My heart will go on—beneficial effects of anti-MiR-30 after myocardial infarction
MicroRNAs (miRNAs) are small, non-coding RNA molecules, approximately 22 nucleotides in length, which act as master regulators of gene expression (1). Mature miRNAs are single stranded RNAs that block translation—or induce degradation of mRNA by base pairing to partially or perfectly complementary sites on their target mRNA, usually in the 3'-UTR (2). Accumulated data have indicated that miRNAs play key roles in the regulation of diverse cellular processes and during cardiovascular disease development and progression, such as atherosclerosis, hypertension, arrhythmias, left ventricular hypertrophy, myocardial infarction (MI), as well as heart failure (HF) (3,4).
Despite all available therapeutic approaches, MI still ranks as the main cause of death worldwide (5). Pathophysiological landmarks of MI include acute myocardial damage, post-ischemic neovascularization, and cardiac remodeling (6). Acute myocardial damage is attributed by ischemic cellular hypoxia, which results in an increase of reactive oxygen species during early reperfusion, endothelial cell (EC) activation, cytokine production in the damaged area, priming and recruiting of neutrophils bordering the infarcted region, and ultimately cardiomyocyte death, endothelial capillary impairment and post-ischemic neovascularization. The remodeling process is initially an adaptive mechanism to maintain adequate cardiac function, but eventually leads to fibrosis, left ventricular dilatation, and HF (7).
Evidentially, all these processes are tightly regulated by miRNAs (6). Primarily, miRNAs participate in the regulation of cardiomyocyte fate, such as the pro-apoptotic miRs-15, -34, -320, and -140; the anti-apoptotic miRs-24 and -214; the pro-proliferative miR-17–92 cluster, miRs-199a and -590; and anti-proliferative miRs-15 and -133. Secondly, several miRNAs have been described to induce (miR-210) or repress (miR-15, -24, -26, and -17–92 cluster) angiogenesis. Furthermore, miRNAs modulate cardiac remodeling via inhibiting (miR-15 and -34) or enhancing (miR-1, -21, -24, -126, -155, -221, and -499) the function of stem and/or progenitor cells.
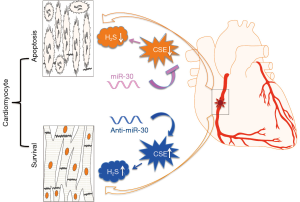
A recent manuscript by Shen and colleagues identifies a novel and crucial role for the miR-30 family in acute MI injury and cardiac function (8). In this study, the authors emphasized on the cardio-protective role of hydrogen sulfide (H2S), predominantly deriving from L-cysteine and being catalyzed by cystathionine-c-lyase (CSE). Their experiments provide additional evidence that the miR-30-CSE-H2S axis contributes to the protection against cardiomyocyte ischemic injury, both in vitro and in vivo, by regulating H2S production.
The authors were able to discover significant deduction of CSE and H2S in MI, which negatively correlates with expression levels of the miR-30 family. Of importance, they could confirm that CSE is a direct target of the miR-30 family. The miR-30 family regulates CSE mRNA and protein levels, thus affects H2S production and hypoxia-induced cardiomyocyte injury. In addition, silencing the miR-30 family not only protects against cardiomyocyte apoptosis during hypoxia, but also increases cardiac function evaluated by echocardiography after MI induction in mice, which turns out to be a CSE-dependent mechanism, as these effects were absent in genetically mutated CSE knockout mice. In contrast, delivery of miR-30b in mice greatly aggravated MI injury. Taken together, the study is able to demonstrate for the very first time that the miR-30 family regulates H2S production by directly targeting CSE. Inhibition of the miR-30 family after MI injury offers clear therapeutic value to ‘keep our heart going on’ (Figure 1).
One limitation to the translational feasibility of the study is that the authors did not evaluate potential off-target effects in other organ systems than the targeted heart (e.g., liver, kidney) in which systemically administered miRNA modulators assimilate to a much higher extent. Further translationally focused pre-clinical studies should take these considerations into account. One promising solution may be local delivery of miRNAs highlighted in the past by Hinkel et al. (9). In their study, locked nucleic acid-modified antisense miR-92a (LNA-92a) was applied either regionally (antegrade or retrograde) with a catheter or systemically (intravenously). They confirmed that LNA-92a reduced miR-92a expression in the infarct zone regardless of the application venue. However, catheter-based delivery, but not intravenous infusion, reduced the infarct size compared with control LNA—treated pigs, which correlated with an improved ejection fraction and left ventricular end-diastolic pressure, while not accumulating in other organs (such as kidney and liver). In addition, as reported by previous studies (10,11), both down-regulated miR-30a and up-regulated miR-30c/d could aggravate myocardial hypertrophy, indicating that distinctive modulation of a miR-30 family subtype would need to be taken into account when developing future therapeutic cardiomyocyte rescue strategies after acute MI in humans. Last but not the least, endogenous H2S level subsequent to miR-30 modulation should be tightly controlled due to its latent toxicity.
The therapeutic value of miRNA in cardiovascular as well as other diseases is obvious. By having unique expression profiles and higher stability in biological samples, miRNAs quickly emerged as novel biomarkers. The ability to regulate multiple genes in various disease-contributing signaling pathways makes them promising future therapeutic targets. Noteworthy, translational applicability to utilization in humans needs to be viewed with caution, especially issues relating to potential immune stimulatory effects, mode of delivery, and off-target effects. These obstacles need to be overcome to bring miRNA therapeutics into mainstream clinical practice.
Acknowledgements
Funding: Research in the Maegdefessel laboratory on non-coding RNAs in cardiovascular diseases is supported by the Swedish Heart-Lung-Foundation (20120615, 20130664, and 20140186), the Ragnar Söderberg Foundation (M55/14), the Swedish Research Council (2015-03140), and the European Research Council (ERC-StG NORVAS).
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Zhijun Han, MD (Department of Laboratory Medicine, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China).
Conflicts of Interest: Yuhuang Li is a CERIC (Center of Excellence for Research in Inflammatory and Cardiovascular Diseases) scholar at the Karolinska Institute, Stockholm, Sweden. The other author has no conflicts of interest to declare.
References
- Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell 2013;153:516-9. [Crossref] [PubMed]
- Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res 2015;116:751-62. [Crossref] [PubMed]
- Romaine SP, Tomaszewski M, Condorelli G, et al. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 2015;101:921-8. [Crossref] [PubMed]
- Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011;469:336-42. [PubMed]
- Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015;12:135-42. [Crossref] [PubMed]
- Fiedler J, Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol 2013;33:201-5. [Crossref] [PubMed]
- Shen Y, Shen Z, Miao L, et al. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-γ-lyase expression. Antioxid Redox Signal 2015;22:224-40. [Crossref] [PubMed]
- Hinkel R, Penzkofer D, Zühlke S, et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013;128:1066-75. [Crossref] [PubMed]
- Pan W, Zhong Y, Cheng C, et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 2013;8:e53950. [Crossref] [PubMed]
- Jentzsch C, Leierseder S, Loyer X, et al. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol 2012;52:13-20. [Crossref] [PubMed]