Emergency department treatment process planning: a field research, case analysis, and simulation approach
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been the most widespread and difficult to prevent/control of any major public health emergency (1,2), has placed an unprecedented burden on health care systems and economies around the globe (3-5). Most countries have adopted the strategy of strict social distancing guidelines, particularly to protect and strengthen health care systems (6). Global dramatic changes have also taken place in medical practices including the introduction of temporary screening and assessment areas outside the footprint of the main hospital structures (7), Which is highlighting gaps around the world in the design and workflow of Emergency Departments (EDs) (8). The EDs, the hospital’s primary outpost in the fight against COVID-19, triage ordinary as well as febrile patients, identify suspected COVID-19 cases early, and implement isolation to control the source of infection and avoid further spread of infectious diseases (9). Failure to recognise the cases and delays in management of an outbreak can pose global health risks (10) and risks to the health of medical staff (11). Due to the vulnerability of the ED environment, the treatment process design and risk mitigation measures are extremely important before the outbreak of infectious diseases (12). Further study is needed to optimise the emergency treatment protocols, in order to reduce contact between patients with suspected or confirmed COVID-19 infection and ordinary patients, and to achieve more effective infection protection.
In severe epidemic situations, process optimisation has always been one of the key tasks for ensuring the continuous improvement of medical quality and safety. Some researchers have proposed the concept of peripheral defence (13), that is, forming a conceptual through the ED design and workflow to segregate potentially infected patients from other nonsuspect cases. Other researchers proposed to separate the ED into physically independent areas, introduce outpatient COVID-19 testing to enhanced surveillance, and reorganize the medical manpower into modular teams (14). There is no denying the effectiveness of these methods, but verifying the effect requires a lot of personal protective equipment, medical personnel, sanitation workers and isolation space, resulting in a heavy burden on hospitals.
Realistically, hospitals need to maintain adequate resources during hospital outbreaks or community pandemics. Herein, we present an alternative approach to address both the tactical and strategic needs of a hospital system by using discrete event simulation and simulation-based optimisation methods (15). Virtual reality technology can be created and designed without the necessity for samples, venues, time, personnel, funding, etc., and can provide managers with ideal decision-making options while reducing costs and promoting major economic and social benefits. Virtual reality technology, most of which are mastered by third-party professional technicians, has been applied to develop medical processes such as clinical operations, optimal staffing, virtual reality surgery simulations for training, and digital hospital medical simulations, etc. (16-19).
This is the first study to use data modelling and simulation experiments to optimise the treatment process for ordinary patients and quarantined people who visit the ED, while minimising manpower, material, financial, and other costs. Simultaneously, our study proposes that simulation tools are an alternative method that does not require external factors, which can assist hospitals or other institutions in strategic evaluation and selection. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1944/rc).
Methods
Study design
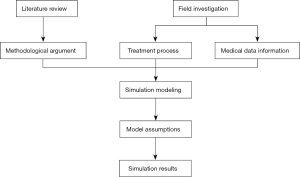
The present study adopted a combination of theory, field investigation, case analysis, and simulation in its research framework design. We reviewed the literature to understand the application of simulation models in the process optimisation of medical systems. Field investigations were used to disclose the treatment process and obtain medical records information from our hospital’s ED.
Based on the findings of the above groundwork, we designed a simulation model and formed its assumptions. The final strategy was obtained through simulation optimisation (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the participants.
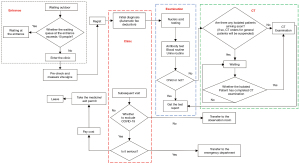
Flow chart of the treatment process of ordinary patients and quarantined people
Our researchers and staff, roleplaying as both ordinary patients and quarantined people, visited the hospital’s ED and underwent the entire treatment process. Together with interviews from doctors and nurses, we were able to optimize the treatment process for the hospital’s ordinary patients and quarantined people coming in for testing (Figures 2,3).

Medical records information
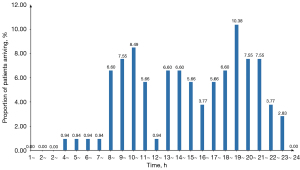
We reviewed 6,364 records from September to November 2020 to determine the nature of patient flow. We found that the arrival times of ordinary patients were unevenly distributed at various times throughout the day. There were two obvious peaks from 9:00 to 11:00 and 19:00 to 21:00, as well as a small peak from 13:00 to 15:00 (Figure 4). The arrival times of quarantined people exhibited a high degree of variability, and data analysis did not reveal any discernible pattern in the frequency distributions. The number of arrivals per day was less than five, and the average daily arrival was 0.8.
We also analysed and fitted data of the time interval between nucleic acid testing and computed tomography (CT) scanning during triage from September to November 2020. We found that the number of priority patients for nucleic acid testing was 74.7% of the number of people who needed both CT and nucleic acid testing, and the number of priority patients for CT testing was 25.3%. Simultaneously, with regard to the time interval from CT examination to report delivery, the Minitab fitting result was distributed in accordance with the lognormal distribution LOGN (3.803, 0.760) (Figure 5). The time interval between initiation of the polymerase chain reaction (PCR) nucleic acid detection equipment and delivery of the nucleic acid test report conformed to the lognormal distribution LOGN (4.983, 0.410) (Figure 6).


Simulation modelling
A simulation model was built based on the treatment process and the treatment records information, and the relevant parameters, attributes, and variables were set according to the real application situations.
Patient attributes and settings are shown in Table S1.
Resources and process time settings are shown in Table S2.
The statistical variables in the simulation model are shown in Table S3.
Model assumptions
Considering the availability of data and the operability of the ED, this study was designed to investigate the following five hypothetical scenarios: (I) CT pre-scheduling strategy; (II) nucleic acid testing priority strategy; (III) nucleic acid detection sample size optimisation strategy; (IV) CT pre-scheduling and nucleic acid priority strategy joint analysis; and (V) nucleic acid priority strategy and test sample size optimisation joint analysis. We simulated the total process time that the patient spends in these five hypothetical scenarios to evaluate the population capacity and risk of cross-infection in the ED.
Evaluation index
In our hospital, the completion time of blood sampling and antibody testing was significantly shorter than the total time needed for nucleic acid testing, and the short transfer time of patients between stations had little effect on the total process time. Thus, these parameters were not specifically analysed in the model. We selected the following evaluation index:
Average total process time for patients (T): the total process time refers to the total time required from the beginning of the pre-check to completion of the return visit. According to the results of the medical records information, the average maximum patient endurance time for the overall process was 257 minutes.
Crowd density (w, person/m2, w ≤1): real-time crowd density refers to the total number of patients waiting for a certain process (including those waiting for consultation rooms, examination, and reports)/total waiting area.
Resource utilisation: the utilisation rate of nucleic acid sample inspection machines (un) ≤95%, and the utilisation rate of the CT room (including routine and ultraviolet disinfection time) (uc) ≤95%.
The times of CT room cross-use (a, times/day): cross-use refers to the use of the CT room by an ordinary patient followed by a quarantined person.
Statistical analysis
The data distribution of continuous variables was presented as average values [± standard deviation (SD)], and categorical variables were presented as frequencies (%). The intervals between CT examinations and nucleic acid sampling were examined with a normal distribution. The average daily arrivals of ordinary and quarantined people were investigated using the Poisson test. Statistical software, including Minitab16 and Arena14, was used for statistical analysis, simulation modelling, and drawing.
Results
Simulation of CT scheduling strategy to reduce the risk of cross-infection
The CT pre-scheduling strategy set up scenarios with different proportions of ordinary and quarantined patients, and simulated the influence of different preparation times in the CT room prior to the arrival of quarantined patients (Tp) on the average total process time (T).
The original strategy was to temporarily suspend the billing of ordinary patients in the system who required CT at triangular distribution (TRIA) {30, 45, 60} before the arrival of the quarantined people until all of the quarantined people in the system had completed their CT testing, and then reopen to ordinary patients. In the CT scheduling strategy, for different epidemic severities (λo=3; λo=5) and different carrying capacities [λc ∈ {30, 80}], when the original strategy and the CT preparation time changed to TRIA {0, 15, 30}, the mean changes of T, TCT, uc, un, a, and Nt were recorded to evaluate the impact of the CT scheduling strategy on reducing the risk of cross-infection. Arena software was employed to perform simulations and was repeated 10 times in 5 days.
The simulation results demonstrated that shortening the preparation time by a TRIA of {0, 15, 30} decreased both the T and TCT. When λo=3, λc≥50 or λo=5, λc≥60, as the preparation time was shortened, TCT and T both decreased. When λc was small, the preparation time TRIA, TCT decreased, and T appeared to increase slightly (Table 1).
Table 1
| No. | Tp (min) | λc | λo | T (min) | TCT (min) | uc (%) | un (%) | a | Nt |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TRIA {30, 45, 60} | 30 | 3 | 264.01 | 61.67 | 58.17 | 24.95 | 3 | 7 |
| 2 | 40 | 3 | 260.36 | 70.65 | 67.23 | 31.99 | 3 | 10 | |
| 3 | 50 | 3 | 266.59 | 77.42 | 76.83 | 42.51 | 3 | 12 | |
| 4 | 60 | 3 | 253.02 | 85.42 | 84.01 | 46.33 | 3 | 14 | |
| 5 | 70 | 3 | 312.55 | 208.87 | 93.7 | 55.18 | 3 | 21 | |
| 6 | 80 | 3 | 430.60 | 418.71 | 95.23 | 55.21 | 3 | 34 | |
| 7 | 30 | 5 | 274.12 | 84.06 | 74.53 | 26.83 | 4.88 | 8 | |
| 8 | 40 | 5 | 250.43 | 97.28 | 84.73 | 31.14 | 4.92 | 9 | |
| 9 | 50 | 5 | 285.60 | 164 | 94.77 | 39.02 | 4.96 | 13 | |
| 10 | 60 | 5 | 410.31 | 367.89 | 97.79 | 45.65 | 5 | 24 | |
| 11 | TRIA {0, 15, 30} | 40 | 3 | 262.04 | 49.65 | 68.32 | 34.4 | 3 | 10 |
| 12 | 50 | 3 | 252.46 | 63.57 | 79.63 | 40.11 | 3.04 | 12 | |
| 13 | 60 | 3 | 253.79 | 81.43 | 88.05 | 47.31 | 3.04 | 14 | |
| 14 | 70 | 3 | 278.02 | 158.59 | 96.05 | 52.21 | 3 | 19 | |
| 15 | 80 | 3 | 425.38 | 390.28 | 94.75 | 63.51 | 3.04 | 34 | |
| 16 | 40 | 5 | 263.85 | 73.3 | 85.32 | 35.24 | 4.92 | 10 | |
| 17 | 50 | 5 | 299.81 | 171.06 | 94.52 | 39.69 | 5.04 | 14 | |
| 18 | 60 | 5 | 378.19 | 333.08 | 97.75 | 44.94 | 4.96 | 22 |
Tp, CT room preparation time for quarantined people before their arrival; λc, average daily visits for ordinary patients; λo, average daily visits of quarantined people; T, total process time for patients; TCT, CT scan and waiting time; uc, CT utilisation; un, PCR utilisation; a, average number of cross-uses of CT; Nt, average number of waiting patients.
Simulation of the nucleic acid priority strategy to reduce the risk of cross-infection
In the nucleic acid testing priority strategy, λc=60, 80, 100, and λo=1 represented the following three scenarios: patients were free to choose the priority order of examination (74.7% of patients preferred nucleic acid testing), the patient’s 100% priority nucleic acid detection, and the patient’s 100% priority CT examination. The mean changes in T, TCT, uc, un, a, and Nt were used to evaluate the impact of different strategies on reducing the risk of cross-infection. Arena software was applied to perform simulations and was repeated 20 times in 5 days.
The simulation results demonstrated that when 60≤λc≤80, and λo=1, the patient’s 100% priority was nucleic acid detection, which effectively shortened T and reduced Nt. Compared to when the patient’s 100% priority was CT examination, Nt was shortened by 26 min (λc=60, λo=1) and 38 min (λc=80, λo=1). When the load capacity was not exceeded (λc=80), larger λc represented a more obvious optimisation effect of the nucleic acid priority strategy on reducing the risk of infection. When λc=100 and λo=1, the patient’s 100% priority nucleic acid detection was better than that of CT examination, but was similar to the patient’s free choice of examination order (Table 2).
Table 2
| No. | Po (%) | λc | λo | T (min) | TCT (min) | uc (%) | un (%) | a | Nt |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74.7 | 60 | 1 | 236.88 | 46.06 | 68.46 | 42.48 | 1 | 14 |
| 2 | 100% | 60 | 1 | 234.11 | 47.10 | 69.07 | 45.18 | 1 | 22 |
| 3 | 0 | 60 | 1 | 262.17 | 48.86 | 69.14 | 44.69 | 1 | 13 |
| 4 | 74.7 | 80 | 1 | 256.87 | 87.89 | 88.83 | 61.45 | 1 | 13 |
| 5 | 100 | 80 | 1 | 248.32 | 93.62 | 88.10 | 62.19 | 1 | 15 |
| 6 | 0 | 80 | 1 | 294.43 | 98.94 | 88.41 | 59.51 | 1 | 19 |
| 7 | 74.7 | 100 | 1 | 416.74 | 375.33 | 93.99 | 76.46 | 1 | 18 |
| 8 | 100 | 100 | 1 | 420.80 | 417.68 | 94.35 | 73.39 | 1 | 22 |
| 9 | 0 | 100 | 1 | 636.70 | 448.49 | 94.20 | 67.53 | 1 | 41 |
Po, proportion of preferential nucleic acid testing; λc, average daily visits for ordinary patients; λo, average daily visits of quarantined people; T, average total process time for patients; TCT, CT scan and waiting time; uc, CT utilisation; un, PCR utilisation; a, average number of cross-uses of CT; Nt, average number of waiting patients.
Nucleic acid detection sample size optimisation strategy to shorten the processing time
Rapid nucleic acid tests can detect multiple samples at the same time and reduce testing times. However, when the number of consultations was small, time was incurred in waiting to reach the minimum number of samples required to run the test. The experiment explored optimisation of the nucleic acid detection sample size (Sn) in different visits to reduce the waiting times for nucleic acid detection results. This study designed three sets of experiments with λo =1, λc=60, 80, 90, Sn ∈ {3, 5}, and conducted simulation experiments that were repeated 20 times in 5 days. The researchers simultaneously recorded the mean changes in T, TCT, Tn, uc, un, a, and Nt for each experiment.
The simulation results demonstrated that when Sn ∈ {3, 5}, as Sn increased, T and Nt exhibited a trend of initially decreasing and then increasing. When Sn=4, T was the shortest and Nt was the smallest, which implied that four samples at a time were ideal for optimising the process in our hospital (Table 3).
Table 3
| No. | Sn | λc | λo | T (min) | TCT (min) | Tn (min) | uc (%) | un (%) | a | Nt |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 60 | 1 | 238.02 | 45.84 | 227.38 | 68 | 61.94 | 1 | 13 |
| 2 | 4 | 60 | 1 | 231.50 | 44.71 | 221.72 | 68.46 | 42.48 | 1 | 13 |
| 3 | 5 | 60 | 1 | 255.24 | 46.55 | 242.82 | 68.51 | 36.87 | 1 | 14 |
| 4 | 3 | 80 | 1 | 255.79 | 99.74 | 255.79 | 74.87 | 78.65 | 1 | 19 |
| 5 | 4 | 80 | 1 | 264.71 | 100.38 | 223.92 | 90.81 | 62.35 | 1 | 20 |
| 6 | 5 | 80 | 1 | 278.02 | 113.29 | 235.78 | 88.95 | 50.54 | 1 | 21 |
| 7 | 3 | 90 | 1 | 372.62 | 269.99 | 254.58 | 93.37 | 88.01 | 1 | 33 |
| 8 | 4 | 90 | 1 | 296.35 | 177.40 | 206.16 | 93.41 | 64.23 | 1 | 25 |
| 9 | 5 | 90 | 1 | 316.77 | 212.81 | 215.47 | 94.12 | 54.15 | 1 | 27 |
Sn, sample size; λc, average daily visits for ordinary patients; λo, average daily visits of quarantined people; T, average total process time for patients; TCT, CT scan and waiting time; Tn, waiting time for nucleic acid testing report after sampling; uc, CT utilisation; un, PCR utilisation; a, average number of cross-uses of CT; Nt, average number of waiting patients.
Joint analysis of CT pre-scheduling and nucleic acid priority strategies
Since λo was primarily affected by the CT pre-scheduling and nucleic acid priority strategies, we conducted a joint analysis of the CT pre-scheduling strategy and the nucleic acid priority strategy, especially considering the urgency of the quarantined cases.
For different arrival rates λo =1, 3 and λc = 60, 80, the experiment altered the CT scheduling strategy (Tpmax =60 min, Tpmin =0 min)* and nucleic acid priority strategy. The mean changes in Tc, TCT, To, Nt, etc. were simultaneously recorded (*considering that the quarantined people treated in the hospital only had a 60-minute drive) using Arena software to perform simulations that were repeated 10 times in 3 days, and a weight system was established to evaluate and select optimisation strategies.
The simulation results demonstrated that with an increase in the number of quarantined people, reducing Tp would have a greater impact on shortening Tc, and with an increase in the number of ordinary patients, increasing Tp appropriately would shorten To (Table 4).
Table 4
| No. | Po (%) | Tp (min) | λc | λo | Ordinary patient | Quarantined people | Nt | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc (min) | TCT (min) | To (min) | Ta (min) | TCT (min) | |||||||
| 1 | 100 | 60 | 60 | 1 | 234.68 | 50.68 | 247.95 | 218.22 | 12.58 | 13 | |
| 2 | 30 | 60 | 1 | 232.97 | 42.36 | 204.53 | 162.98 | 21.6 | 13 | ||
| 3 | 0 | 60 | 1 | 231.64 | 40.10 | 206.35 | 154.4 | 32.92 | 13 | ||
| 4 | 60 | 60 | 3 | 255.04 | 127.99 | 220.07 | 185.83 | 18.23 | 14 | ||
| 5 | 30 | 60 | 3 | 242.51 | 94.39 | 194.96 | 161.97 | 16.46 | 13 | ||
| 6 | 0 | 60 | 3 | 239.29 | 76.26 | 220.92 | 185 | 18.05 | 13 | ||
| 7 | 75 | 60 | 60 | 1 | 247.42 | 46.70 | 275.57 | 244.06 | 14.8 | 14 | |
| 8 | 30 | 60 | 1 | 237.13 | 38.72 | 246.20 | 208.77 | 21.74 | 13 | ||
| 9 | 0 | 60 | 1 | 240.18 | 44.39 | 237.79 | 184.9 | 31.35 | 13 | ||
| 10 | 60 | 60 | 3 | 258.52 | 101.79 | 238.98 | 207.86 | 14.58 | 14 | ||
| 11 | 30 | 60 | 3 | 257.62 | 86.05 | 238.96 | 204.56 | 18.75 | 14 | ||
| 12 | 0 | 60 | 3 | 249.50 | 65.02 | 222.34 | 185.75 | 16.74 | 14 | ||
| 13 | 50 | 60 | 60 | 1 | 254.92 | 51.63 | 232.02 | 203.02 | 14.47 | 14 | |
| 14 | 30 | 60 | 1 | 248.51 | 46.92 | 240.48 | 201.54 | 24 | 14 | ||
| 15 | 0 | 60 | 1 | 248.74 | 42.59 | 215.66 | 165.3 | 33.5 | 14 | ||
| 16 | 60 | 60 | 3 | 265.61 | 110.53 | 215.55 | 183.78 | 16.43 | 15 | ||
| 17 | 30 | 60 | 3 | 265.13 | 87.63 | 244.10 | 209.75 | 17.59 | 15 | ||
| 18 | 0 | 60 | 3 | 249.92 | 63.78 | 229.65 | 190.91 | 19.91 | 14 | ||
| 19 | 25 | 60 | 60 | 1 | 258.96 | 46.55 | 229.14 | 202.17 | 13.31 | 14 | |
| 20 | 30 | 60 | 1 | 254.23 | 45.43 | 261.78 | 221.96 | 25.03 | 14 | ||
| 21 | 0 | 60 | 1 | 254.54 | 42.69 | 277.76 | 224.41 | 34.16 | 14 | ||
| 22 | 60 | 60 | 3 | 291.26 | 112.21 | 223.98 | 190.93 | 16.51 | 16 | ||
| 23 | 30 | 60 | 3 | 267.80 | 77.06 | 220.85 | 187.36 | 15.36 | 15 | ||
| 24 | 0 | 60 | 3 | 269.70 | 77.37 | 258.59 | 218.82 | 19.35 | 15 | ||
| 25 | 0 | 60 | 60 | 1 | 258.50 | 47.45 | 233.72 | 204.77 | 14.26 | 14 | |
| 26 | 30 | 60 | 1 | 254.16 | 43.22 | 252.53 | 212.55 | 23.26 | 14 | ||
| 27 | 0 | 60 | 1 | 256.41 | 43.66 | 299.29 | 249.03 | 31.3 | 14 | ||
| 28 | 60 | 60 | 3 | 301.64 | 112.38 | 253.14 | 219.69 | 15.99 | 17 | ||
| 29 | 30 | 60 | 3 | 289.35 | 89.64 | 247.39 | 210.76 | 20.04 | 16 | ||
| 30 | 0 | 60 | 3 | 268.08 | 67.24 | 229.82 | 193.73 | 18.6 | 15 | ||
| 31 | 100 | 60 | 80 | 1 | 237.80 | 99.60 | 190.93 | 159.94 | 14.78 | 18 | |
| 32 | 30 | 80 | 1 | 230.75 | 82.56 | 174.52 | 131.29 | 22.27 | 17 | ||
| 33 | 0 | 80 | 1 | 244.08 | 82.12 | 272.51 | 217.17 | 30.21 | 18 | ||
| 34 | 75 | 60 | 80 | 1 | 251.61 | 98.70 | 186.12 | 152.97 | 14.76 | 19 | |
| 35 | 30 | 80 | 1 | 254.61 | 96.23 | 192.15 | 154.42 | 21.35 | 19 | ||
| 36 | 0 | 80 | 1 | 250.89 | 83.15 | 228.13 | 176.75 | 31.68 | 18 | ||
| 37 | 50 | 60 | 80 | 1 | 267.3 | 91.48 | 232.89 | 203.15 | 14.33 | 20 | |
| 38 | 30 | 80 | 1 | 264.17 | 89.72 | 221.86 | 179.30 | 24.12 | 19 | ||
| 39 | 0 | 80 | 1 | 268.15 | 96.30 | 266.73 | 207.66 | 33.39 | 20 | ||
| 40 | 25 | 60 | 80 | 1 | 279.38 | 92.86 | 238.86 | 208.28 | 15.06 | 21 | |
| 41 | 30 | 80 | 1 | 265.64 | 72.14 | 271.79 | 230.41 | 22 | 20 | ||
| 42 | 0 | 80 | 1 | 269.3 | 74.47 | 248.29 | 194.52 | 30.49 | 20 | ||
| 43 | 0 | 60 | 80 | 1 | 288.91 | 88.40 | 246.84 | 215.00 | 14.67 | 21 | |
| 44 | 30 | 80 | 1 | 291.63 | 103.14 | 241.78 | 200.34 | 22.95 | 22 | ||
| 45 | 0 | 80 | 1 | 273.45 | 78.19 | 249.16 | 191.32 | 31.69 | 20 | ||
Po, proportion of preferential nucleic acid testing; Tp, CT room preparation time for quarantined people before their arrival; λc, average daily visits for ordinary patients; λo, average daily visits of quarantined people; Tc, total process time for ordinary patients; TCT, CT scan and waiting time; To, total process time of quarantined people; Ta, time from CT test to getting both reports; Nt, average number of waiting patients.
Considering that Tp and the nucleic acid/CT priority strategies jointly affected the time for nucleic acid samples to reach the laboratory and the effects of their interaction had a non-linear relationship with the results, the experiment used a weighting method [Wc= Uc* λc/(Uc* λc + Uo* λo), Wo= Uo* λo/(Uc* λc + Uo* λo)] to evaluate the combined impact of CT pre-scheduling and nucleic acid priority strategies on Tp. According to the experimental hypothesis, the weighted results showed that Tp met the optimal time queue {60, 30, 0} when Uc=1 and Uo=25, at different visits (λc=60, 80, λo=1, 3), and nucleic acid priority strategies were applied (Po=100%, 75%, 50%, 25%, 0%) (Figure 7).

Within the load capacity of the medical system, the experiment compared the impact of the nucleic acid priority and the CT pre-scheduling strategies on Tw in different medical visits. When the number of visits remained the same, the nucleic acid priority strategy always reduced the total process time and the reduction was greater than that achieved with the CT pre-scheduling strategy. When the system adopted the nucleic acid priority strategy, a more obvious impact of Tp on T was observed with increased patient frequency (Figure 8).

At the same time, the experiment considered the severity of the epidemic and attempted to design an optimal strategy for when Uc:Uo=1:25, 1:10, and 1:1. The choice of optimal strategy was greatly affected by the severity of the epidemic. When Uo was lower, shortening Tp was conducive to shortening T (Figure 9).

Joint analysis of the nucleic acid priority strategy and test sample size optimisation
Based on the above results, we conducted a joint analysis of two variables that influence Tn, i.e., nucleic acid priority strategy (Po) and the average test Sn. Considering the peak visit hours and the long duration of patient visits, this study selected all patient visits from 7:00 p.m. to 11:00 p.m. from the above experimental data and performed 20 repeated experiments. This experiment was performed based on the following: (I) under the premise that all ordinary patients chose the nucleic acid priority strategy, the experiment established Sn ∈ {2, 6} and conducted five sets of experiments to record Tc, Tn, and TCT, in order to compare the optimal detection Sn. (II) In the combined analysis of the nucleic acid priority strategy and average sample size, the experiment set Sn ∈ {2, 6}, Po=90%, 70%, and eight sets of experiments recorded Tc, Tn, and TCT to compare the optimal strategies (Table 5).
Table 5
| Sn | Po (%) | T (min) | TCT (min) | Tn (min) |
|---|---|---|---|---|
| 2 | 100 | 343.10 | 123.64 | 388.29 |
| 3 | 243.43 | 117.43 | 232.30 | |
| 4 | 226.79 | 142.76 | 65.08 | |
| 5 | 244.39 | 110.53 | 183.73 | |
| 6 | 227.07 | 111.58 | 204.01 | |
| 2 | 90 | 287.35 | 97.15 | 344.54 |
| 2 | 70 | 341.58 | 112.10 | 402.58 |
| 3 | 90 | 218.80 | 101.69 | 213.53 |
| 3 | 70 | 235.85 | 91.31 | 200.08 |
| 4 | 90 | 203.53 | 108.92 | 173.14 |
| 4 | 70 | 226.47 | 108.02 | 175.30 |
| 5 | 90 | 211.76 | 94.85 | 171.10 |
| 5 | 70 | 226.77 | 98.85 | 185.62 |
Sn, sample size; Po, proportion of preferential nucleic acid testing; T, average total process time for patients; TCT, CT scan and waiting time; Tn, waiting time for nucleic acid testing report after sampling.
According to the simulation results, when Po=100%, as Sn increased, Tc exhibited a trend of initially decreasing and then increasing. The optimal average sample detection volume under the current peak of visits was four (Sn=4). Although Sn=6, Tc was the lowest because the total process time of most ordinary patients was increased and not included in the system. Furthermore, as the results of the previous groups of experimental data tended to be more obvious, this study did not consider Sn=6. At the same time, when Sn ∈ {2, 5}, Po dropped to 90%, Tc decreased significantly, but when it dropped to 70%, the downward trend was less obvious. It may be that during peak hours, the excessive accumulation of nucleic acid samples increased the invalid waiting times. Therefore, a moderately small reduction in Po could optimise the allocation of resources, while a large reduction in Po would make the time stacking effect caused first by the patient’s CT and then by the nucleic acid testing more obvious.
Discussion
Strategies for entry personnel to control and prevent infection
Previous study has shown that controlling the density of personnel can reduce the risk of cross-infection (20). The ED could play a role in active surveillance; however, the costs, benefits, and implications of hospital-based surveillance have yet to be studied (21). A quick assessment area could be set up at the entrance of the ED to reduce access and contact opportunities for non-essential patients and staff (22). For example, by installing monitoring devices, the system dynamically recognises the number of people entering, and when the density of patients exceeds the threshold, it sends an early warning to the staff to restrict patients from entering the outpatient and the ED, and also alerts those waiting outside the entrance.
Optimise the information system to automatically initiate the CT pre-scheduling strategy
The above results showed that the longer the preparation time before the arrival of quarantined people, the longer the waiting time for ordinary patients and the shorter the waiting time for quarantined people. At the same time, simulation tools can assist in evaluating the results of different strategies and in making strategic choices based on the hospital’s preferences. Our study findings suggest that it is necessary to establish effective communication channels between the ED and the administrative, clinical, and medical technical departments. For example, the administrative department should relay the arrival times of quarantined people in advance, whereby the hospital information system algorithm can determine the time when ordinary patients should stop using the CT room and quarantined people may start to use the CT room independently. This will help to improve the efficiency of CT use and reduce patient waiting times, while at the same time preventing cross-use.
Nucleic acid priority strategy to reduce the risk of cross-infection
The nucleic acid priority strategy results demonstrated that when 60≤λc≤80 and λo=1, the patient’s 100% priority nucleic acid detection effectively shortened T and reduced Nt. Owing to the long time it takes to complete the nucleic acid sampling and deliver the report for review, CT detection can be completed during this time; hence, the nucleic acid priority strategy was beneficial to increase the time coincidence of Tn and TCT, thereby shortening T. When λc=100, the patient’s 100% priority nucleic acid detection was superior to that of the CT examination, but was less dissimilar to the patient’s free choice of examination order. It may be that when the number of visits increased, TCT increased rapidly, which reduced the degree of coincidence with Tn. However, when λc>100, the advantage of the nucleic acid priority strategy was not prevalent because of the rapid growth of TCT. Therefore, when the number of consultations is relatively stable, the hospital can guide ordinary patients to perform nucleic acid testing first followed by CT testing, which is more conducive to shortening T. However, when the number of visits fluctuates greatly, the system can appropriately reduce Po; for example, 90% of patients prioritise nucleic acid testing and 10% of patients prioritise CT testing. Furthermore, this study also found that when the number of visits remained the same, shortening the processing time for a single patient was also conducive to reducing the density of people in the system and reducing the risk of cross-infection. Hospitals can select the optimal strategy according to the actual scenario.
Sample sizes per batch testing optimisation process
According to the sample size analysis results, based on an actual scenario in our hospital, four samples per batch were found to be beneficial for optimising the process and reducing the risk of infection. However, the sample size of each batch is not uniform; it is very common for 10 samples to be tested in the whole population and five samples to be tested in a large group in China. Other health institutions can also use simulation methods to determine the optimal sample size for nucleic acid testing according to the severity of the epidemic and the need of nucleic acid testing, thereby saving time and budget costs.
Efficient review strategy for nucleic acid test results
Research and data analysis showed that after completing nucleic acid testing, issuing test reports took 2–3 hours in some cases, causing patients to remain in the outpatient clinic and thus increasing the risk of cross-infection. We propose the use of automatic prompts (23), such as SMS and WeChat, to alert doctors to speed up the review, and use adherence to this practice as one of the criteria for the evaluation of a doctor’s performance.
Refined patient classification, precise prevention, and control
As a precaution during the epidemic, medical institutions should formulate a clear classification of emergency patients (especially those with fever), determine a targeted treatment process for grading patients, and optimize the ED according to this scheme.
Conclusions
The simulation tool is a viable and effective approach for identifying workflow inefficiencies, which can assist in evaluating the results of various strategies according to different needs. This will facilitate the discovery and validation of options for improvement through what-if scenario testing, as hospitals can choose strategies based on their own needs and obtain the most benefits with the least cost. This method is worthy of replication and promotion by similar hospitals or fields.
For the ED of our hospital, when the number of consultations was relatively stable, the hospital was able to guide ordinary patients to undergo nucleic acid testing first followed by CT testing, which was more conducive to shortening T. In cases where the number of visits fluctuated greatly, the system could appropriately reduce the proportion of priority nucleic acid testing (Po), and four samples per batch were beneficial for optimising this process. However, regardless of the strategy adopted, it was adjusted according to the actual scenario observed in the ED. According to the national public health emergency response level, decision makers can predict the risk level and difficult challenges of infectious diseases, and carry out the simulation design of emergency plan according to the actual situation of the hospital to customize the workflow optimization strategy.
Acknowledgments
Funding: This study was supported by the Fund provided by 2020 Clinical Technology Innovation Project of Shanghai Shenkang Hospital Development Center (No. SHDC12020611), the Shanghai Hospital Association Hospital Management Research Fund (No. X2020166), the National Social Science Fund (No. 19BGL245), and Science and Technology Innovation Project of Shanghai Jiao Tong University School of Medicine (No. WK2110).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1944/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1944/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1944/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shen J, He Q, Shen T, et al. "An integrated system, three separated responsibilities", a new fever clinic management model, in prevention and control of novel coronavirus pneumonia. World J Clin Cases 2021;9:9050-8. [Crossref] [PubMed]
- Ates AA, Alomari T, Bhardwaj A, et al. Differences in endodontic emergency management by endodontists and general dental practitioners in COVID-19 times. Braz Oral Res 2020;34:e122. [Crossref] [PubMed]
- Rodriguez HC, Gupta M, Cavazos-Escobar E, et al. Umbilical cord: an allogenic tissue for potential treatment of COVID-19. Hum Cell 2021;34:1-13. [Crossref] [PubMed]
- Campanella S, Arikan K, Babiloni C, et al. Special Report on the Impact of the COVID-19 Pandemic on Clinical EEG and Research and Consensus Recommendations for the Safe Use of EEG. Clin EEG Neurosci 2021;52:3-28. [Crossref] [PubMed]
- Bhowmick NA, Oft J, Dorff T, et al. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer 2020;27:R281-92. [Crossref] [PubMed]
- Khan M, Adil SF, Alkhathlan HZ, et al. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020;26:39. [Crossref] [PubMed]
- Miller NM, Jones I, Russ S, et al. A model for rapid emergency department expansion for the COVID-19 pandemic. Am J Emerg Med 2020;38:2065-9. [Crossref] [PubMed]
- Nadarajan GD, Omar E, Abella BS, et al. A conceptual framework for Emergency department design in a pandemic. Scand J Trauma Resusc Emerg Med 2020;28:118. [Crossref] [PubMed]
- Hu X, Liu S, Wang B, et al. Management practices of emergency departments in general hospitals based on blockage of chain of infection during a COVID-19 epidemic. Intern Emerg Med 2020;15:1545-52. [Crossref] [PubMed]
- The Neglected Dimension of Global Security. The Neglected Dimension of Global Security. National Academy of Sciences; 2016.
- Wilder-Smith A, Low JG. Risk of respiratory infections in health care workers: lessons on infection control emerge from the SARS outbreak. Southeast Asian J Trop Med Public Health 2005;36:481-8. [PubMed]
- Ong ME. War on SARS: a Singapore experience. CJEM 2004;6:31-7. Erratum in: CJEM 2004;6:79. [Crossref] [PubMed]
- Cliff A, Smallman-Raynor M. Oxford Textbook of Infectious Disease Control.Oxford Textbook Infect Dis Control. 2013:1-69.
- Tan RMR, Ong GY, Chong SL, et al. Dynamic adaptation to COVID-19 in a Singapore paediatric emergency department. Emerg Med J 2020;37:252-4. [Crossref] [PubMed]
- Tellis R, Starobinets O, Prokle M, et al. Identifying Areas for Operational Improvement and Growth in IR Workflow Using Workflow Modeling, Simulation, and Optimization Techniques. J Digit Imaging 2021;34:75-84. [Crossref] [PubMed]
- OʼHara S. Planning intensive care unit design using computer simulation modeling: optimizing integration of clinical, operational, and architectural requirements. Crit Care Nurs Q 2014;37:67-82. [Crossref] [PubMed]
- Shim SJ, Kumar A, Jiao R. Using computer simulation for optimal staffing: A case for the patient registration process of a hospital. Technol Health Care 2017;25:385-90. [Crossref] [PubMed]
- Holford N, Ma SC, Ploeger BA. Clinical trial simulation: a review. Clin Pharmacol Ther 2010;88:166-82. [Crossref] [PubMed]
- Li B, Wang H, Li G, et al. A patient-specific modelling method of blood circulatory system for the numerical simulation of enhanced external counterpulsation. J Biomech 2020;111:110002. [Crossref] [PubMed]
- Gilligan P, Quirke M, Winder S, et al. Impact of admission screening for methicillin-resistant Staphylococcus aureus on the length of stay in an emergency department. J Hosp Infect 2010;75:99-102. [Crossref] [PubMed]
- Liang SY, Theodoro DL, Schuur JD, et al. Infection prevention in the emergency department. Ann Emerg Med 2014;64:299-313. [Crossref] [PubMed]
- Sangal RB, Scofi JE, Parwani V, et al. Less social emergency departments: implementation of workplace contact reduction during COVID-19. Emerg Med J 2020;37:463-6. [Crossref] [PubMed]
- Zhou S, Ma X, Jiang S, et al. A retrospective study on the effectiveness of Artificial Intelligence-based Clinical Decision Support System (AI-CDSS) to improve the incidence of hospital-related venous thromboembolism (VTE). Ann Transl Med 2021;9:491. [Crossref] [PubMed]
(English Language Editor: A. Kassem)