Comparison of bone marrow sparing intensity modulated radiotherapy (IMRT) and three-dimensional conformal radiotherapy (3DCRT) in carcinoma of anal canal: a prospective study
Introduction
The scenario of treatment of anal canal carcinoma changed when Nigro et al. (1) reported his case series with three patients with chemoradiation (CRT). After the successful incorporation of definitive CRT in anal cancers, the literature on anal canal carcinoma has seen a shift from improving local control and colostomy free survival to decreasing the treatment related toxicity. Intensity modulated radiotherapy (IMRT) in this respect has been a major field of research in the treatment protocols. The large volume of bone marrow (BM) irradiated during conventional pelvic-inguinal radiation is likely to cause significant hematological toxicity, because up to 50% of a patient’s total hematopoietically active BM is within the conventional treatment fields (2). BM stem cells are highly radiosensitive, and the destruction of these cells during pelvic radiation is a principal cause of acute myelosuppression (3). In our study, we compared two different techniques of radiation in the CRT protocols of anal cancers, the three-dimensional conformal radiation therapy (3DCRT) and the BM sparing IMRT. The primary end point of this study was to compare the acute toxicity between the two treatment groups in the form of hematological and gastrointestinal events. In addition to it, dosimetric comparison between the plans generated by the two techniques was also done in-terms of doses to organs at risk. Secondary end points were need for blood transfusion, parental funds, anti motility and antispasmodic drugs and local control evaluation at the end of 6 months.
Material and methods
Study was conducted in Department of Radiotherapy at regional cancer research center P.G.I.M.E.R. Chandigarh, from Nov 2011 to May 2013. Twenty patients were enrolled in the study and randomized between the two arms of group A with radiation delivered with 3DCRT and group B where IMRT was used for radiation delivery. All patients received concurrent chemotherapy with 5-FU (375 mg/m2) and cisplatin (30 mg/m2) weekly. A radiation dose of 45 Gy/25 fractions/5 weeks was delivered in both the arms. Patients were evaluated with weekly records of complaints, physical examination, complete blood counts, and need for supportive care.
Patients in both the treatment groups were simulated in Phillips CT simulator where planning CECT with immobilization in prone position was taken at 2.5 mm slice thickness. Target delineation was done according to the RTOG consensus guidelines for anorectum (4), where gross total volume (GTV), clinical target volume (CTV), planning target volume (PTV) were delineated along with the organs at risk; the pelvic BM as seen on CT image, bowel bag, urinary bladder and femoral heads. Inguinal lymph nodes were electively treated in all the groups.
Group A: three-dimensional conformal radiation therapy (3DCRT) arm
A 4-field forward 3DCRT plan with multi leaf collimators was made for each patient in this arm. Plans were evaluated and on approval, treatment started, 6 or 15 MV photons were used in the treatment.
Group B: intensity modulated radiotherapy (IMRT) arm
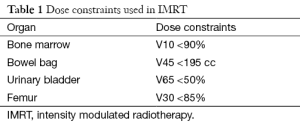
Patients in this group were treated by IMRT technique. For all IMRT plans, 6 MV photons were utilized on the Eclipse Planning System. Radiation treatment was designed to be delivered by a Varian Linear Accelerator (Varian Medical Systems, Palo Alto, CA). Dynamic IMRT plans were generated for this group with dose constraints as shown in Table 1.
Full table
Residual disease in both the treatment arms was managed by boost external radiation to local growth, interstitial brachytherapy, or surgery depending on disease status, response to radiation and patients’ willingness for surgery (data not shown).
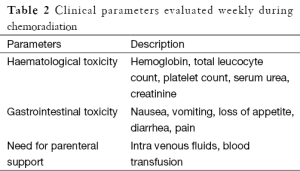
Patients were followed up on weekly basis during radiation. Para meters evaluated weekly are shown in Table 2. Toxicity evaluation was done using Common Toxicity Criteria for Adverse Events (CTCAE) version 3 (5).
Full table
In addition to that dosimetric comparisons were made between the two treatment arms. The mean percentage volume of urinary bladder, and small bowel receiving more than 30 and 40 Gy (V30 and V40) were calculated from the plans. Also the mean percentage volume of delineated BM receiving more than 10 and 20 Gy (V10 and V20) was calculated and compared between the two treatment groups.
Statistical analysis
Descriptive statistics was used for demographic characterization. ANOVA was used for identifying heterogeneity between the two arms. Fisher exact test was used to know the significance of difference in acute toxicities. Statistical analysis was performed using the statistical package for social sciences (SPSS) software V 19.0. A P value of <0.05 was taken as significant.
Results
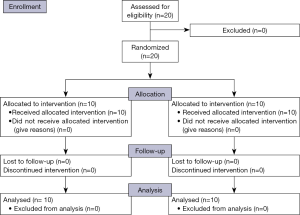
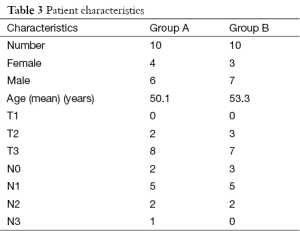
Twenty patients with carcinoma anal canal were divided equally into two groups. All patents completed their treatment and were eligible for analysis (consort diagram in Figure 1). The differences in male to female ratio was not significant (P=0.639), the median age in 3DCRT group was 50.1 years, and in IMRT group was 53.3 years which was not significant (P=0.725). Baseline characteristics of patients are shown in Table 3. The incidence of lymph node-positive disease and the overall AJCC stage also did not differ significantly between the two groups (Table 3).
Full table
Toxicity evaluation
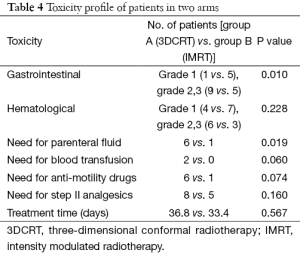
Acute toxicity rates suggested a lower incidence of hematological and gastrointestinal toxicity with the use of IMRT. Parameters like incidence of worst hematological toxicity, grade II (GII) and GIII was seen in 40% [4] vs. 30% [3] and 20% [2] vs. 0% [0] respectively, in 3DCRT and IMRT group (Table 4). However these did not come as statistically significant (P=0.228). Difference in gastrointestinal toxicity, however, was significantly better in favor of IMRT group than 3DCRT group for GIII toxicity. Incidence of worst gastrointestinal toxicity during treatment in terms of GII was 30% [3] vs. 50% [5] and GIII was 60% [6] vs. 0% [0] in 3DCRT and IMRT group respectively (P=0.01).
Full table
Other parameters indicating better tolerance of treatment with IMRT arm than 3DCRT arm were lesser need for administration of parenteral fluid 10% [1] vs. 60% [6] (P=0.019); lesser need for blood transfusion 0% [0] vs. 20% [2] (P=0.060) in IMRT arm than in 3DCRT arm respectively. Patient requiring supportive care during treatment like need for anti-motility drugs and WHO. Step II analgesics also favored IMRT arm as shown in Table 4. All this was reflected in earlier treatment completion in patients of Arm B (33.40 days) than in Arm A patients (36.8 days), although difference was not statistically significant (P=0.569) (Table 4).
Dosimetric evaluation
Comparison of OAR sparing between IMRT and conventional 3D plans
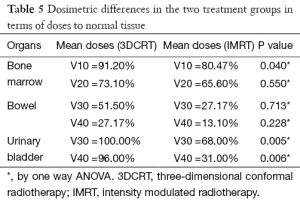
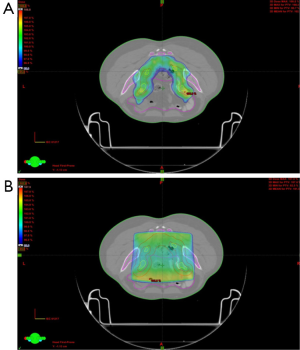
Use of IMRT in group B patients showed a difference in the volume of bladder and bowel receiving ≥30 and ≥40 Gy patients as compared to conventional 3D plans. The mean volume of bladder receiving ≥30 and 40 Gy respectively was 100% and 96% for group A (3DCRT) as compared to 68% and 31% for the group B (IMRT) (P<0.05) (Table 5). For bowel, although, the V30 and V40 for 3DCRT vs. IMRT respectively were 51% and 27% vs. 27% and 13%, statistical significance was not reached (P>0.05). There was also less mean BM receiving ≥10 Gy (80.4%) and ≥20 Gy (65.6%) for group B using IMRT, than in 3DCRT (group A) were it was 91% and 73% respectively. Although for V10 it was significant (P=0.04), it did not reach statistical significance for the V20 (P=0.550) (Table 5). Figure 2 is showing a typical dose color wash obtained with (I) 3DCRT and (II) IMRT in patients of our study.
Full table
Local control
With a median follow up of 9.2 months for 3DCRT and 8 months for IMRT, there was no difference in terms of local control in the two treatment groups. In both the arms six patients were showing complete response (CR) and four of the patients were showing partial response (PR).
Discussion
We initiated a comparison to evaluate any advantage of use of BM sparing IMRT in anal cancer patients over 3DCRT both clinically and dosimetrically. We have shown that using BM sparing IMRT, throughout the entire treatment reduces threshold radiation doses to the small bowel, bladder, and BM as compared to 3DCRT treatment technique. The mean values that we obtained for these critical structures with a prescription dose of 45 Gy treatments are comparable to those obtained by Milano et al. (6) in their IMRT treatment arm with a dose of 45 Gy.
In our study, BM sparing IMRT has provided statistically reduced mean and threshold doses to the organs at risk. The mean V10 and V20 for BM in our patient subgroup were 80% and 65%, respectively, compared to the 73% and 59% obtained by Milano et al. (6). A reduction in the volume of bladder and genitalia receiving higher doses was also achieved with the use of IMRT vs. 3DCRT in the delivery of the final radiation boost who received it with external beam radiation (data not shown).
Iliac BM is a relatively large structure and reducing the dose to this structure, which surrounds the anal canal, is a problem even in the most conformal of techniques. More sophisticated techniques such as more rigorous IMRT constraints or using the field reductions recommended by the RTOG in all patients, may improve hematological toxicity. However, the use of concurrent mitomycin C may negate any potential benefit of IMRT BM sparing. A benefit in hematological toxicity with IMRT may be achieved in patients receiving cisplatin and 5-FU, a CRT regimen that has been studied in phase II settings (7-12). In our study also, we have used cisplatin concurrently with radiation instead of mitomycin C, as the former’s toxicity profile is much favorable.
Incidence of worst hematological toxicity, GII and GIII was seen in 90% vs. 50% respectively in group A vs. group B. However this did not come as statistically significant (P=0.228). Difference in gastrointestinal toxicity, however was significantly better in favor of group B (IMRT) than group A (3DCRT). Incidence of worst gastrointestinal toxicity during treatment in terms of GIII was 60% (6) vs. 0% (0) in Arm A and Arm B respectively (P=0.01). This decrease in toxicity has led to a lesser incidence of need for administration of parenteral fluid 10% [1] vs. 60% [6] (P=0.019); lesser need for blood transfusion 0% [0] vs. 20% [2] (P=0.060) favor the use of IMRT. Other parameters like need for WHO. Step II analgesics and use of anti-motility drugs also favored IMRT arm.
Treatment breaks in anal cancer therapy have been associated with inferior disease-related outcomes. RTOG 92-08 examined toxicity rates and radiation dose in anal cancer patients. In this investigation, 59.4 Gy was delivered over 8.5 weeks, including a mandated 2-week treatment break to relieve treatment-related toxicity. Although not powered for comparison, overall, disease-free, and colostomy-free survival rates were lower than results from previous chemo-radiotherapy trials using lower radiation doses with no treatment break. The authors concluded that treatment breaks should be avoided to maximize disease-related outcomes (13).
A recent analysis of anal cancer patients treated at Memorial Sloan-Kettering Cancer Centre also suggested that frequent treatment breaks or inability to complete radiation because of toxicity are predictors for disease recurrence. Patients were treated primarily using conventional radiation techniques, with 77% of their cohort receiving at least one treatment break. Failure to complete radiation therapy was a significant predictor for anal cancer relapse (14). In our study, the difference in earlier completion of treatment was not statistically significant; IMRT versus 3DCRT arm (36.8 vs. 33.4 days) respectively probably because of ours being a lesser powered study.
Our data thus, supports the hypothesis that concurrent chemotherapy and IMRT seem to be at the favourable end of the research as far as decreasing acute toxicity rates and overall treatment time is concerned in anal canal cancer patient.
Conclusions
In conclusion, our series suggests that IMRT-based CRT therapy may result in reduction in normal tissue doses and corresponding acute toxicity rates compared to 3DCRT and historic controls; potentially leading to fewer toxicity-related treatment interruptions. Based on our single-institution experience, early treatment related outcomes seem encouraging. The fact that in this study, cisplatin was used instead of mitomycin C also throws a light into the feasibility of use of cisplatin in anal cancer patients, majority of who are of old and expected to develop more toxicity, especially, hematological. However, a more powered study with more number of patients is required and long-term local controls need to be established before incorporation of BM sparing IMRT as a routine practice in carcinoma anal canal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. [PubMed]
- Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol 1961;5:255-8. [PubMed]
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [PubMed]
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824-30. [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [PubMed]
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354-61. [PubMed]
- Martenson JA, Lipsitz SR, Wagner H Jr, et al. Initial results of a phase II trial of high dose radiation therapy, 5-fluorouracil, and cisplatin for patients with anal cancer (E4292): an Eastern Cooperative Oncology Group study. Int J Radiat Oncol Biol Phys 1996;35:745-9. [PubMed]
- Rich TA, Ajani JA, Morrison WH, et al. Chemoradiation therapy for anal cancer: radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol 1993;27:209-15. [PubMed]
- Doci R, Zucali R, La Monica G, et al. Primary chemoradiation therapy with fluorouracil and cisplatin for cancer of the anus: results in 35 consecutive patients. J Clin Oncol 1996;14:3121-5. [PubMed]
- Peiffert D, Giovannini M, Ducreux M, et al. High-dose radiation therapy and neoadjuvant plus concomitant chemotherapy with 5-fluorouracil and cisplatin in patients with locally advanced squamous-cell anal canal cancer: final results of a phase II study. Ann Oncol 2001;12:397-404. [PubMed]
- Hung A, Crane C, Delclos M, et al. Cisplatin-based combined modality therapy for anal carcinoma: a wider therapeutic index. Cancer 2003;97:1195-202. [PubMed]
- Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol 2003;69:201-7. [PubMed]
- Konski A, Garcia M Jr, John M, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys 2008;72:114-8. [PubMed]
- Roohipour R, Patil S, Goodman KA, et al. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum 2008;51:147-53. [PubMed]