | 301.5–48,240 | 301.5 |
| Testosterone | y=0.00591x + 1.31 | 0.9962 | 100.1–16,010 | 100.1 |
LC-MS/MS, liquid chromatography-tandem mass spectrometry; R2, correlation coefficient; LLOQ, the lower limit of quantification.
Table 3
| Analyte | Spiked concentration (ng·mL−1) | Intraday (n=6) | Interday (n=18) | |||||
|---|---|---|---|---|---|---|---|---|
| Measured concentration (mean ± SD) | Precision (%RSD) | Accuracy (%RE) | Measured concentration (mean ± SD) | Precision (%RSD) | Accuracy (%RE) | |||
| Metoprolol tartrate | 50.27 | 52.35±4.34 | 8.29 | 3.22 | 52.44±4.07 | 7.76 | 3.4 | |
| 507.2 | 508.65±20.38 | 3.99 | 0.29 | 513.35±44.91 | 8.75 | 1.21 | ||
| 2,029 | 2,179.50±236.91 | 10.87 | 7.42 | 2,207.28±249.50 | 11.3 | 8.79 | ||
| Omeprazole | 50.15 | 50.76±4.08 | 8.03 | 1.21 | 51.92±5.21 | 10.03 | 3.52 | |
| 501.5 | 511.72±25.77 | 5.04 | 2.04 | 522.12±29.00 | 5.55 | 4.11 | ||
| 2,006 | 2,214.00±225.72 | 10.2 | 10.37 | 2,214.89±206.29 | 9.31 | 10.41 | ||
| Phenacetin | 101.6 | 97.56±12.60 | 12.91 | −3.98 | 98.06±10.74 | 10.95 | −3.49 | |
| 1016 | 1,121.83±121.55 | 10.83 | 10.42 | 1,134.22±114.18 | 10.07 | 11.64 | ||
| 4,062 | 4,336.83±414.37 | 9.55 | 6.77 | 4,247.72±328.48 | 7.73 | 4.57 | ||
| Tolbutamide | 603 | 606.77±28.81 | 4.75 | 0.62 | 615.63±31.77 | 5.16 | 2.09 | |
| 6,030 | 6,331.33±223.41 | 3.53 | 5 | 6,252.83±389.48 | 6.23 | 3.7 | ||
| 24,120 | 23,702.33±2,733.00 | 11.53 | −1.73 | 23,898.72±2,361.35 | 9.88 | −0.92 | ||
| Testosterone | 200.1 | 195.23±11.37 | 5.8 | −2.43 | 198.84±19.36 | 9.74 | −0.63 | |
| 2,001 | 2,049.83±150.07 | 7.32 | 2.44 | 2,058.78±156.35 | 7.59 | 2.89 | ||
| 8,005 | 8,375.17±312.41 | 3.73 | 4.62 | 8,362.00±367.24 | 4.39 | 4.46 | ||
LC-MS/MS, liquid chromatography-tandem mass spectrometry; SD, standard deviation; RSD, relative standard deviation; RE, relative error.
Table 4
| Analyte | Spiked concentration (ng·mL−1) | Extraction recovery (mean ± SD) (%) | Matrix effect (mean ± SD) (%) |
|---|---|---|---|
| Metoprolol tartrate | 50.27 | 72.36±1.87 | 105.33±2.84 |
| 507.2 | 73.60±3.69 | 99.37±5.08 | |
| 2,029 | 71.40±4.63 | 109.74±9.69 | |
| Omeprazole | 50.15 | 75.70±3.18 | 97.55±3.20 |
| 501.5 | 75.75±2.23 | 100.58±2.57 | |
| 2,006 | 77.57±5.18 | 96.46±3.43 | |
| Phenacetin | 101.6 | 91.62±11.08 | 102.44±7.38 |
| 1,016 | 81.69±6.32 | 104.04±3.69 | |
| 4,062 | 85.73±1.70 | 100.75±2.43 | |
| Tolbutamide | 603 | 85.96±5.79 | 111.12±10.74 |
| 6,030 | 87.64±5.77 | 101.40±3.93 | |
| 24,120 | 84.82±6.06 | 104.39±7.24 | |
| Testosterone | 200.1 | 92.94±6.85 | 106.46±6.71 |
| 2,001 | 91.54±4.07 | 113.62±12.99 | |
| 8,005 | 95.18±1.89 | 98.70±2.35 |
SD, standard deviation.
Enzyme kinetic profile of the probe cocktail substrates
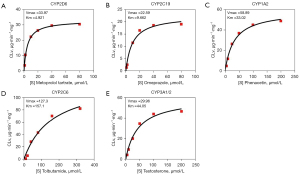
An enzyme kinetic study was conducted to optimize the probe substrate concentrations. The kinetic profiles of five probe reactions are represented in Figure 2. As shown in Figure 2, the Km values of MT, OMP, PNT, TOL, and T were 4.921, 9.662, 33.02, 157.1, and 44.05 µM, respectively.
Determination of the IC50 values
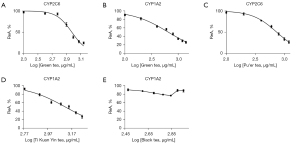
The IC50 values of the four teas were determined. As shown in Figure 3, the aqueous extracts of green tea strongly inhibited CYP1A2 and CYP2C6, as shown by IC50 value of less than 1,000 µg mL−1; the aqueous extracts of Ti Kuan Yin tea weakly inhibited CYP1A2, with the IC50 value being 1,304 µg·mL−1; the aqueous extracts of black tea with intermediate concentrations had only a slight inhibitory effect on CYP1A2, with the IC50 value being 840,314 µg·mL−1; and the aqueous extracts of Pu’er tea strongly inhibited CYP2C6, with the IC50 value being 738.7 µg·mL−1. However, none of the aqueous extracts from the four teas significantly altered the activity of CYP2D6, CYP2C19, and CYP3A1/2 in vitro. More detailed results can be found in the Supplemental Material.
Discussion
Selection of aqueous tea extracts and cytochrome P450 enzymes
According to their processing technique, teas can be divided into green, black, oolong (Ti Kuan Yin tea), and dark (Pu’er tea) teas. Among them, green tea is non-fermented, oolong tea is semi-fermented, black tea is fully fermented, and dark tea is microbial fermented. Owing to variation in their processing techniques, which include the steps of withering, fermentation, fixation, rolling, and drying, the chemical compositions and contents of these four teas differ (24). Furthermore, the chemical composition of tea can be affected by brewing temperature, time, and water quality (25,26). Therefore, in this study, the extraction process utilized was strictly controlled, and the experimental procedure remained consistent, so as to reduce the variable factors as much as possible. The edible part of tea is the aqueous extract, which contains water-soluble components, mainly catechins, polyphenols, and polysaccharides. Considering that the aqueous extracts of tea allow for research into the interaction between CY0450 enzymes and a mixture of water-soluble components rather than a single compound, they are suitable research objects.
The CYP450 superfamily includes many isoforms, such as CYP3A4, CYP2C9, CYP1A2, CYP2C19, CYP2D6, and CYP2E1. Chlorzoxazone is the specific probe of CYP2E1 (27). It may affect the metabolism of CYP3A4 substrates and further interact with CYP1A2 substrates; thus, its use in multi-substrate probe cocktails should be avoided (28). Furthermore, all of the analytes in this study were detected in the positive ion mode, while chlorzoxazone should be detected in the negative ion mode. Also, ESI polarity switching should be used during the run (29,30). Therefore, given the interaction between substrates and the limitation of using equipment (AB Sciex-API3000, USA) without polarity switching, CYP2E1 was not included in the present study.
LC-MS/MS evaluation
Considering the advantages of LC-MS/MS, including its high selectivity and specificity, a cocktail approach was developed to monitor the activity of several CYPs in a single experiment. The LC-MS/MS method was used to directly determine the concentrations of the probe drugs, from which the inhibitory effects of tea extracts with multiple components could be inferred. To ensure reliability during normal use, the analytical method was validated through a series of experiments, including those to measure the specificity, linearity, sensitivity, matrix effects, recovery, precision, accuracy, and stability; the results satisfied the measurement requirements. This demonstrated that the analytical method was suitable for its intended purpose and was accurate, specific, and precise over the specified range at which an analyte would be analyzed. The results showed that the IC50 values of green tea were 652.2 µg·mL−1 on CYP1A2 and 953.6 µg·mL−1 on CYP2C6, the IC50 value of Pu’er tea was 738.7 µg·mL−1 on CYP2C6, the IC50 value of Ti Kuan Yin tea was 1,304.0 µg·mL−1 on CYP1A2, and the IC50 value of black tea was 840,314.0 µg·mL−1 on CYP1A2; these values were reliable.
Optimization of the incubation conditions
In our experiment, different protein concentrations were evaluated. The protein concentration in an incubation system can have a major effect on estimates of the inhibitory potency of inhibitors and contribute to variations between studies (31-33). Therefore, in this study, the lowest possible microsomal protein concentration was used to reduce the probability of non-specific binding of substrates to microsomal proteins in in vitro experiments of metabolic inhibition (34). As shown in Figure S8, the depletion of all five probe drugs was linear, with the protein concentrations increasing (0.1–0.8 mg·mL−1) after incubation for 30 min. Considering protein binding as well as reaction rates, a protein concentration of 0.5 mg·mL−1 was used.
For optimization of the incubation time, the linear relationship between the incubation time and the depletion of all five probe drugs within 5 to 60 min was evaluated. As shown in Figure S9, the results demonstrated that the depletion of all five probe drugs was linear with the increase in incubation time from 5 to 30 min (35,36), and each probe drug depleted by less than 30%. However, the depletion rate of testosterone decreased after 30 minutes, which indicated that inhibition of testosterone would occur if the incubation time were too long. Moreover, due to the instability of enzymes in vitro, the reaction time should be appropriately short. Therefore, 30 minutes was chosen as the incubation time. In addition, results of previous reports have indicated that the concentration of organic solvents in the incubation mixture should be kept below 0.5–1% (v/v) to maintain the activity of CYPs at at least 80% (37,38). The methanol concentration was therefore set to <1% (v/v) in our incubation system.
Inhibition of CYP450 isoform activity by the four types of tea
The effect of plant extracts on CYP450 enzymes is currently being researched. Most studies have used the ReA% in the experimental and control groups for statistical testing, comparing whether significant differences are displayed or calculating IC50 values to determine whether inhibition or induction has occurred. Foti et al. (39) reported that there is a significant inhibitory effect of drugs on CYP450 if the ReA% is less than 10%. Furthermore, some studies (40,41) initially used the ReA% to evaluate the inhibition of CYP450 by herbs, and then, if the inhibition was greater than 70% at high concentrations, used a series of different concentrations to determine the IC50. Liu et al. (42) proposed that strong inhibition was observed with IC50 <1,000 µg·mL−1, but there was only a weak inhibitory effect on enzymes with IC50 >1,000 µg·mL−1.
In this study, three concentration levels of tea (low, intermediate, and high) were used to investigate whether the ReA% values show significant differences between the experimental and control groups, with an inhibitory effect indicated by P<0.05. As shown in Tables S1-S5, preliminary evaluation of the inhibitory effects of the teas on the five CYP450 isoforms showed that all three concentrations of green tea, the high-concentration of Ti Kuan Yin tea, and the intermediate-concentration of black tea could inhibit CYP1A2, whereas the high-concentration of green tea as well as the high- and intermediate-concentration of Pu’er tea samples had inhibitory effects on CYP2C6. However, none of the four teas had inhibitory effects on CYP2D6, CYP2C19, or CYP3A1/2. The aqueous extracts of green tea strongly reduced the activity of CYP1A2 and CYP2C6; the aqueous extracts of Ti Kuan Yin tea weakly inhibited CYP1A2; the aqueous extracts of black tea had only a slight inhibitory effect on CYP1A2; and the aqueous extracts of Pu’er tea had a strong inhibitory effect on CYP2C6.
Due to interspecies differences, in vitro studies have suggested variation in the degree to which tea extracts (mainly green tea) inhibit the activity of CYP1A1, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2D6, and CYP3A4 (43,44). Rat models have shown reduced enzyme activity of CYP2C, CYP2E1, and CYP3A with green and black tea treatment (45,46), as well as increased CYP3A activity with oolong tea ingestion (47). Given the regional differences and slight changes in tea processing techniques, the results obtained in this study may differ from those of previous studies. The four teas used in our study inhibited different CYPs: green tea inhibited CYP1A2 and CYP2C6; black tea inhibited CYP1A2; Pu’er tea (oolong tea) inhibited CYP2C6; and Ti Kuan Yin (dark tea) inhibited CYP1A2.
The chemical components of tea mainly consist of phenols, alkaloids, and amino acids, and some of these compounds have been linked to health benefits, and give tea its bitter taste and astringent quality (48). Most phenolic compounds found in tea are polyphenols, which are represented by catechins. Catechins and their derivatives have significant inhibitory effects on the activity on CYPs, with gallated catechins able to inhibit most CYPs (21,49,50). Tea alkaloids are generally purine alkaloids, of which caffeine is the most abundant alkaloid in all six categories of tea (51,52). Caffeine also inhibits the activity of CYPs, particularly CYP1A2 (53). Furthermore, tea extract contains a considerable number of amino acids, including theanine, which is a non-proteinic amino acid that is unique to tea. Theanine alone does not change CYP activity directly, but modulates the biodistribution or damage for liver protection (54). The chemical components of tea vary considerably according to the places of production, character of the cultivar, manufacturing style, cultivation method, and brewing. Therefore, phenols, alkaloids, and amino acids may directly or indirectly affect the activity of CYPs (51,52). In the present study, tea extract, rather than a single component, was examined to comprehensively reflect the synergistic inhibitory effects of multiple components on the activity of CYPs.
Our study also has limitations that should be noted. For instance, regarding the effects of tea on the selection of the method, the composition of tea was not investigated to check for the presence of potential CYP inhibitors. Further studies should be carried out on the compositional changes in tea extract to identify the key components that relieve the burden on the liver. Moreover, subsequent studies with human liver microsomes and in vivo studies are needed to examine the effects of tea on CYP450 enzymes under physiological conditions and provide scientific information on potential in vivo tea-drug interactions.
Conclusions
In this study, we developed a highly sensitive LC-MS/MS method with a suitable linear range and short analysis time for the simultaneous determination of five probe drugs (MT, OMP, PNT, TOL, and T) in RLMs. This method was applied to assess the inhibitory effects of aqueous extracts of four types of tea on CYP450 enzymes in vitro. These results suggested that aqueous extracts of green tea have potential influences on the metabolism of drugs mediated by CYP1A2, such as PNT, theophylline, paracetamol, caffeine, and imipramine. We found that drugs metabolized by CYP2C6 (ibuprofen, TOL, warfarin, phenytoin, and irbesartan) are influenced by the aqueous extracts of green and Pu’er teas. Caution should therefore be applied during the concomitant use of tea with these drugs.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81373969) and the Key Scientific Research and Development Program of Sichuan Province (No. 2019YFS0066).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-5490/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-5490/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-5490/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5490/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article does not contain any studies with human participants or animals performed by any of the authors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brody H. Tea. Nature 2019;566:S1. [Crossref] [PubMed]
- Ohishi T, Goto S, Monira P, et al. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med Chem 2016;15:74-90. [Crossref] [PubMed]
- Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial Properties of Green Tea Catechins. Int J Mol Sci 2020;21:1744. [Crossref] [PubMed]
- Yang K, Gao ZY, Li TQ, et al. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int J Biol Macromol 2019;122:95-103. [Crossref] [PubMed]
- Cao J, Han J, Xiao H, et al. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients 2016.
- Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD009934. [Crossref] [PubMed]
- Yang CS, Zhang J. Studies on the Prevention of Cancer and Cardiometabolic Diseases by Tea: Issues on Mechanisms, Effective Doses, and Toxicities. J Agric Food Chem 2019;67:5446-56. [Crossref] [PubMed]
- Kotzé-Hörstmann LM, Sadie-Van Gijsen H. Modulation of Glucose Metabolism by Leaf Tea Constituents: A Systematic Review of Recent Clinical and Pre-clinical Findings. J Agric Food Chem 2020;68:2973-3005. [Crossref] [PubMed]
- Liu C, Guo Y, Sun L, et al. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct 2019;10:2061-74. [Crossref] [PubMed]
- Tang P, Shen DY, Xu YQ, et al. Effect of Fermentation Conditions and Plucking Standards of Tea Leaves on the Chemical Components and Sensory Quality of Fermented Juice. Journal of Chemistry 2018;2018:4312875. [Crossref]
- Templeton I, Peng CC, Thummel KE, et al. Accurate prediction of dose-dependent CYP3A4 inhibition by itraconazole and its metabolites from in vitro inhibition data. Clin Pharmacol Ther 2010;88:499-505. [Crossref] [PubMed]
- Chen XM, Wei M, Zhang HM, et al. Effect of vanillin and ethyl vanillin on cytochrome P450 activity in vitro and in vivo. Food Chem Toxicol 2012;50:1897-901. [Crossref] [PubMed]
- Han YL, Li D, Ren B, et al. Evaluation of impact of Herba Erigerontis injection, a Chinese herbal prescription, on rat hepatic cytochrome P450 enzymes by cocktail probe drugs. J Ethnopharmacol 2012;139:104-9. [Crossref] [PubMed]
- de Castro WV, Mertens-Talcott S, Derendorf H, et al. Grapefruit juice-drug interactions: Grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci 2007;96:2808-17. [Crossref] [PubMed]
- Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 1997;32:210-58. [Crossref] [PubMed]
- Administration FaD. Draft Guidance for Industry on Drug Interaction Studies-Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations;Availability. Federal Register;77.
- Murray M. Role of CYP pharmacogenetics and drug-drug interactions in the efficacy and safety of atypical and other antipsychotic agents. J Pharm Pharmacol 2006;58:871-85. [Crossref] [PubMed]
- Preissner S, Kroll K, Dunkel M, et al. SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res 2010;38:D237-43. [Crossref] [PubMed]
- Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest 1997;99:2545-53. [Crossref] [PubMed]
- Krishnan R, Raghunathan R, Maru GB. Effect of polymeric black tea polyphenols on benzo(a)pyrene [B(a)P]-induced cytochrome P4501A1 and 1A2 in mice. Xenobiotica 2005;35:671-82. [Crossref] [PubMed]
- Satoh T, Fujisawa H, Nakamura A, et al. Inhibitory Effects of Eight Green Tea Catechins on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activities. J Pharm Pharm Sci 2016;19:188-97. [Crossref] [PubMed]
- Chen X, Sun CK, Han GZ, et al. Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression. World J Gastroenterol 2009;15:1829-35. [Crossref] [PubMed]
- FDA. Bioanalytical Method Validation Guidance for Industry. Available online: http://www.fda.gov. Accessed July 2015.
- Wang Y, Kan Z, Wang D, et al. Differences in Chemical Composition among Commercially Important Cultivars of Genus Camellia. J Agric Food Chem 2019;67:5457-64. [Crossref] [PubMed]
- Xu YQ, Zou C, Gao Y, et al. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chem 2017;236:142-51. [Crossref] [PubMed]
- Franks M, Lawrence P, Abbaspourrad A, et al. The Influence of Water Composition on Flavor and Nutrient Extraction in Green and Black Tea. Nutrients 2019;11:80. [Crossref] [PubMed]
- Lucas D, Ferrara R, Gonzalez E, et al. Chlorzoxazone, a selective probe for phenotyping CYP2E1 in humans. Pharmacogenetics 1999;9:377-88. [Crossref] [PubMed]
- Palmer JL, Scott RJ, Gibson A, et al. An interaction between the cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A). Br J Clin Pharmacol 2001;52:555-61. [Crossref] [PubMed]
- Zhang T, Zhu Y, Gunaratna C. Rapid and quantitative determination of metabolites from multiple cytochrome P450 probe substrates by gradient liquid chromatography-electrospray ionization-ion trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002;780:371-9. [Crossref] [PubMed]
- Kim HJ, Lee H, Ji HK, et al. Screening of ten cytochrome P450 enzyme activities with 12 probe substrates in human liver microsomes using cocktail incubation and liquid chromatography-tandem mass spectrometry. Biopharm Drug Dispos 2019;40:101-11. [Crossref] [PubMed]
- Bjornsson TD, Callaghan JT, Einolf HJ, et al. The conduct of in vitro and in vivo drug-drug interaction studies: a PhRMA perspective. J Clin Pharmacol 2003;43:443-69. [Crossref] [PubMed]
- Margolis JM, Obach RS. Impact of nonspecific binding to microsomes and phospholipid on the inhibition of cytochrome P4502D6: implications for relating in vitro inhibition data to in vivo drug interactions. Drug Metab Dispos 2003;31:606-11. [Crossref] [PubMed]
- Tran TH, Von Moltke LL, Venkatakrishnan K, et al. Microsomal protein concentration modifies the apparent inhibitory potency of CYP3A inhibitors. Drug Metab Dispos 2002;30:1441-5. [Crossref] [PubMed]
- Nirogi R, Palacharla RC, Uthukam V, et al. Chemical inhibitors of CYP450 enzymes in liver microsomes: combining selectivity and unbound fractions to guide selection of appropriate concentration in phenotyping assays. Xenobiotica 2015;45:95-106. [Crossref] [PubMed]
- Nguyen V, Espiritu M, Elbarbry F. Development and validation of a sensitive and specific LC-MS/MS cocktail assay for CYP450 enzymes: Application to study the effect of catechin on rat hepatic CYP activity. Biomed Chromatogr 2020;34:e4789. [Crossref] [PubMed]
- Lee JT, Pao LH, Hsiong CH, et al. Validated liquid chromatography-tandem mass spectrometry method for determination of totally nine probe metabolites of cytochrome P450 enzymes and UDP-glucuronosyltransferases. Talanta 2013;106:220-8. [Crossref] [PubMed]
- Busby WF Jr, Ackermann JM, Crespi CL. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab Dispos 1999;27:246-9. [PubMed]
- Easterbrook J, Lu C, Sakai Y, et al. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos 2001;29:141-4. [PubMed]
- Foti RS, Wahlstrom JL, Wienkers LC. The in vitro drug interaction potential of dietary supplements containing multiple herbal components. Drug Metab Dispos 2007;35:185-8. [Crossref] [PubMed]
- Usia T, Iwata H, Hiratsuka A, et al. CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine 2006;13:67-73. [Crossref] [PubMed]
- Appiah-Opong R, Commandeur JN, Axson C, et al. Interactions between cytochromes P450, glutathione S-transferases and Ghanaian medicinal plants. Food Chem Toxicol 2008;46:3598-603. [Crossref] [PubMed]
- Liu KH, Kim MJ, Jeon BH, et al. Inhibition of human cytochrome P450 isoforms and NADPH-CYP reductase in vitro by 15 herbal medicines, including Epimedii herba. J Clin Pharm Ther 2006;31:83-91. [Crossref] [PubMed]
- Albassam AA, Markowitz JS. An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis). Planta Med 2017;83:496-508. [Crossref] [PubMed]
- Markowitz JS, Zhu HJ. Limitations of in vitro assessments of the drug interaction potential of botanical supplements. Planta Med 2012;78:1421-7. [Crossref] [PubMed]
- Yao HT, Hsu YR, Li ML. Beverage-Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats. Membranes (Basel) 2020;10:233. [Crossref] [PubMed]
- Yao HT, Hsu YR, Lii CK, et al. Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in Wistar rats. Food Chem Toxicol 2014;70:120-7. [Crossref] [PubMed]
- Niwattisaiwong N, Luo XX, Coville PF, et al. Effects of Chinese, Japanese and Western tea on hepatic P450 enzyme activities in rats. Drug Metabol Drug Interact 2004;20:43-56. [Crossref] [PubMed]
- Nowogrodzki A. How climate change might affect tea. Nature 2019;566:S10-1. [Crossref] [PubMed]
- Misaka S, Kawabe K, Onoue S, et al. Effects of green tea catechins on cytochrome P450 2B6, 2C8, 2C19, 2D6 and 3A activities in human liver and intestinal microsomes. Drug Metab Pharmacokinet 2013;28:244-9. [Crossref] [PubMed]
- Muto S, Fujita K, Yamazaki Y, et al. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res 2001;479:197-206. [Crossref] [PubMed]
- Horie H, Kohata K. Analysis of tea components by high-performance liquid chromatography and high-performance capillary electrophoresis. J Chromatogr A 2000;881:425-38. [Crossref] [PubMed]
- Tang GY, Meng X, Gan RY, et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int J Mol Sci 2019;20:6196. [Crossref] [PubMed]
- Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet 2000;39:127-53. [Crossref] [PubMed]
- Sadzuka Y, Sugiyama T, Nagamine M, et al. Efficacy of theanine is connected with theanine metabolism by any enzyme, not only drug metabolizing enzymes. Food Chem Toxicol 2006;44:286-92. [Crossref] [PubMed]


