
Personalized tooth-supported digital guide plate used in the treatment of trigeminal neuralgia with balloon compression
Introduction
Trigeminal neuralgia (TN) is one of the most common cerebral nerve diseases and is common in people over 50 years old (1-3). Pain during TN attacks is extremely intense and intolerable (1-3). The chronic neuropathic pain disorder associated with TN is characterized by spontaneous paroxysmal pain, which is burning, electric shock-like, or stabbing, and is located in the innervation region of the trigeminal nerve (1-3). According to an epidemiological survey, women are more likely than men to experience TN (4,5). As the disease progresses, patients often develop severe anxiety and/or depression when experiencing intense pain (6,7). Great physical and mental suffering is experienced by patients with TN. The third edition of the International Classification of Headache Disorders discusses 3 types of TN: classical, secondary, and idiopathic (8). Treatment methods mainly include drug and surgical treatment. Drugs, such as carbamazepine and oxcarbazepine, have been considered the first-line agents of symptom control. Surgical treatment includes microvascular decompression (MVD), trigeminal ganglion percutaneous balloon compression (BC), percutaneous trigeminal ganglion radiofrequency thermocoagulation, stereotactic radiosurgery (SRS), and glycerol rhizotomy (1-3,7,9). Among these procedures, BC surgery, which is simple and safe and causes minimal trauma, has rapid and positive treatment effects and has been widely used in the clinical setting (10-12). Additionally, it is an ablative procedure that may be repeated over time and can be performed on aged patients or those who cannot tolerate or do not desire MVD (13,14).
In 1980, Mullan et al. first introduced BC as a type of TN treatment (13,15). The remaining primary challenge in BC is how to safely, effectively, quickly, and accurately access the foramen ovale (FO). The insertion method proposed by Hartel in 1913, which has been used for a long time (16), involves 3 the adoption of cutaneous reference points (the cheek point, zygomatic point, and mid-pupillary point) to guide the cannulation of the FO (16). Although experienced surgeons can easily complete an FO insertion, it is very difficult for novice surgeons to do so, resulting in longer operation times and additional patient trauma and surgical complications. This partially blind insertion technique may place important structures at high risk. Moreover, anatomical variations in the FO or the partial or complete ossification of the pterygospinous ligament and pterygoid ligament near the FO may block FO insertion (17,18). These factors may lead to longer insertion times or insertion failure, thereby also increasing patient trauma and serious postoperative complications. Furthermore, repeated FO insertion attempts may cause severe complications, such as more severe trauma, increased numbness, and bleeding (19). The therapeutic effect and complications of percutaneous BC largely depend on the accuracy and safety of FO insertion. To solve this problem, C-arm fluoroscopy and computed tomography (CT) are often used to obtain high-quality intraoperative data. However, this method cannot achieve real-time guidance during the operation, instead requiring surgeons to rely on their own experience and risking additional harm to patients. This method also exposes surgeons to environmental radiation. Various other methods, such as navigation (20-22) and guide plates (23-25), have also been used in the clinical setting. Many previous studies have used a guide plate for radiofrequency or microsphere compression treatment, but without exception, they have used a facial contour-based design (24,26-28). Although the application of these guide plates can significantly reduce difficulty and improve surgical accuracy, due to the flexibility of the maxillofacial soft tissue, there are some related shortcomings, such as unstable retention, the need for intraoperative artificial fixation, and even repeated adjustment of position, which can affect the accuracy and success rate of FO insertion.
Teeth are an ideal anatomical structure for plate retention, and tooth-supported digital guide plates are widely used in all kinds of oral surgery. The position of teeth in the oral cavity is stable, there is no need to accommodate the maxillofacial soft tissue, and a change in body position will not affect tissue morphology. Thus, teeth are an ideal anatomical structure for retention, and as such we used them as a fixed method, thereby eliminating the necessity for the operator to adjust or help fix the guide plate. By relying only on the teeth, the position of the cannula can be kept constant, which reduces human-inflicted displacement of the needle and cannula.
Therefore, to lessen patient trauma, reduce operating difficulty and duration, improve operating efficiency, and better protect the surgeon, in this pilot study we sought to describe personalized tooth-supported digital guide plates in the compression treatment of TN with BC. We present the following article in accordance with the Transparent Reporting of Evaluations and Nonrandomized Designs (TREND) reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4827/rc).
Methods
Ethics approval and informed consent
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (No. SH9H-2020-TI88-2). All participants signed a consent form.
Patient enrollment
Patients were recruited from the Oral Surgery Department of the Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Between January 2019 and November 2020, 15 patients with TN (11 females and 4 males) were enrolled after a review of their medical history and clinical examination. The inclusion criteria (14,29) were as follows: (I) the diagnostic criteria of the International Society for Pain (29) were used in the patient’s TN diagnosis, and bilateral TN was excluded; (II) age greater than 50 years; (III) intracranial space-occupying lesions were excluded by routine CT or magnetic resonance imaging (MRI); (IV) no surgical treatment history for TN; and (V) patient could tolerate general anesthesia and TN BC operation. We summarized the basic characteristics of all patients with TN according to the enrollment criteria.
Examination and design for digital guide plate-guided BC operation through the mouth
All participants underwent a magnetic resonance tomographic angiography (MRTA) examination [Siemens Magnetom Trio, Munich, Germany; repetition time (TR): 7.9 ms; echo time (TE): 2 ms; reverse rotation angle: 70 degrees; thickness: 1 mm; interval: 0 mm; and 512×320 pixel matrix, 0.43 mm × 0.43 mm] to exclude space-occupying lesions in the cerebellopontine angle area. Additionally, all patients underwent an enhanced CT examination (light speed, GE Healthcare, Chicago, IL, USA GE; pitch: 1.375–1; thickness: 1.25 mm; interval: 1.25 mm; and bed speed: 27.5 mm/rot) from the cranial vault to the mandibular margin. A model of the maxillary teeth was obtained with alginate impression material and cast in place with anhydrite for each patient. Following this preoperative examination, the CT data were converted into three-dimensional (3D) reconstructions using Mimics 17.0 software via automatic segmentation methods (Materialise, Leuven, Belgium). First, the CT data was imported to Mimics 17.0 software. Next, according to the different thresholds of image data, the bone tissue was separated from the surrounding soft tissue. Thus, we obtained the imaging data of all cranial and maxillofacial bones, which can be reconstructed in the 3D model using Mimics 17.0 software. With the help of this software, the required data to obtain the 3D model of the specified bone tissue can be processed. Accurate 3D surface data were obtained using a laser scanner to scan the gypsum model of the maxillary teeth. Finally, the 3D CT reconstruction data were matched with the 3D data obtained from the maxillary teeth model.
A laser scanner was applied to scan the BC needle and cannula (Shenzhen Shineyard Medical Device Co., Ltd., Shenzhen, China; CTZ-15 L) to obtain accurate surface and 3D data. All models were exported in standard tessellation language (STL) format into the Mimics 17.0 software, where we could control the direction of the needle at will. During the design process, we placed the tip of the needle at the FO of the affected side, and the long axis of the needle pointed to the junction of the semilunar ganglion indentation and the nerve root. After determining the needle direction, the maxillary teeth were set as the retention surface, and the digital guide plate was designed, including retainers, connectors, and insertion and dislocation pathways. The surgical guide plate was 3D printed based on this design and then placed into the patient’s mouth to check the position and stability. After proper trimming, the guide plate was sterilized with low-temperature plasma for standby use. The same surgeon (Dr. Min-Jie Chen) operated on all patients.
Duration of insertion and number of needle adjustments
To verify the accuracy and time savings of this insertion process, we counted the guide plate time (from guide placement to the needle and cannula reaching the FO) and guide plate navigation time (from the beginning of insertion to the needle entering the FO) for each patient (Figure 1). We also counted the insertion and adjustment times for each patient.
Evaluation indices before and after digital guide plate-guided BC surgery
In this study, we compared 6 different time points for each participant before the operation (T0), immediately after the operation (T1), 1 day after the operation (T2), 1 week after the operation (T3), 1 month after the operation (T4), and 3 months after the operation (T5). At each time point, we counted the trigger point, the highest frequency of pain attack, the longest attack time, the Barrow Neurological Institute (BNI) (30,31) pain intensity score, and the visual analog scale (VAS) score. Participants were categorized as follows based on their daily attack frequency (AF): grade 0 (AF =0), grade 1 (1< AF ≤20), grade 2 (20< AF ≤40), grade 3 (40< AF ≤60), and grade 4 (AF >60). We also categorized participants’ pain attack duration (AD) as follows: grade 0 (painless), grade 1 (0< AD ≤10 s), grade 2 (10 s< AD ≤60 s), grade 3 (60 s< AD ≤180 s), grade 4 (180 s< AD ≤300 s), and grade 5 (AD >300 s).
The VAS and BNI pain intensity scores of TN episodes 2 days before digital guide plate-guided BC surgery and 1 day, 1 week, 1 month, and 3 months after surgery were recorded.
Statistical analysis
Data analysis was performed with SPSS 23.0 software (IBM Corp., Armonk, NY, USA). The comparison between preoperative and postoperative trigger point conditions was evaluated with McNemar’s test. The AF, AD, and BNI pain intensity were compared between preoperative and postoperative timepoints using the Wilcoxon matched-pair signed-rank test. The VAS scores were evaluated with paired sample t-tests between preoperative and postoperative timepoints (Kolmogorov-Smirnov test was used for normally distributed data). The Bonferroni correction was conducted for multiple comparisons. A P value <0.05 was considered statistically significant (2-sided).
Results
Basic patient information
Sociodemographic and medical characteristics of the 15 patients included based on the inclusion and exclusion criteria are shown in Table 1.
Table 1
| General data | Enrollment state |
|---|---|
| Age (years) | 66.7±8.61 |
| Gender (male/female) | 4/11 |
| Preoperative general condition evaluation (pass/fail) | 15/0 |
| Secondary TN (yes/no) | 0/15 |
| The course of TN (years) | 4.7±2.74 |
| Location of the disease (right/left) | 8/7 |
| Branches of the affected trigeminal nerve (V2.3/V1.2.3) | 13/2 |
| Vascular nerve compression (yes/no) | 0/15 |
| Medical treatment history (yes/no) | 15/0 |
| Surgical treatment history (yes/no) | 0/15 |
| Maxillary residual teeth | 9.1±2.09 |
TN, trigeminal neuralgia.
Comparison of preoperative situation and postoperative follow-up
In the study, guide plate insertion was completed within 60 seconds for all participants. Guide plate navigation time was limited to 5 seconds (Table 2). All 15 participants were directly punctured in the mouth without an extraoral incision. With the application of a personalized tooth-supported digital guide plate, successful insertion into the FO was achieved in 1 attempt for all 15 participants, as confirmed by CT scan. Then, we threaded the catheter and slowly inflated the balloon with contrast agent. No adjustments to the needle and catheter we required by 5 of the 15 participants. No participants required more than 3 adjustments. All 15 participants obtained the ideal compression effect (pear-shaped balloon with a small nipple protruding into the posterior fossa) after the needle and balloon were dislocated and fine-tuned. No postoperative complications occurred such as cerebrospinal fluid leakage, intracranial infection, or visual acuity change.
Table 2
| Operation time | Mean ± SD |
|---|---|
| Guide plate time (s) | 48.5±7.70 |
| Guide plate navigation time (s) | 3.7±0.82 |
FO, foramen ovale; s, second.
Table 3 displays the longitudinal changes of the trigger point, AF per day, AD, and BNI pain intensity score across the 6 timepoints. A total of 14 participants had no postoperative pain at T1, T2, T3, T4, or T5 during the follow-up. Just 1 participant had tolerable postoperative pain relief, which increased to aggravated and intolerable pain at T3. Carbamazepine was prescribed, and relief was achieved. The comparison showed that the trigger point, AF per day, AD, and BNI pain intensity score were significantly improved (all P<0.05) (Specific P values are shown in Table S1).
Table 3
| Time | Trigger point | Attack frequency per day | Attack duration | BNI pain intensity score | |||
|---|---|---|---|---|---|---|---|
| (Yes/No) | (Grade 0/1/2/3/4) | (Grade 0/1/2/3/4/5) | (Grade 1/2/3/4/5) | ||||
| Preoperation (T0) | 15/0 | 0/0/6/4/5 | 0/2/5/3/4/1 | 0/0/1/2/12 | |||
| immediately after surgery (T1) | 1/14 | NA | NA | NA | |||
| 1 day after surgery (T2) | 1/14 | 14/1/0/0/0 | 14/1/0/0/0/0 | NA | |||
| 1 week after surgery (T3) | 1/14 | 14/1/0/0/0 | 14/1/0/0/0/0 | 14/1/0/0/0 | |||
| 1 month after surgery (T4) | 0/15 | 15/0/0/0/0 | 15/0/0/0/0/0 | 14/0/1/0/0 | |||
| 3 months after surgery (T5) | 0/15 | 15/0/0/0/0 | 15/0/0/0/0/0 | 14/0/1/0/0 |
BNI, Barrow Neurological Institute; NA, not applicable.
This study compared pre- and postoperative VAS scores (Table 4). The VAS scores at T2, T3, T4, and T5 significantly decreased compared with T0 (all P<0.001) and gradually decreased further with the prolongation of follow-up time.
Table 4
| Time | VAS score | P value |
|---|---|---|
| Mean ± SD | ||
| Preoperation (T0) | 9.4±0.59 | NA |
| Immediately after surgery (T1) | NA | NA |
| 1 day after surgery (T2) | 1.8±0.89 | <0.001 |
| 1 week after surgery (T3) | 0.8±0.30 | <0.001 |
| 1 month after surgery (T4) | 0.5±0.20 | <0.001 |
| 3 months after surgery (T5) | 0.4±0.22 | <0.001 |
VAS score, visual analog scale score; NA, not applicable; SD, standard deviation.
Exemplary case
A 66-year-old Chinese male with hypertension was diagnosed with left TN (v2.3). He had received carbamazepine treatment, which had ceased efficacy after 2 years. An MRTA examination showed that the left trigeminal nerve root was compressed by blood vessels, but the patient feared MVD and chose BC instead.
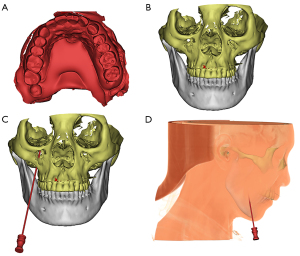
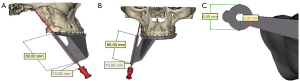
Before the operation, we performed a maxillofacial CT scan and obtained a plaster model of the patient’s maxillary teeth. Then, Mimics 17.0 software was used to construct a 3D model by matching and merging the CT scan data with the maxillary teeth model. We then performed the preoperative design and surgical simulation of the guide plate (Figure 2), which was then designed and 3D printed (Figure 3). The direction and angle of the digital guide can be changed during compression, allowing the removal of the needle from the dislocation path and the adjustment of cannula direction and depth to obtain the ideal balloon position and shape.
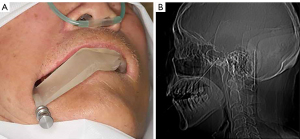
The patient underwent surgery under general anesthesia [intravenous anesthesia without endotracheal intubation and electrocardiogram (ECG) monitoring], routine surgical disinfection, and towel laying. Oral sterilization was performed (1% iodine tincture 2 min 2 times, and 1% iodine tincture cotton ball disinfection 2 times). Then, the sterilized digital guide plate was placed in the mouth and accurately positioned on the maxillary teeth. The needle was directed along the insertion pathway and reached the specified insertion depth. Then, CT was performed to determine whether the FO had been reached. If so, a balloon was placed along the cannula so that it passed through the FO, entered Meckel’s cave, and reached the specified insertion depth according to the guide plate design. Then, 0.7 mL iodohydrin was injected to fill the balloon, after which it was compressed for 3 minutes (Figure 4). After compression, the balloon, cannula, and guide plate were removed from the patient’s mouth, gauze was applied, and the patient was sent to the anesthesia recovery room.
Discussion
The BC approach is recognized as an effective treatment for TN. It has the advantages of minimal trauma, a high effective rate, and a low recurrence rate (28,32,33). Furthermore, its postoperative complications are milder than those of other traumatic operations (10,14,32,33). The FO insertion is the first step and basis of BC and is often the most time-consuming component of the process (32,34). The Hartel anterior approach is the most commonly used insertion method (16), but is not suitable for all anatomical variations in this region (17,32,35). Especially for novice surgeons, BC is a time- and labor-intensive process, and less experience corresponds to a higher failure rate (32). The development of digital medicine provides us with new ideas and methods to solve this problem. Previous studies have reported various other methods, such as the use of navigation (20-22,36) and guide plates (23-25,37-39) in the clinical setting. We also used methods such as electromagnetic navigation and mask-like guide plates for radiofrequency thermocoagulation. We found that due to the yielding of soft tissue and the uncertainty of the intraoperative mandibular position, these methods lack sufficient stability. Therefore, we used a dental model and CT data fusion to provide retention for the guide plate and improve the stability of the guide plate, in combination with the application of an intraoral approach to jointly reduce the trauma of patients.
Although the CT scan may expose the patient to additional radiation, it also offers unique advantages. As previously reported, 2–4% of patients have significant anatomic variations in the FO, making puncture an especially difficult procedure (17,28,40). A preoperative CT scan can determine FO size and exclude space-occupying diseases in the process of trigeminal nerve deviation. Based on the patient’s CT and teeth model, we designed a personalized digital guide plate to reduce the subjective influence of the operator caused by a change in surgeon; reduce the difficulty, trauma, and duration of FO insertion; and improve the success rate of FO insertion. Simultaneously, with the help of a plate, the operator is able to operate with both hands and is distanced from the radiation source when taking films, which in turn reduces the operator’s exposure to and damage from radiation due to the better protection provided. The application of a guide plate greatly reduces the insertion and anesthesia duration. Therefore, BC can be performed under intravenous anesthesia without tracheal intubation (32), which further reduces patient trauma.
By using the guide plate, the needle and cannula can puncture directly through the oral mucosa. Therefore, there is no need for postoperative extraoral incisions or sutures, which reduces both surgical trauma and operation time. Because the tooth-supported guide is located in the oral environment, insertion is performed in the mouth, which creates a second type of surgical incision. Therefore, stricter disinfection measures are needed to prevent intracranial infection. Many TN patients cannot perform daily oral cleaning and preoperative periodontal treatment due to fear of pain attacks, which leads to contamination of the oral environment. Therefore, patients’ oral cavities must be thoroughly disinfected. Additionally, the retainer portion of the tooth-supported fixed guide plate must be positioned to obtain stable tooth retention, increase the wrapping of the gingival area, and reduce the probability of contact between the periodontal area and the guide rail. Furthermore, the guide rail should be located far away from the retainer. Due to our application of these measures, no patients exhibited intracranial infection symptoms.
To reduce the influence of the operator on the research results, the 15 patients included in this study were operated on by the same surgeon, the corresponding author of this study, Dr. Min-Jie Chen, who performs at least 50 such operations every year. An experienced surgeon can ensure the smooth completion of the operation and avoid failure of the operation due to ineffective guide plates. The tooth-support digital guide plate used in this study was fixed on the intraoral dentition with repeatable retention stability. First, the direction and depth of the guide plates are unique. Second, the stability of the guide plates allows for stable retention. These 2 characteristics determined that the operator can only operate according to the preoperative design, without the possibility of adjustment based on his or her own experience. In all operations, all guides were effective. Therefore, we believe that this guide can provide more convenience for novice surgeons.
A pear-shaped inflated balloon is considered the most important factor in the treatment of TN (13,33-35). In our study, due to our preoperative simulation design, the pear shape was achieved on the first attempt for 5 of 15 participants and after adjustment for 10 of 15 participants. As a next step, we plan to reconstruct Meckel's cave and trigeminal ganglion with MRI technology. The matching and merging of CT and MRI data allows for FO insertion in 1 step (i.e., after the needle and cannula are in place), and the ideal pear shape can be obtained without cannula adjustment.
The new technology introduced in this study can reduce not only patient injury in the puncture process but also radiation injury to the operator during surgery. Although this study focused on the application of a new technology, it had some limitations, such as a small sample size, lack of a control group, and the lack of additional follow-up time. These problems need to be addressed in a future study to obtain more reliable and accurate data and conclusions. One issue is that the guides are patient-specific, cannot be reused, and as such there is an associated cost. However, the improvement and further application of this new technology will reduce its cost to patients. Finally, the guide was placed on the teeth of the entire maxilla. Therefore, a compromised condition of tooth loss, dentition defector, or inadequate dental treatment, especially edentulous jaws, may make the application of the guide more difficult. To address this problem, we needed to obtain patients’ data 3–5 days before the operation to avoid sudden changes in the condition of the teeth. A problem with a single tooth will not affect the stability of the whole guide plate, as appropriate grinding can adjust the corresponding position of the guide plate. For patients with edentulous jaws, we may implement the ‘complete denture’-like guide, but the stability is poor. In future research, we will identify a more stable guide plate fixation method.
Conclusions
Using this new technology, we can significantly reduce the difficulty of FO insertion and decrease patient trauma and postoperative complications. This procedure eliminates the need for an extraoral incision for FO insertion, which reduces patient trauma and improves postoperative appearance. This study may help to improve the success rate and accuracy of this procedure, but a multicenter, large sample, randomized controlled trial is needed. Additionally, long-term follow-up is essential.
For surgeons, personalized tooth-supported digital guide plates makes the operation easier to perform especially for novice surgeons, and it protects surgeons from radiation.
Acknowledgments
Funding: The study was supported by the Key Biological and Pharmaceutical Projects of Shanghai Science and Technology Commission (No. 16411953300) and the Science and Technology Commission of Shanghai Municipality (No. 21Y11903500).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4827/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4827/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4827/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4827/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (No. SH9H-2020-TI88-2). All participants signed a consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cruccu G, Di Stefano G, Truini A. Trigeminal Neuralgia. N Engl J Med 2020;383:754-62. [Crossref] [PubMed]
- Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 2020;19:784-96. [Crossref] [PubMed]
- Zakrzewska JM, Linskey ME. Trigeminal neuralgia. BMJ 2015;350:h1238. [Crossref] [PubMed]
- Katusic S, Beard CM, Bergstralh E, et al. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol 1990;27:89-95. [Crossref] [PubMed]
- Maarbjerg S, Gozalov A, Olesen J, et al. Trigeminal neuralgia--a prospective systematic study of clinical characteristics in 158 patients. Headache 2014;54:1574-82. [Crossref] [PubMed]
- Zakrzewska JM, Wu J, Mon-Williams M, et al. Evaluating the impact of trigeminal neuralgia. Pain 2017;158:1166-74. [Crossref] [PubMed]
- Allsop MJ, Twiddy M, Grant H, et al. Diagnosis, medication, and surgical management for patients with trigeminal neuralgia: a qualitative study. Acta Neurochir (Wien) 2015;157:1925-33. [Crossref] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.
- Noorani I, Lodge A, Vajramani G, et al. The Effectiveness of Percutaneous Balloon Compression, Thermocoagulation, and Glycerol Rhizolysis for Trigeminal Neuralgia in Multiple Sclerosis. Neurosurgery 2019;85:E684-92. [Crossref] [PubMed]
- Grewal SS, Kerezoudis P, Garcia O, et al. Results of Percutaneous Balloon Compression in Trigeminal Pain Syndromes. World Neurosurg 2018;114:e892-9. [Crossref] [PubMed]
- Scranton RA, Shah K, Cohen-Gadol AA. Alternative customized instrumentation and technique for percutaneous balloon compression rhizotomy for trigeminal neuralgia. J Neurosurg 2019;132:1938-41. [Crossref] [PubMed]
- Noorani I, Lodge A, Vajramani G, et al. Comparing Percutaneous Treatments of Trigeminal Neuralgia: 19 Years of Experience in a Single Centre. Stereotact Funct Neurosurg 2016;94:75-85. [Crossref] [PubMed]
- Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg 1983;59:1007-12. [Crossref] [PubMed]
- Stomal-Słowińska M, Słowiński J, Lee TK, et al. Correlation of clinical findings and results of percutaneous balloon compression for patients with trigeminal neuralgia. Clin Neurol Neurosurg 2011;113:14-21. [Crossref] [PubMed]
- Mullan S, Duda EE, Patronas NJ. Some examples of balloon technology in neurosurgery. J Neurosurg 1980;52:321-9. [Crossref] [PubMed]
- Härtel F. Die Leitungsanästhesie und Injections-behandlung des Ganglion Gasseri und der Trigeminusstämme. Die Leitungsanästhesie und Injections-behandlung des Ganglion Gasseri und der Trigeminusstämme. Berlin, Heidelberg: Springer, 1913:1-100.
- Bohnstedt BN, Tubbs RS, Cohen-Gadol AA. The use of intraoperative navigation for percutaneous procedures at the skull base including a difficult-to-access foramen ovale. Neurosurgery 2012;70:177-80. [PubMed]
- Tubbs RS, May WR Jr, Apaydin N, et al. Ossification of ligaments near the foramen ovale: an anatomic study with potential clinical significance regarding transcutaneous approaches to the skull base. Neurosurgery 2009;65:60-4; discussion 64. [PubMed]
- Abdennebi B, Mahfouf L, Nedjahi T. Long-term results of percutaneous compression of the gasserian ganglion in trigeminal neuralgia (series of 200 patients). Stereotact Funct Neurosurg 1997;68:190-5. [Crossref] [PubMed]
- Chen MJ, Gu LX, Zhang WJ, et al. Electromagnetic navigation-guided radiofrequency thermocoagulation in trigeminal neuralgia: technical note with three case reports. J Neurol Surg A Cent Eur Neurosurg 2013;74:251-7. [Crossref] [PubMed]
- Lin J, Zhang Y, Peng R, et al. Preoperative Imaging and Microscopic Navigation During Surgery Can Avoid Unnecessarily Opening the Mastoid Air Cells Through Craniotomy Using the Retrosigmoid Approach. World Neurosurg 2019;121:e15-21. [Crossref] [PubMed]
- Pérez-Bovet J, Caro Cardera JL, Rimbau Muñoz J. Frameless navigation-guided percutaneous rhizotomy of the trigeminal nerve: an appraisal of the literature. Neurosurg Rev 2022;45:405-10. [Crossref] [PubMed]
- Oertel MF, Sarnthein J, Regli L, et al. Peroral Trigeminal Rhizotomy Using a Novel 3-Dimensional Printed Patient-Specific Guidance Tool. Oper Neurosurg (Hagerstown) 2021;21:491-6. [Crossref] [PubMed]
- Peng Y, Xie Z, Chen S, et al. Evaluation of the effects of personalized 3D-printed jig plate-assisted puncture in trigeminal balloon compression. Br J Neurosurg 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Han ZX, Chen MJ, Zhang WJ, et al. Tooth-supported personalized template-assisted foramen ovale puncture system for trigeminal neuralgia treatment. J Clin Neurosci 2020;82:71-5. [Crossref] [PubMed]
- Zhang LG, Deng MH, Long X, et al. 3D printing navigation template-guided percutaneous radiofrequency thermocoagulation for V2 trigeminal neuralgia treatment. Hua Xi Kou Qiang Yi Xue Za Zhi 2018;36:662-6. [PubMed]
- Deng M, Cai H, Fang W, et al. Three-dimensionally printed personalized guide plate for percutaneous radiofrequency thermal coagulation in idiopathic trigeminal neuralgia. Int J Oral Maxillofac Surg 2018;47:392-4. [Crossref] [PubMed]
- Aydoseli A, Akcakaya MO, Aras Y, et al. Neuronavigation-assisted percutaneous balloon compression for the treatment of trigeminal neuralgia: The technique and short-term clinical results. Br J Neurosurg 2015;29:552-8. [Crossref] [PubMed]
- Nugraha B, Gutenbrunner C, Barke A, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain 2019;160:88-94. [Crossref] [PubMed]
- Steinberg JA, Sack J, Wilson B, et al. Tentorial sling for microvascular decompression in patients with trigeminal neuralgia: a description of operative technique and clinical outcomes. J Neurosurg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Zhang C, Mei L, Xia M, et al. CT-guided radiofrequency treatment of trigeminal neuralgia at different temperatures through foramen rotundus. Am J Transl Res 2021;13:3102-10. [PubMed]
- De Córdoba JL, García Bach M, Isach N, et al. Percutaneous Balloon Compression for Trigeminal Neuralgia: Imaging and Technical Aspects. Reg Anesth Pain Med 2015;40:616-22. [Crossref] [PubMed]
- Li MW, Jiang XF, Niu CS. Efficacy of and risk factors for percutaneous balloon compression for trigeminal neuralgia in elderly patients. Br J Neurosurg 2021;35:280-4. [Crossref] [PubMed]
- Lichtor T, Mullan JF. A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg 1990;72:49-54. [Crossref] [PubMed]
- Unal TC, Unal OF, Barlas O, et al. Factors Determining the Outcome in Trigeminal Neuralgia Treated With Percutaneous Balloon Compression. World Neurosurg 2017;107:69-74. [Crossref] [PubMed]
- Allam AE, Khalil AAF, Eltawab BA, et al. Ultrasound-Guided Intervention for Treatment of Trigeminal Neuralgia: An Updated Review of Anatomy and Techniques. Pain Res Manag 2018;2018:5480728. [Crossref] [PubMed]
- Kienzler JC, Tenn S, Chivukula S, et al. Linear accelerator-based radiosurgery for trigeminal neuralgia: comparative outcomes of frame-based and mask-based techniques. J Neurosurg 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Bichay TJ, Mayville A. The Continuous Assessment of Cranial Motion in Thermoplastic Masks During CyberKnife Radiosurgery for Trigeminal Neuralgia. Cureus 2016;8:e607. [Crossref] [PubMed]
- Chen MJ, Gu LX, Zhang WJ, et al. Fixation, registration, and image-guided navigation using a thermoplastic facial mask in electromagnetic navigation-guided radiofrequency thermocoagulation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e43-8. [Crossref] [PubMed]
- Mendes PD, Martins da Cunha PH, Monteiro KKO, et al. Percutaneous Foramen Ovale Puncture: Usefulness of Intraoperative CT Control, in the Eventuality of a Narrow Foramen. Stereotact Funct Neurosurg 2021;99:75-8. [Crossref] [PubMed]
(English Language Editors: B. Meiser and J. Jones)


