
Predictive accuracy of an ADC map for hemorrhagic transformation in acute ischemic stroke patients after successful recanalization with endovascular therapy
Introduction
Endovascular therapy (EVT) has become the standard treatment for acute ischemic stroke (AIS) caused by large vessel occlusion in the anterior circulation (1-3). However, despite highly successful recanalization rates, more than half of patients do not achieve a favorable outcome [modified Rankin Scale (mRS) score ≤2] at 3 months after thrombectomy (1-4). Early neurological deterioration and unfavorable outcomes are reportedly associated with hemorrhagic transformation (HT), which can be caused by the disruption of the blood-brain barrier due to reperfusion of ischemic brain tissue (5,6). Preoperative evaluation of the risk of HT may guide therapeutic strategies and improve the safety of EVT.
The severity of hypoperfusion and the volume of infarction core are important predictors of HT (6-8). To predict HT, perfusion parameters have previously been used to assess the degree of ischemia (8,9), but the vascular recanalization situation of most analyzed patients was unclear, and only a few studies combined ischemic severity and the corresponding volume for the prediction. The apparent diffusion coefficient (ADC) value is associated with the severity of the perfusion deficit (10) and has been used to quantify ischemia severity in the prediction of HT after recanalization with EVT (9). Given the onion-like distribution of successively decreasing ADC values from the periphery toward the center of the infarct core (10), we hypothesized that a combination of ADC thresholds and corresponding volumes may predict the occurrence of HT. In this study, we aimed to evaluate the predictive role of the volume below each ADC threshold value for HT following EVT. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2255/rc).
Methods
Data selection
We retrospectively analyzed the consecutive patient data from January 2018 to June 2020 collected prospectively from stroke database of Henan Provincial People’s Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by ethics committee of Henan Provincial People’s Hospital (No. 2017-034-01). Individual consent for this retrospective analysis was waived. Patients were selected according to the following criteria: (I) those diagnosed with AIS with internal carotid artery (ICA), M1, or M2 middle cerebral artery (MCA) segment occlusion; (II) the time from symptom onset to groin puncture was less than 24 hours; (III) National Institutes of Health Stroke Scale (NIHSS) score ≥6; (IV) completion of an acute ischemic stroke workup including diffusion weighted imaging (DWI) at admission and non-contrast computed tomography (NCCT), magnetic resonance angiography (MRA), or computed tomography angiography (CTA) follow-up within a week after EVT; (V) modified thrombolysis in cerebral infarction score (mTICI) ≥ 2b after EVT; (VI) no hemorrhagic foci was observed on the preoperative magnetic resonance imaging (MRI); and (VII) no reocclusion of target vessel was found on follow-up MRA or CTA.
Image acquisition
All patients underwent a 3.0T MRI (Skyra, Siemens, Germany) exam using the following sequences: DWI [spin echo, echo time (TE) =64 ms, repetition time (TR) =4,140 ms, field of view (FOV) =230 mm, matrix =160×160, and 6.0-mm section thickness with a 1.2-mm intersection gap], ADC (spin echo, TE =64 ms, TR =4,140 ms, FOV =230 mm, matrix =160×160, and 6.0-mm section thickness with a 1.2-mm intersection gap), fluid attenuated inversion recovery (FLAIR) (turbo spin echo, TE =81 ms, TR =7,500 ms, FOV =230 mm, matrix =320×256, time of inversion (TI) =2,300 ms, and 6.0-mm section thickness with 1.2-mm intersection gap), T2 (turbo spin echo, TE =96 ms, TR =5,000 ms, FOV =230 mm, matrix =384×384, and 6.0-mm section thickness with a 1.2-mm intersection gap), and MRA [time of flight (TOF)-MRA; fast field echo, TE =3.45 ms, TR =21 ms, FOV =200 mm, matrix =320×288, and 0.7-mm section thickness with a −6.3-mm intersection gap]. The follow-up exam included at least NCCT and CTA (SOMATOM Definition AS, Siemens, Germany) or MRA.
Image analysis
The baseline and follow-up imaging analyses were respectively performed by two interventional neuroradiologists who were blinded to the other clinical and imaging information using Olea Sphere 23.0 (Olea medical S.A.S, La Ciotat, France) software. Inconsistent cases were determined by discussion between the two blind evaluators. The infarction core was defined as the area with a high preoperative DWI signal and ADC <0.6×10−3 mm2/s. Artifacts and ischemic infarction in non-target vessel territory were eliminated manually. The preoperative volume of the ischemic lesions with ADC values below thresholds ranging from 0.3×10−3 to 0.6×10−3 mm2/s at 0.1 intervals was measured. The mTICI grade of the occluded vessel was evaluated on digital subtraction angiography (DSA) before and after recanalization. CT was performed within a week after EVT to diagnose HT caused by blood-brain barrier damage due to severe ischemic based on the European Cooperative Acute Stroke Study (ECASS) scoring system (11). Follow-up CTA or MRA was used to identify target vessel patency after successful recanalization with EVT.
Statistical analysis
Patients were stratified into ‘HT’ and ‘non-HT’ groups. All data processing, statistical analysis, and plotting were conducted using R 4.1.0 software (R Foundation for Statistical Computing, Vienna, Austria). The intraclass correlation coefficient (ICC) or Cohen Kappa Statistic was used to analyze the inter- and intra-observer agreement for the diagnosis of HT. We used the Student’s t-test, Mann-Whitney U test, and Chi-square test to compare the clinical variable differences between the two groups. Receiver operator characteristic (ROC) curves were generated for thresholded ADC lesion volumes, and the areas under the curve (AUCs) were determined to compare their individual test characteristics in predicting HT. To determine which factors were associated with HT, multivariate logistic regression analysis were performed with clinical variables with statistically significant differences in univariate analysis, factors significantly associated with HT in previous studies and volume of optimal ADC threshold. All P values were two-tailed, and variables were considered significant at P<0.05.
Results
From a total of 424 patients, 119 met the inclusion criteria and were included in this research. No adverse event associated with MRI examination occurred. The inter-observer agreement for diagnosing HT was good (κ=0.862). Forty-two (35.29%, HT group) patients had HT on follow-up CT, including 10 patients with hemorrhagic infarct (HI) 1, 14 patients with HI2, as well as nine cases with parenchymal hematoma (PH) 1 and PH2, respectively. The mean time between baseline MRI and CT diagnosis of HT was 72 h. The remaining 77 (64.71%, non-HT group) patients did not show HT on the follow-up CT scan. Figure 1 shows the flow of participants throughout the study.
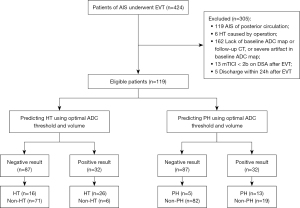
There were no differences between the HT and non-HT groups in terms of age, sex, the proportion of patients with hypertension, and the use of antiplatelet and anticoagulant drugs before EVT. Furthermore, the onset-to-recanalization times were similar between the two groups. In the HT group, more patients had diabetes mellitus, atrial fibrillation, and tandem occlusion, in addition to larger numbers of passes and intravenous tissue plasminogen activator (IV tPA) treatment. The NIHSS scores at admission were similar between patients with and without HT. However, the NIHSS score at discharge was significantly higher and the rate of patients with a mRS of 0–2 at 3 months was lower in the HT group. The details are shown in Table 1.
Table 1
| Patient characteristics | HT group (n=42) | Non-HT group (n=77) | P values |
|---|---|---|---|
| Sex, men | 26 (61.90) | 55 (71.43) | 0.287 |
| Age, y | 63.67 (SD, 12.199) | 61.82 (SD, 13.082) | 0.452 |
| Smoking | 15 (35.71) | 35 (45.45) | 0.304 |
| Hypertension | 24 (57.14) | 44 (57.14) | 1.00 |
| Diabetes mellitus | 20 (47.62) | 22 (28.57) | 0.038 |
| Atrial fibrillation | 20 (47.62) | 17 (22.08) | 0.04 |
| Previous stroke or of TIA | 10 (23.81) | 22 (28.57) | 0.576 |
| Preoperative use of antiplatelets | 4 (9.52) | 9 (11.69) | 0.718 |
| Preoperative use of anticoagulants | 3 (7.14) | 1 (1.30) | 0.247 |
| Admission NIHSS score | 13 (11.0–15.0) | 13 (11.0–15.5) | 0.924 |
| Volume of ischemic lesion with ADC <0.6 (×10−3 mm2/s) | 37.9645 (15.7635–90.1765) | 13.431 (5.5945–26.1915) | <0.001 |
| Volume of ischemic lesion with ADC <0.5 (×10−3 mm2/s) | 20.007 (8.320–70.626) | 5.673 (1.731–12.096) | <0.001 |
| Volume of ischemic lesion with ADC <0.4 (×10−3 mm2/s) | 8.498 (2.288–25.236) | 0.805 (0–3.096) | <0.001 |
| Volume of ischemic lesion with ADC <0.3 (×10−3 mm2/s) | 0.554 (0–4.480) | 0 (0–0) | <0.001 |
| Occlusion site | <0.001 | ||
| ICA | 18 (42.86) | 25 (32.47) | |
| MCA | 10 (23.81) | 45 (58.44) | |
| Tandem occlusion | 14 (33.33) | 7 (9.09) | |
| IV tPA treatment | 19 (45.24) | 15 (19.48) | 0.003 |
| Number of passes | 2 (1.0–3.0) | 1 (1.0–2.0) | <0.001 |
| Complete reperfusion (mTICI 3) | 29 (69.05) | 50 (64.94) | 0.650 |
| Onset-to-baseline ADC time, min | 335.5 (260.75–494.0) | 332 (138.0–621.5) | 0.666 |
| Baseline ADC to final reperfusion time, min | 166 (139.25–209.5) | 168 (128–200.5) | 0.519 |
| Onset to final reperfusion time, min | 518 (428.75–678.75) | 509 (325.50–786.5) | 0.619 |
| NIHSS score at discharge | 9 (6.0–18.5) | 6 (2.0–9.0) | <0.001 |
| mRS 0–2 at 3 months | 14 (33.33) | 51 (66.23) | 0.001 |
HT, hemorrhagic transformation; TIA, transient ischemic attack; NIHSS, National Institutes of Health Stroke Scale; ADC, apparent diffusion coefficient; ICA, internal carotid artery; MCA, middle cerebral artery; IV tPA, intravenous tissue plasminogen activator; mTICI, modified Thrombolysis in Cerebral Infarction scale; mRS, modified Rankin Scale.
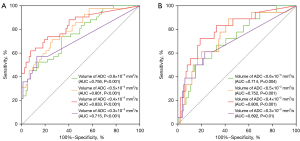
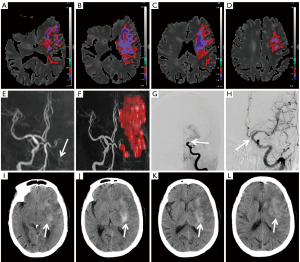
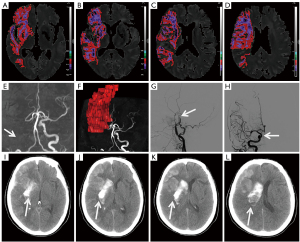
The median volume of the HT group was larger than that of the non-HT group (P<0.001) for ADC thresholds <0.6×10−3, 0.5×10−3, 0.4×10−3, and 0.3×10−3 mm2/s. The volume of ADC <0.4×10−3 mm2/s showed a better ability to differentiate the occurrence of HT (including HI and PH) from non-occurrence and the occurrence of the PH subtype from non-occurrence, but with different accuracies [AUC value of 83.3% (95% confidence interval (CI): 75.3%, 91.3%) for HT and a lower AUC value of 80% (95% CI: 68.5%, 91.5%) for PH]. Under this ADC threshold, the cutoff volumes for predicting HT and PH were both 6.46 mL; the sensitivity and specificity were 61.9% (95% CI: 45.65%, 76.01%) and 92.21% (95% CI: 83.21%, 96.79%) with positive and negative predictive values of 81.25% (95% CI: 62.96%, 92.14%) and 81.61% (95% CI: 71.55%, 88.81%), respectively, for predicting HT, and 72.22% (95% CI: 46.41%, 89.29%) sensitivity and 81.19% (95% CI: 71.93%, 88.02%) specificity with positive and negative predictive values of 40.63% (95% CI: 24.22%, 59.21%) and 94.25% (95% CI: 86.50%, 97.87%) for predicting PH, respectively. However, the volumes of ADC <0.6×10−3 mm2/s, ADC <0.5×10−3 mm2/s, and ADC <0.3×10−3 mm2/s showed comparatively inferior differentiation abilities for HT (AUC 71.5–80.1%) and PH (AUC 69.2–75.2%) prediction (Figure 2A,2B). Figures 3,4 show the ADC threshold and volume distribution in the respective preoperative infarct areas of patients with HI2 and PH2 on follow-up CT after EVT. Tables S1,S2 in the supplemental material display the contingency tables on the relationship between the actual HT and predicted results of the ADC map.
The multivariate logistic regression analysis with selected clinical variables and volume below optimal ADC threshold of 0.4×10−3 mm2/s shows larger volume of ADC <0.4×10−3mm2/s [odds ratio (OR), 34.164; 95% CI: 6.825–171.022; P<0.001], atrial fibrillation (OR, 5.807; 95% CI: 1.234–27.339 P=0.026), intravenous thrombolysis (OR, 7.898; 95% CI: 2.032–30.697; P=0.003) were all independent predictors of HT (Table 2). And the OR value for the dichotomous volume according to 6.46 mL of ADC below 0.4×10−3 mm2/s is higher than that of other potential risk factors.
Table 2
| Patient characteristics | B | SE | OR (95% CI) values | P values |
|---|---|---|---|---|
| Hypertension | 0.211 | 0.603 | 1.235 (0.379–4.031) | 0.726 |
| Diabetes mellitus | 0.046 | 0.659 | 1.047 (0.288–3.808) | 0.944 |
| Atrial fibrillation | 1.759 | 0.790 | 5.807 (1.234–27.339) | 0.026 |
| Volume of ischemic lesion with ADC <0.4 (×10-3mm2/s) >6.456 mL | 3.531 | 0.822 | 34.164 (6.825–171.022) | <0.001 |
| Number of passes | 0.345 | 0.252 | 1.412 (0.862–2.312) | 0.170 |
| IV tPA treatment | 2.067 | 0.693 | 7.898 (2.032–30.697) | 0.003 |
| Onset to final reperfusion time | −0.002 | 0.001 | 0.998 (0.996–1.001) | 0.185 |
| Occlusion site | 0.94 | |||
| Tandem occlusion | Reference | |||
| ICA | −1.247 | 0.857 | 0.287 (0.054–1.540) | 0.145 |
| MCA | −1.936 | 0.892 | 0.144 (0.025–0.829) | 0.030 |
HT, hemorrhagic transformation; EVT, endovascular therapy; SE, standard error; OR, odds ratio; ADC, apparent diffusion coefficient; IV tPA, intravenous tissue plasminogen activator; ICA, internal carotid artery; MCA, middle cerebral artery.
Discussion
In the present study, we obtained a volume of ADC <0.4×10−3 mm2/s reaching 6.46 mL as the optimal threshold for predicting both HT and PH but with different accuracies in AIS patients following successful recanalization with EVT. The multivariate logistic regression analysis shows volume below optimal threshold is independent predictors of HT.
Endothelial cell damage, basement membrane degradation, and vascular remodeling caused by an inflammatory response to cerebral infarction and ischemia/reperfusion injury after recanalization lead to the disruption of the blood-brain barrier coupled with other mechanisms, which is an important pathological basis for HT after EVT (12,13). We observed that the volume and ischemia severity of the preoperative infarction core were both associated with the occurrence of HT after recanalization. According to present guidelines, only infarction volume is taken into consideration to avoid HT when selecting eligible patients for mechanical thrombectomy (14). Our study added the ADC value to quantify the ischemia severity.
For ischemia severity, several researchers have shown that the perfusion parameters of cerebral blood volume (CBV) <0.5 mL/100 g, relative CBV (rCBV) <1.09, relative cerebral blood flow (rCBF) <4.5%, time-to-peak (TTP) >0.27 s, and ADC <300×10–6 mm2/s in the infarct area andthe mean relative mean transit time (rMTT) value within the area of rMTT >1.3 can be used to predict HT (8,9,15-18). Previous reports have also applied a combination of ischemia severity and the corresponding volume to predict HT, such as the volume of time-to-maximum (Tmax) >14 s or the volume of very low CBV 2.5 (<2.5th percentile threshold) area greater than 2 mL (8,9). However, most of these studies were published before the popularization of EVT, and the majority of enrolled patients were either untreated or treated with intravenous thrombolysis alone. In our research, only patients who underwent EVT and achieved successful recanalization were enrolled, which avoided infarct expending due to no reperfusion and better reflected the influence of preoperative ischemia on the blood-brain barrier and HT.
Laredo et al. reported that patients in the HT group after EVT had a larger region of very low CBV and more patients in this group had exceedingly low CBV regions (19). Another study showed that rADC <0.65 in the infarct core is the optimal point to predict HT after EVT (20). There are currently no reports on the predictive value of combining different ischemia severity and the corresponding volume only using EVT patients. In our research, the occurrence of HT was predicted by analyzing the brain tissue volume measured below the specific ADC thresholds. The results indicated a higher predictive value for HT than other imaging methods utilized in previous research (8,15). In addition, the ADC sequence in preoperative MRI was used to quantify the ischemia severity, which is non-invasive and more routine relative to perfusion imaging.
Previous studies have reported that the admission NIHSS score, delayed recanalization, baseline glucose, hypertension, cardiogenic stroke, and intravenous thrombolysis are independent risk factors for HT after recanalization (13,15,21). In our study, intravenous thrombolysis, atrial fibrillation, and larger volumes of ADC <0.4×10−3 mm2/s were found to be independent predictors of HT. The bigger OR of volume below ADC <0.4×10−3 mm2/s indicates the feasibility of optimal ADC parameter and volume threshold in our ROC result for predicting HT.
Our study has some limitations that should be noted. Firstly, this was a retrospective and single-center study. Secondly, the sample size was small, especially for the PH subtype, so only a logistic regression analysis for HT was created. Thirdly, the choice of post-processing software may have influenced the optimal ADC value.
Conclusions
The risk of HT after thrombectomy can be predicted using a combination of ischemia severity and the corresponding volume in an ADC map. A volume of ADC <0.4×10−3 mm2/s provides a higher predictive value than the total volume of the infarct core in ROC analysis. In addition to the total infarct volume, preoperative assessment of the volume in severely ischemic area may provide more information for HT risk prediction. These findings may provide a reference for clinicians to identify patients at risk of HT before EVT, which will contribute to more precise and individualized treatment.
Acknowledgments
Funding: This work was supported by the Key Research and Development Program of Henan Province (Scientific and Technological Project of Henan Province) (No. 202102310037), the Provincial and Ministerial Joint Project of Henan Provincial Medical Science and Technology (No. SBGJ2018063), and the Research and Popularization Project of Appropriate Intervention Techniques for High-risk Population of Stroke in China (No. GN-2018R0007).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2255/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2255/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2255/coif). All authors report that this work was supported by the Key Research and Development Program of Henan Province (Scientific and Technological Project of Henan Province) (No. 202102310037), the Provincial and Ministerial Joint Project of Henan Provincial Medical Science and Technology (No. SBGJ2018063), and the Research and Popularization Project of Appropriate Intervention Techniques for High-risk Population of Stroke in China (No. GN-2018R0007). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by ethics committee of Henan Provincial People’s Hospital (No. 2017-034-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-31. [Crossref] [PubMed]
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018;378:11-21. [Crossref] [PubMed]
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378:708-18. [Crossref] [PubMed]
- Wollenweber FA, Tiedt S, Alegiani A, et al. Functional Outcome Following Stroke Thrombectomy in Clinical Practice. Stroke 2019;50:2500-6. [Crossref] [PubMed]
- Yu S, Ma SJ, Liebeskind DS, et al. Reperfusion Into Severely Damaged Brain Tissue Is Associated With Occurrence of Parenchymal Hemorrhage for Acute Ischemic Stroke. Front Neurol 2020;11:586. [Crossref] [PubMed]
- Kaesmacher J, Kaesmacher M, Maegerlein C, et al. Hemorrhagic Transformations after Thrombectomy: Risk Factors and Clinical Relevance. Cerebrovasc Dis 2017;43:294-304. [Crossref] [PubMed]
- Raychev R, Saver JL, Jahan R, et al. The impact of general anesthesia, baseline ASPECTS, time to treatment, and IV tPA on intracranial hemorrhage after neurothrombectomy: pooled analysis of the SWIFT PRIME, SWIFT, and STAR trials. J Neurointerv Surg 2020;12:2-6. [Crossref] [PubMed]
- Adebayo OD, Culpan G. Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis. Eur Stroke J 2020;5:4-16. [Crossref] [PubMed]
- Suh CH, Jung SC, Cho SJ, et al. MRI for prediction of hemorrhagic transformation in acute ischemic stroke: a systematic review and meta-analysis. Acta Radiol 2020;61:964-72. [Crossref] [PubMed]
- Fiehler J, Knab R, Reichenbach JR, et al. Apparent diffusion coefficient decreases and magnetic resonance imaging perfusion parameters are associated in ischemic tissue of acute stroke patients. J Cereb Blood Flow Metab 2001;21:577-84. [Crossref] [PubMed]
- Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-25. [Crossref] [PubMed]
- Kanazawa M, Takahashi T, Nishizawa M, et al. Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke. J Atheroscler Thromb 2017;24:240-53. [Crossref] [PubMed]
- Otsu Y, Namekawa M, Toriyabe M, et al. Strategies to prevent hemorrhagic transformation after reperfusion therapies for acute ischemic stroke: A literature review. J Neurol Sci 2020;419:117217. [Crossref] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344-418. [Crossref] [PubMed]
- Souza LC, Payabvash S, Wang Y, et al. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis 2012;33:8-15. [Crossref] [PubMed]
- Jain AR, Jain M, Kanthala AR, et al. Association of CT perfusion parameters with hemorrhagic transformation in acute ischemic stroke. AJNR Am J Neuroradiol 2013;34:1895-900. [Crossref] [PubMed]
- Yassi N, Parsons MW, Christensen S, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013;44:3039-43. [Crossref] [PubMed]
- Aviv RI, d'Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 2009;250:867-77. [Crossref] [PubMed]
- Laredo C, Renú A, Llull L, et al. Elevated glucose is associated with hemorrhagic transformation after mechanical thrombectomy in acute ischemic stroke patients with severe pretreatment hypoperfusion. Sci Rep 2020;10:10588. [Crossref] [PubMed]
- Shinoda N, Hori S, Mikami K, et al. Prediction of hemorrhagic transformation after acute thrombolysis following major artery occlusion using relative ADC ratio: A retrospective study. J Neuroradiol 2017;44:361-6. [Crossref] [PubMed]
- Guo Y, Yang Y, Zhou M, et al. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous recombinant tissue plasminogen activator: a systematic review and meta-analysis. Stroke Vasc Neurol 2018;3:203-8. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


