Predictive value of BRCA1 expression on the efficacy of chemotherapy based on anti-microtubule agents: a pooled analysis across different malignancies and agents
Introduction
Despite the development of monoclonal antibodies and small molecule pathway inhibitors, chemotherapy remains the go-to treatment for patients with cancer, including neoadjuvant chemotherapy, adjuvant chemotherapy and palliative chemotherapy. Excitingly, an improvement in the efficacy of chemotherapy has been observed in recent years. However, its sensitivity varies from one patient to another. With the advances of molecular biological techniques, we have gained a deeper understanding of the pathogenesis and proliferation of the tumor at the molecular level. Thus, focus on the molecular characteristics of the disease to guide treatment choice has increased; one example is the trending use of molecular markers to predict activity of chemotherapeutic agents.
Anti-microtubule agents act by binding to soluble and/or polymerized tubulin in the microtubules ultimately affecting microtubule function. Vinca alkaloids and taxanes are two families of anti-microtubule agents wildly used in clinics including solid tumors and hematological malignancies, such as non-small cell lung cancer, breast cancer and ovarian cancer (1). Taxanes, a class of diterpenes derived from the plants of the genus Taxus (yews), are mitotic inhibitors that stabilize and protect the microtubule polymer from disassembly, causing chromosomes to be unable to forma metaphase spindle conformation, blocking progress of mitosis, and triggering cell death (2,3). Vinca alkaloids, such as vinorelbine, restrain mitosis and apoptosis by binding to tublin and preventing its assembly into microtubules (4).
Breast cancer susceptibility gene 1 (BRCA1), a scaffold protein, was first identified as an early-onset breast and ovarian cancer susceptibility gene (5). It has multiple roles not only in DNA damage repair but also in cell cycle regulation and apoptosis through association with other proteins (6). It has been reported that BRCA1 correlated positively with taxanes sensitivity, which functions as a sensitizer to apoptosis induced by anti-microtubule agents (7). A number of investigations have found that BRCA1 may be used as a predictive biomarker of response to anti-microtubule agents (5). Yang et al. (8) reported the potential role of BRCA1 in predicting sensitivity of NSCLC, and found that patients with high/positive BRCA1 had better ORR. However, existing evidence is conflicting. We conducted a systematic review to evaluate the associations of expression of BRCA1 and the efficacy of anti-microtubule agents on cancer patients.
Materials and methods
Literature search
Literature search was conducted using PubMed and Embase from their dates of inception to Oct 23, 2014. The search strategy employed was a combination of: BRCA1 or “Breast cancer susceptibility gene 1” or “Breast cancer 1” and chemotherapy or paclitaxel or docetaxel or vinorelbine. Language was limited to English and Chinese.
Inclusion criteria and exclusion criteria
Articles retrieved from the search were independently reviewed by two reviewers (Mingzhe Zhang & Jianrong Zhang), and any discrepancies were resolved by discussion with the third reviewer (Jianfei Shen). The following criteria was used to select publications: (I) cancer patients, regardless of tumor type, should be included; (II) only studies that detected BRCA1 expression by immunohistochemistry (IHC) or reverse transcriptase polymerase chain reaction (QPCR) were included; (III) original papers must contain enough data to calculate the objective response rate (ORR); studies that failed to meet all of the above criteria were excluded from analyses. Reviews, animal or cell line studies were also excluded.
Data collection and quality assessment
Publication characteristics including first author’s name, publication year, patients’ original country, middle/mean age of study sample, first-line chemotherapeutic agents with doses and sample type, detection method of BRCA1, sample size, and disease stage were extracted from each eligible publication. End points of interest were ORR, overall survival (OS), and progression-free survival (PFS). Each included study was scored by two independent reviewers (Shengyi Zhong and Yang Liu).
Statistical analysis
To estimate ORR, patients were divided into two groups: patients that responded to treatment (responders) and patients that did not respond to treatment (non-responders). Responders were defined as complete response (CR) or partial response (PR). Non-responders included stable disease (SD) and progressive disease (PD). Disease control ratio (DCR), was defined as CR, PR and SD. The pooled odds ratio (OR) and its 95% confidence intervals (CIs) were calculated by the methods proposed by Mantel and Haenszel (9), or DerSimonian R and Laird N (10). Time-to-event data OS and PFS, hazard ratios (HRs) and associated 95% CI were estimated using the methods reported by Parmar (11). Heterogeneity between the studies was determined by Qtestand I2 metric (I2=0–25%: no heterogeneity; I2=25–50%: moderate heterogeneity; I2=50–75%: large heterogeneity; I2=75–100%: extreme heterogeneity) (12). The fixed-effect model was applied in the initial analysis, and if significant heterogeneity existed, the random-effect model was used. Begg’s test was used to evaluate the publication bias. P<0.05 indicated significant publication bias (13). All P values were two-tailed, REVIEW MANAGER (version 5.3 for Windows; the Cochrane Collaboration, Oxford, UK) and STATA version 11.1 (Stata Corporation, USA) were used to perform most data analyses.
Results
Eligible studies
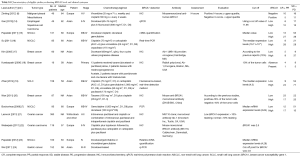
Our search of PubMed database revealed 1,045 potentially relevant articles, 976 studies were immediately excluded upon review of their title and abstract. A total of 69 full text articles were carefully screened, 33 of which were excluded due to lack of sufficient data for extraction, another 20 articles were then excluded due to containing other therapeutic and unable to separate the results of BRCA1 and BRCA2. Finally a total of 13 studies were selected for analysis. Figure 1 summarizes the flow chart. Among these studies, the object response rate (ORR) was provided in 9 studies (5,7,14-20), the remaining 4 studies provided only OS or PFS (21-24). Characteristics of all involved studies are summarized in Table S1.
Full table
Characteristics of eligible studies
Our meta-analysis contained 13 studies involving a total of 1,490 cancer patients who had been treated with anti-microtubule agents as first- or second-line chemotherapy treatment. In all included studies, the major components of the chemotherapy regimen were anti-microtubule agents (including taxanes, paclitaxel, docetaxel and vinorelbine). Of the 13 included studies, 4 were for non-small-cell lung cancer, 3 were for breast cancer, and 2 were for ovarian cancer; the remaining four were for malignant pleural mesothelioma, esophageal squamous cell carcinoma, small cell lung cancer and gastric cancer. Of the 13 studies, 7 were from an East-Asian population (14,16-20,24), the other 6 studies were from a European population (5,7,15,21-23). Characteristics of included studies are summarized in Table S1.
BRCA1 level and the clinical outcome of chemotherapy
The ORR was reported in 9 of the included studies consisting of a total of 729 patients. By synthesis, we observed greater ORR in population with high BRCA1 expression vs. low expression (OR 1.63, 95% CI: 0.92 to 2.88, P=0.09) but the heterogeneity was severe (P=0.01; I2=61%) (Figure 2). No significant difference was observed in PFS (high vs. low expression, HR 0.93, 95% CI: 0.75 to 1.15, P=0.49; heterogeneity, P<0.01, I2=75%) and OS (high vs. low expression, HR 0.77, 95% CI: 0.57 to 1.04, P=0.09; heterogeneity, P=0, I2=74%) (Figure 3). When analyzing the DCR, 4 studies consisting of 233 patients were included for comparison. No significant difference between the two groups was found (high vs. low expression, OR=0.83, 95% CI: 0.38 to 1.80, P=0.63; I2= 17%, P=0.63 for heterogeneity).


Subgroup analyses
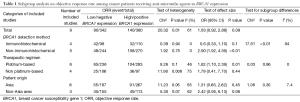
After stratification by testing methods, a significantly higher ORR in the population with high BRCA1 expression was shown in the subgroup using mRNA as a measure approach (OR 2.90, 95% CI: 1.92 to 4.39, Chi2 =0.39, P<0.01) whereas the difference in the subgroup using IHC was not significant (OR 0.60, 95% CI: 0.33 to 1.10, Chi2 =1.92, P=0.10). The interaction between the two subgroups was significant (Chi2 =17.61, P<0.01). However, results stratified by therapeutic regimens revealed a similar tendency between subgroups (for platinum-based studies, high vs. low expression, OR 1.62, 95% CI: 1.10 to 2.39, Chi2 =9.26, P=0.01; for non-platinum-based studies, high vs. Low expression, OR 1.79, 95% CI: 0.41 to 7.70, Chi2 =11.90, P=0.44); but there was no significant interaction between stratifications (Chi2 =0.7, P=0.4). A potential association between BRCA1 and efficacy was found in the non-Asian population but not in the Asian population (for non-Asian population studies, high vs. low expression, OR 2.42, 95% CI: 0.95 to 6.13, Chi2 =11.23, P=0.06; for Asian studies, high vs. low expression, OR 1.31, 95% CI: 0.65 to 2.62, Chi2 =5.3, P=0.45) but the interaction was not significant (Chi2 =1.08, P=0.3). Details about the results on subgroup analysis are shown in Table 1 and Figure 4.
Full table

Discussion
Due to its ubiquitous presence and importance in all cells, microtubules are one of the most validated intracellular targets in oncology (25). Because of this, the mechanism of resistance to anti-microtubule agents earn widespread concerns and many studies have reported on the subject. Several mechanisms explain the resistance, including decrease of the cellular accumulation mediated by P-glycoprotein (26) exportation and altered expression or post-translational modification of tubulin or other microtubule regulatory proteins (27).
Recently, some studies have reported on the relationship and mechanism between BRCA1 expression and chemotherapy outcomes for carcinoma, but the results were controversial. Therefore a meta-analysis is needed to incorporate all available results to give further insight on this conflicting issue. After combining the available data of the included studies, our results were in concordance with our initial hypothesis that increased BRCA1 expression might be associated with higher sensitivity of anti-microtubule agents and longer PFS/OS. However, the effect sizes of all syntheses were not statistically significant.
Since significant heterogeneity was observed in the overall analyses, we carried out additional subgroup analyses. Interestingly, our results show that using non-immunohistochemical (PCR and Relative cDNA quantification) detection methods offer the notable result that high BRCA1 expression was associated with higher sensitivity of anti-microtubule agents whereas the difference in the subgroup using IHC was not significant (for non-immunohistochemical study, high vs. low expression, OR 2.90, 95% CI: 1.92 to 4.39, P<0.01; I2=0; for immunohistochemical studies, high vs. low expression, OR 0.60, 95% CI: 0.33 to 1.10, P=0.10; I2=0), and the heterogeneity of the subgroup was extreme(P<0.01, I2=94%). This result implies that PCR and Relative cDNA quantification maybe a more accurate evaluation method compared to IHC in determining the expression of BRCA1.
IHCis a process that exploits the principle of antibodies binding specifically to antigens in biological tissues to detect antigens (e.g., proteins) (28). The detection target of IHC is the proteins which are at the last destination to take a leading role in biological effects, so it has clinical significance but it also carries obvious limitations. Firstly, an antibody may not be specific to the object protein since one antibody may combine with a variety of proteins. Secondly, many factors can cause a false positive or a false negative result. For example, the concentration and the effect of an antibody, whether reagent covers the tissue, and the incubation time of the antibody can cause a false negative result, whereas improper selection of antigen retrieval method, antibody titer deduced or failure can result in false negative results (29). Another limitation is that IHC only carries out semi-quantitative assessment of the protein expression, and the judgments of the pathologist are inevitably subjective. By contrast, q-PCR based mRNA level detection is a quantitative determination. It has the advantages of: (I) accurate quantification; (II) reliable sensitivity and specificity; (III) reducing pollution and automation, etc. Because of this, we believe that detection based on mRNA might be a preferred technique over IHC. We are looking forward to future research to further prove our conclusion and explore the cause.
Another issue that captivated our attention is the confounding effect of cisplatin on the predictive value of BRCA1. Several cell studies, based on clinical trials, demonstrated high/positive BRCA1 expression could resist platinum-based chemotherapy. But cisplatin and anti-microtubule agents are often combined in cancer therapy due to their differing mechanisms of action. The question now is how we might determine whether an anti-microtubule agent the proper choice according to BRCA1 expression. In subgroup analysis based on therapeutic regimen, no subgroup difference was found between the platinum-based population and then on platinum-based population. According to this result, we believe the existence of platinum in chemotherapy regimen did not offer confounding effect to BRCA1 expression. The predictive value of BRCA1 for anti-microtubule agents is valid.
This is the first study to address the interaction between BRCA1 expression and the outcome of anti-microtubule agents in cancer patients. However, there are several limitations. First, it was based on retrospective analysis; prospective analysis is needed to further clarify these issues. Second, although our purpose is the prediction of BRCA1 for paclitaxel, we cannot eliminate the effects of the combination of the chemotherapeutic agents. In addition, we are unable to study the effects on each cancer separately and are therefore unable to distinguish the individual role of BRCA1. Further studies are necessary to validate our results.
In conclusion, although the predictive value of BRCA1 expression on the anti-microtubule chemotherapy remained uncertain based on overall results, our exploratory analyses suggested that detection using mRNA might be a preferred technique over IHC, however, further validation is required to substantiate our findings.
Acknowledgements
Funding: This work was supported by Science and Technology Planning Project of Guangdong Province, China (Grant numbers: 2007B031515017; 2008A030201024); Science and Technology Planning Project of Guangzhou, China (Grant numbers: 2007Z1-E0111; 2007Z3-E0261).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res 2014;16:209. [Crossref] [PubMed]
- Wang S, Qiu J, Shi Z, et al. Nanoscale drug delivery for taxanes based on the mechanism of multidrug resistance of cancer. Biotechnol Adv 2015;33:224-41. [Crossref] [PubMed]
- Gelmon K. The taxoids: paclitaxel and docetaxel. Lancet 1994;344:1267-72. [Crossref] [PubMed]
- Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res 1991;51:2212-22. [PubMed]
- Kennedy RD, Quinn JE, Mullan PB, et al. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst 2004;96:1659-68. [Crossref] [PubMed]
- Zimling ZG, Sørensen JB, Gerds TA, et al. A biomarker profile for predicting efficacy of cisplatin-vinorelbine therapy in malignant pleural mesothelioma. Cancer Chemother Pharmacol 2012;70:743-54. [Crossref] [PubMed]
- Boukovinas I, Papadaki C, Mendez P, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One 2008;3:e3695. [Crossref] [PubMed]
- Yang Y, Xie Y, Xian L. Breast cancer susceptibility gene 1 (BRCA1) predict clinical outcome in platinum- and toxal-based chemotherapy in non-small-cell lung cancer (NSCLC) patients: a system review and meta-analysis. J Exp Clin Cancer Res 2013;32:15. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Gao Y, Zhu J, Zhang X, et al. BRCA1 mRNA expression as a predictive and prognostic marker in advanced esophageal squamous cell carcinoma treated with cisplatin- or docetaxel-based chemotherapy/chemoradiotherapy. PLoS One 2013;8:e52589. [Crossref] [PubMed]
- Papadaki C, Tsaroucha E, Kaklamanis L, et al. Correlation of BRCA1, TXR1 and TSP1 mRNA expression with treatment outcome to docetaxel-based first-line chemotherapy in patients with advanced/metastatic non-small-cell lung cancer. Br J Cancer 2011;104:316-23. [Crossref] [PubMed]
- Su C, Zhou S, Zhang L, et al. ERCC1, RRM1 and BRCA1 mRNA expression levels and clinical outcome of advanced non-small cell lung cancer. Med Oncol 2011;28:1411-7. [Crossref] [PubMed]
- Kim SJ, Miyoshi Y, Taguchi T, et al. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin Cancer Res 2005;11:8425-30. [Crossref] [PubMed]
- Kurebayashi J, Yamamoto Y, Kurosumi M, et al. Loss of BRCA1 expression may predict shorter time-to-progression in metastatic breast cancer patients treated with taxanes. Anticancer Res 2006;26:695-701. [PubMed]
- Zhao H, Zhang H, Du Y, et al. Prognostic significance of BRCA1, ERCC1, RRM1, and RRM2 in patients with advanced non-small cell lung cancer receiving chemotherapy. Tumour Biol 2014;35:12679-88. [Crossref] [PubMed]
- Wan YY, Hui HX, Wang XW, et al. The correlation between chemotherapeutic efficacy and breast cancer susceptibility gene 1 and class IIIβ-tubulin protein expression in non-small cell lung cancer patients. Zhonghua Nei Ke Za Zhi 2011;50:469-73. [PubMed]
- Lesnock JL, Darcy KM, Tian C, et al. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group Study. Br J Cancer 2013;108:1231-7. [Crossref] [PubMed]
- Weberpals JI, Tu D, Squire JA, et al. Breast cancer 1 (BRCA1) protein expression as a prognostic marker in sporadic epithelial ovarian carcinoma: an NCIC CTG OV.16 correlative study. Ann Oncol 2011;22:2403-10. [Crossref] [PubMed]
- Papadaki C, Sfakianaki M, Ioannidis G, et al. ERCC1 and BRAC1 mRNA expression levels in the primary tumor could predict the effectiveness of the second-line cisplatin-based chemotherapy in pretreated patients with metastatic non-small cell lung cancer. J Thorac Oncol 2012;7:663-71. [Crossref] [PubMed]
- Wei J, Costa C, Ding Y, et al. mRNA expression of BRCA1, PIAS1, and PIAS4 and survival after second-line docetaxel in advanced gastric cancer. J Natl Cancer Inst 2011;103:1552-6. [Crossref] [PubMed]
- Klute K, Nackos E, Tasaki S, et al. Microtubule inhibitor-based antibody-drug conjugates for cancer therapy. Onco Targets Ther 2014;7:2227-36. [PubMed]
- Parekh H, Wiesen K, Simpkins H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem Pharmacol 1997;53:461-70. [Crossref] [PubMed]
- Stordal B, Davey R. A systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: role of BRCA1. Curr Cancer Drug Targets 2009;9:354-65. [Crossref] [PubMed]
- Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol 2014;51:42-87. [Crossref] [PubMed]
- Zhang WQ. The application of immunohistochemistry in pathological diagnosis. Anhui Medical and Pharmaceutical Journal 2012;16:1700-2.



