
Multimodal treatments of brain arteriovenous malformations: a comparison of microsurgical timings after endovascular embolization
Introduction
Brain arteriovenous malformations (bAVMs) are anomalies of dilated arteries and veins without capillary networks, allowing high-flow arterial blood to shunt directly into the venous system (1-3). The combination of endovascular embolization followed by microsurgical resection is commonly referred in the treatment of bAVMs as a traditional multi-modal approach, in which embolization is generally considered as an adjunct procedure to microsurgical resection. Appropriate embolization by taking out pedicle feeders in anticipation of a subsequent surgical resection would significantly reduce arterial inflow to the bAVMs to facilitate safer resection (4-6), creating distinct arachnoid planes for more effective nidus dissection and maximizing the protection of surrounding eloquent structures (7,8).
Despite the established concept of multi-modality approach, the timing of the subsequent microsurgery remains undefined. During a multi-staged operation (MO), preoperative embolization is presumed to gradually change the hemodynamics of high-flow lesions and minimize the risk of hemorrhage and parenchymal hyperemia (4,9). Nevertheless, potential risks exist during the interval between embolization and definitive resection, including hemorrhage and epilepsy (10-12). Single-staged hybrid operation (HO) is an emerging multimodal setup to enable performing embolization and microsurgical resection in one treatment session, which has been proven by many to be feasible in the definitive treatment of complex bAVMs (13-16). The timing of subsequent microsurgery is the essential difference between multi-stage and single-stage approaches. To our knowledge, no existing literature has compared the two approaches in a single study. In correspondence, this study was undertaken with the aim to compare the clinical outcomes between multi-staged and single-staged HOs in the treatment of bAVMs. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-811/rc).
Methods
Study design and population
Patients with bAVMs were retrospectively retrieved and reviewed from the database of a multicenter prospective cohort study (NCT03774017) from June 2016 to June 2020. Those who received treatments of both embolization and microsurgical resection were enrolled in this study. Patients with bAVMs of Spetzler-Martin Grade (SM) V or higher were excluded, as per our institution’s treatment algorithm was a relative contraindication to undergo surgical resection.
A case-control study, with MO in one group and single-staged HO in another, was conducted with a 1:1 matching ratio and matched according to morphological characteristics of bAVMs. Microsurgery and embolization were performed by a multidisciplinary team comprised of both neurosurgeons and neuro-interventionists. In MO group, patients received elective microsurgery after single or multiple embolizations, with an interval duration ranging from days to months. In the HO group, patients underwent surgical resection immediately after embolization in a hybrid operating room in a single session.
The risks of HO and MO were explained in detail to patients and families. Preference from patient was taken account into our treatment algorithm. The management strategy was dependent on objective conditions and neurosurgeons in different medical centers. The HO followed a single study protocol across the multi-centers in the aforementioned prospective study (17). The paradigms of HO and MO were objectively recorded. This study was conducted following the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Beijing Tiantan Hospital (No. KY2016-034-02). Written consent was provided by all participants or their legal representatives.
Outcome evaluation
Clinical outcomes were compared between MO and HO group. Primary outcomes included neurological deficits (NDs), defined as a score of the modified Rankin Scale (mRS) >2. NDs presented at three months after microsurgery was regarded as the short-term, and those that lasted for ≥6 months were regarded as long-termed. Our secondary outcomes included residual bAVM, post-embolization hemorrhage, immediate post-operative complications, and mortality. Residual bAVM was defined as persistent nidus in post-operative angiograms. Post-embolization hemorrhage was defined as the diagnosed intracranial hemorrhage (high-density volume >5 mL on CT scan) due to bAVMs occurring during the interval between embolization and microsurgery. Immediate post-operative complications were defined as infarction, seizure, and intracranial or pulmonary infection within seven days after surgery, and surgery-related mortality was defined as a result of microsurgery or operation-related complications within 30 days after resection. Two experienced neurosurgeons (C Zeng, M Wang) independently evaluated the clinical outcomes.
Data collection and follow-up
Data were extracted from the database of the cohort. General information, such as demographics, personal and treatment history, and comorbidity history were included. bAVM-specific data included presenting symptoms, past procedural history, angio-architectural, localization and morphological information of the bAVMs, and Spetzler-Martin grades. Presenting symptoms were summarized into four categories: hemorrhage, seizure, neurological dysfunction, and incidence (with headache included). Hemorrhagic events were carefully distinguished between the primary symptom and the complication of conducted endovascular embolization. Neuro-images provided information on morphology, spatial relation with eloquence, and angioarchitecture of lesions. According to the morphological data, the bAVM volume was calculated by (width × height × length)/2 (18). Operative duration and hematoma volume were used for evaluations of operative risk and difficulty. Clinical outcomes, such as postoperative complications, residual bAVMs, neurological outcomes, and procedure-specific mortality, were acquired from the evaluation of discharge and outpatient follow-ups in the 3rd, 6th, and 12th months after microsurgery.
Statistical analyses
IBM® SPSS® Statistics (Version 26, IBM, NY, United States) was used for all statistical analyses of this study. Data were categorized into categorical and continuous variables. Descriptive analyses were reported, with categorical variables in proportion and continuous variables in mean ± standard deviation (SD) and median ± interquartile range (IQR). A 1:1 case-control matched analysis was adopted with regards to bAVM size, eloquent location, and deep venous drainage, to reduce the heterogeneity and bias in baseline characteristics between groups. The variables in two groups were assessed with the standardized mean difference, which was calculated as the difference in the means or proportions of a variable divided by the pooled estimate of the SD of the variable (19). A standardized mean difference <0.1 indicates a negligible difference; 0.1–0.3 indicates a small difference; 0.3–0.5 indicates a moderate difference; >0.5 indicates a considerable difference (19). McNemar tests were conducted to compare categorical variables between groups. Paired t-tests or Wilcoxon tests were used to compare continuous variables between groups. Univariate and multivariate logistic regression analyses were performed to obtain the independent predictors of NDs. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each variable. Statistical significance was defined as P<0.05.
Results
Five hundred and forty-four patients were involved in the prospective multicentered registry. Twelve patients harboring bAVMs in SMG V and 334 patients being cured by a single modality with either endovascular or microsurgery were excluded from this study. A total of 198 patients met inclusion and exclusion criteria and were enrolled in the study, including 78 (39.4%) patients in MO group and 120 (60.6%) patients in HO group (Figure 1). Prior to 1:1 case-control matching, patients who underwent the HO were more likely to present with poor mRS (mRS >2, P=0.014) and larger bAVM volume (P=0.046) as compared to the MO group. Sixty-six pairs of cases were matched in HO group (male to female is 44:22) and MO group (male to female is 38:28). The demographic and clinical characteristics of two groups were shown in Table 1. Intergroup comparisons of baseline were performed on demography, primary symptoms, neurological function on admission, and morphological and angio-architectural features of lesions. There was no significant difference observed in baselines between groups.
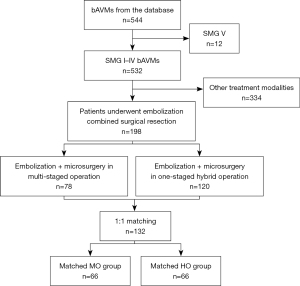
Table 1
| Variables | After matching | |||
|---|---|---|---|---|
| MO (n=66) | HO (n=66) | P value | Standardized mean difference† | |
| Age, mean ± SD [range], years | 29.0±13.92 [7–58] | 29.3±12.37 [3–66] | 0.891 | 0.023 |
| Sex (male), n (%) | 44 (66.7) | 38 (57.6) | 0.345 | 0.189 |
| Prior radiosurgery, n (%) | 3 (4.5) | 2 (3.0) | >0.999 | 0.079 |
| Primary symptom, n (%) | ||||
| Hemorrhage | 37 (56.1) | 33 (50.0) | 0.618 | 0.122 |
| Seizure | 18 (27.3) | 22 (33.3) | 0.585 | 0.131 |
| Neurological dysfunction | 6 (9.1) | 5 (7.6) | >0.999 | 0.054 |
| Incidence | 8 (12.1) | 9 (13.6) | >0.999 | 0.045 |
| Headache | 7 (10.6) | 2 (3.0) | 0.180 | 0.205 |
| Admission mRS score | ||||
| Mean ± SD | 1.2±1.29 | 1.2±1.02 | 0.833 | 0.000 |
| Poor neurological status (mRS >2), n (%) | 4 (6.1) | 4 (6.1) | >0.999 | 0.000 |
| Spetzler-Martin grade, n (%) | ||||
| I | 6 (9.1) | 6 (9.1) | >0.999 | 0.000 |
| II | 24 (36.4) | 23 (34.9) | >0.999 | 0.033 |
| III | 26 (39.4) | 26 (39.4) | >0.999 | 0.000 |
| IV | 10 (15.2) | 11 (16.7) | >0.999 | 0.041 |
| bAVM morphology and angioarchitecture | ||||
| Maximum diameter (median ± IQR), cm | 3.7±1.83 | 3.7±1.43 | 0.878 | 0.027 |
| Volume (median ± IQR), cm3 | 13.6±17.97 | 13.8±16.11 | 0.477 | 0.012 |
| bAVM location, n (%) | >0.999 | 0.059 | ||
| Supratentorial | 62 (93.9) | 61 (92.4) | ||
| Infratentorial | 4 (6.1) | 5 (7.6) | ||
| Eloquence, n (%) | 34 (51.5) | 34 (51.5) | >0.999 | 0.000 |
| Deep perforator supply, n (%) | 8 (12.1) | 12 (18.2) | 0.481 | 0.171 |
| Deep venous drainage, n (%) | 16 (24.2) | 16 (24.2) | >0.999 | 0.000 |
†, standardized mean difference <0.1, negligible difference; 0.1–0.3, small difference; 0.3–0.5, moderate difference; >0.5, considerable difference. MO, multi-staged operation; HO, hybrid operation; SD, standard deviation; mRS, modified Rankin Scale; bAVM, brain arteriovenous malformation; IQR, interquartile range.
Outcomes
Clinical outcomes were shown in detail in Table 2. For the MO paradigm, 23.9% of patients received ≥2 secessions of embolizations prior to microsurgery. The mean interval between different secessions of embolizations was 11.2±5.1 weeks. The average length between the last embolization and microsurgery was 4.9±3.7 weeks. A total of seven patients (10.6%) in MO group encountered post-embolization hemorrhage in a total interval of 170.7 patient years, correlating to an annual hemorrhagic risk of 4.1% per year. No hemorrhage occurred during the immediate interval between embolization and microsurgery in the HO group. During the microsurgical operation, HO group had less blood loss (734.1±620.7 vs. 915.9±1,049.3 mL, P=0.267) and significantly shorter duration of operation (5.5±2.5 vs. 7.5±4.1 hours, P=0.001) compared to MO group.
Table 2
| Variables | Total (n=132) | MO (n=66) | HO (n=66) | P value |
|---|---|---|---|---|
| Embolization degree†, n (%) | ||||
| <30% | 55 (41.7) | 27 (40.9) | 28 (42.4) | >0.999 |
| 30–60% | 38 (28.8) | 22 (33.3) | 16 (24.2) | 0.417 |
| >60% | 39 (29.5) | 17 (25.8) | 22 (33.3) | 0.522 |
| Microsurgical characteristics | ||||
| Blood loss (mean ± SD), mL | 825.0±863.61 | 915.9±1,049.29 | 734.1±620.74 | 0.267 |
| Microsurgical duration (mean ± SD), h | 6.5±3.53 | 7.5±4.09 | 5.5±2.53 | 0.001* |
| Post-embolization hemorrhage, n (%) | 7 (5.3) | 7 (10.6) | 0 (0.0) | 0.023* |
| Post-surgical complications, n (%) | 30 (22.7) | 18 (27.3) | 12 (18.2) | 0.286 |
| Intracranial hemorrhage | 4 (3.0) | 3 (4.6) | 1 (1.5) | 0.625 |
| Cerebral ischemia | 1 (0.8) | 1 (1.5) | 0 (0.0) | >0.999 |
| Seizure | 5 (3.8) | 3 (4.6) | 2 (3.0) | >0.999 |
| Intracranial infection | 17 (12.9) | 10 (15.2) | 7 (10.6) | 0.549 |
| Pulmonary infection | 6 (4.6) | 3 (4.6) | 3 (4.6) | >0.999 |
| NDs, n (%) | ||||
| Discharge | 24 (18.2) | 15 (22.7) | 9 (13.6) | 0.263 |
| 3-month | 12 (9.1) | 10 (15.2) | 2 (3.0) | 0.021* |
| 6-month | 7 (5.3) | 5 (7.6) | 2 (3.0) | 0.375 |
| bAVMs residue, n (%) | ||||
| 3-month | 3 (2.3) | 3 (4.6) | 0 (0.0) | 0.248 |
| 6-month | 2 (1.5) | 2 (3.0) | 0 (0.0) | 0.480 |
†, embolization degree was recorded according to the maximum degree among stages for MO group, and intraoperative embolization degree for HO group. *, P<0.05, significant difference. MO, multi-staged operation; HO, hybrid operation; SD, standard deviation; NDs, neurological deficits; bAVMs, brain arteriovenous malformations.
Postoperative complications were seen in 18 patients from the MO group and 12 patients from the HO group (27.3% vs. 18.2%, P=0.286, shown in Table 2). Postoperative infection was the most frequent complication with morbidity of 12.9% (n=17), and there was no significant difference between the MO and HO groups (15.2% vs. 10.6%, P=0.549). Intracranial hemorrhage was observed in 3 cases (4.6%) in the MO group and 1 case (1.5%) in the HO group (P=0.625). The overall risk of postoperative complications was similar between the MO and HO groups. Length of stay inpatient was 19.3±7.9 days on average, including 19.1±8.8 days in the MO group and 19.6±6.9 days in the HO group (P=0.700).
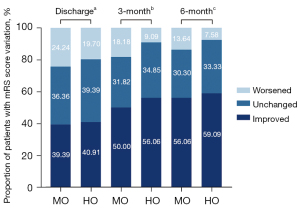
When comparing preoperative and postoperative mRS, neurological function improved or unchanged in 75.8% and 80.3% in the MO group and the HO group at discharge, respectively. During a mean follow-up of 26.2±12.5 months, the difference between pre- and post-operative neurological function was similar between the MO and HO groups at 3 months (81.8% vs. 90.9% with improved or unchanged neurological function respectively, P=0.128), and at 6 months (86.4% vs. 92.4% with improved or unchanged neurological function respectively, P=0.258, Figure 2). NDs occurred in 24 patients (18.2%) at discharge, including 15 (22.7%) in the MO group and 9 (13.6%) in the HO group (P=0.176). The risk of NDs was significantly higher in MO group than HO group at the 3-month follow-up (15.2% vs. 3.0%, P=0.015). This difference was attenuated to non-significance at 6-months (MO vs. HO, 7.6% vs. 3.0%, P=0.437).
In angiographic follow-ups, complete obliterations were achieved in 95.5% of cases (n=63) in MO group and 100% of cases in HO groups at 3 months (Fisher exact test, P=0.243). At 6 months, the incidence of residues was 3.0% (n=2) and 0% (n=0) in MO and HO group (Fisher exact test, P=0.240), respectively. There was no operation-related death occurred in the groups.
Independent predictors of NDs
Variables associated with short-term NDs were analyzed. In the univariate analysis, poor neurological status, bAVM maximum diameter and HO modality were correlated with the occurrence of short-term NDs. After adjusting for age, sex, eloquence and deep venous drainage, poor neurological status (OR, 7.612; 95% CI: 1.633–35.486; P=0.010) and bAVM maximum diameter (OR, 2.010; 95% CI: 1.167–3.461; P=0.012) were confirmed as risk factors for short-term NDs. HO modality (OR, 0.110; 95% CI: 0.017–0.737; P=0.023) was confirmed as the protective factor for short-term NDs (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 1.035 | 0.989–1.083 | 0.140 | 1.053 | 0.998–1.110 | 0.060 | |
| Male | 1.243 | 0.354–4.363 | 0.734 | 1.193 | 0.253–5.636 | 0.824 | |
| Onset symptom | |||||||
| Hemorrhage | 1.267 | 0.381–4.215 | 0.700 | – | – | – | |
| Seizure | 1.337 | 0.342–5.226 | 0.676 | – | – | – | |
| Neurological dysfunction | 2.467 | 0.468–13.015 | 0.287 | – | – | – | |
| Poor neurological status | 8.810 | 2.322–33.430 | 0.001 | 7.612 | 1.633–35.486 | 0.010* | |
| bAVM location | |||||||
| Supratentorial | Ref | Ref | Ref | – | – | – | |
| Infratentorial | 0.786 | 0.090–6.876 | 0.828 | – | – | – | |
| bAVM maximum diameter | 1.773 | 1.128–2.788 | 0.013 | 2.010 | 1.167–3.461 | 0.012* | |
| Eloquence | 3.102 | 0.800–12.022 | 0.102 | 4.748 | 0.921–24.486 | 0.063 | |
| Deep venous drainage | 0.600 | 0.124–2.894 | 0.525 | 0.525 | 0.081–3.394 | 0.499 | |
| Treatment modality | |||||||
| MO | Ref | Ref | Ref | Ref | Ref | Ref | |
| HO | 0.175 | 0.037–0.833 | 0.029 | 0.110 | 0.017–0.737 | 0.023* | |
*, P<0.05, significant difference. NDs, neurological deficits; OR, odds ratio; CI, confidence interval; bAVM, brain arteriovenous malformation; MO, multi-staged operation; HO, hybrid operation.
Analyzing variables related to long-term NDs, the univariate analysis demonstrated that poor neurological status was associated with the occurrence of long-term NDs. After adjusting for age, sex, bAVM maximum diameter, eloquence and deep venous drainage, poor neurological status (OR, 28.138; 95% CI: 4.129–191.770; P=0.001) were shown as the significant predictor of long-term NDs (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 1.051 | 0.991–1.115 | 0.098 | 1.061 | 0.986–1.143 | 0.114 | |
| Male | 1.558 | 0.291–8.353 | 0.604 | 1.568 | 0.167–14.707 | 0.693 | |
| Onset symptom | |||||||
| Hemorrhage | 2.308 | 0.431–12.344 | 0.328 | – | – | – | |
| Seizure | 2.721 | 0.317–23.372 | 0.362 | – | – | – | |
| Neurological dysfunction | 2.810 | 0.296–16.659 | 0.368 | – | – | – | |
| Poor neurological status | 32.222 | 5.464–190.031 | <0.001 | 28.138 | 4.129–191.770 | 0.001* | |
| bAVM location | |||||||
| Supratentorial | Ref | Ref | Ref | – | – | – | |
| Infratentorial | 0.410 | 0.044–3.834 | 0.435 | – | – | – | |
| bAVM maximum diameter | 1.105 | 0.996–1.034 | 0.119 | 1.618 | 0.750–3.488 | 0.220 | |
| Eloquence | 1.271 | 0.273–5.913 | 0.760 | 1.141 | 0.163–8.009 | 0.894 | |
| Deep venous drainage | 1.267 | 0.234–6.868 | 0.784 | 1.125 | 0.118–10.762 | 0.918 | |
| Treatment modality | |||||||
| MO | Ref | Ref | Ref | Ref | Ref | Ref | |
| HO | 0.381 | 0.071–2.039 | 0.260 | 0.438 | 0.053–3.643 | 0.445 | |
*, P<0.05, significant difference. NDs, neurological deficits; OR, odds ratio; CI, confidence interval; bAVM, brain arteriovenous malformation; MO, multi-staged operation; HO, hybrid operation.
Discussion
From our prospective multicenter registry, 198 out of 544 patients who have received microsurgical resection combined with pre-/intra-operative embolization were enrolled. Patients were divided into HO and MO groups according to the treatment received in one-stage or multi-stage. One-hundred and thirty-two of them were 1:1 case-control matched into HO and MO group (n=66 cases for each group). Patients in HO group were obviously protected from the post-embolization hemorrhage due to the elimination of intervals between endovascular and microsurgical treatments. Significant advantages were achieved by HO group in microsurgical duration and neurological outcome at 3 months. Besides, single-staged HO showed its potential in decreasing the volume of intraoperative blood loss, incidences of postoperative complications and residue.
Multimodality treatment consisting of endovascular embolization and microsurgical resection has been routinely utilized in the treatment of bAVMs (20). Compared to the traditional multi-stage approach, HO with embolization and microsurgical resection in one single setting has unique advantages in regard to treatment workflow and patient convenience. However, quantification of this advantage has been lacking in current literature. It has been established that pre-surgical embolization may gradually reduce bAVM arterial supply and enable complete surgical resection while minimizing the risk of normal perfusion pressure breakthrough (NPPB), which was proposed as the leading cause of postoperative hemorrhage, especially in large bAVMs (5,21,22). Martin et al. and Young et al. proposed that stepwise occlusion of the arteriovenous shunting of large-size or high-flow bAVMs may also normalize cerebral hemodynamics and improve disturbed vascular reactivity (23,24).
Conversely, one might argue that committing patients to multi-stage embolization with repeated anesthesia is not without significant risk, and the prolonged interval before definitive resection of the bAVMs is also suboptimal, as hemorrhagic risk might be increased with embolization of bAVMs in short term (11,12). Corroborating the aforementioned point, we have observed that 10.2% of all postembolization patients in MO group experienced a post-embolization hemorrhage in the interval. The morbidity of the hemorrhagic events was reported to be 5.9–20% (11,25,26), with accumulative risk following additional endovascular therapies (27,28). Several studies had proved hemorrhagic risk between the procedures of multi-staged treatments (29,30). Notably, the single-staged mode in HO eliminated this risk by minimizing of embolization-microsurgery interval. Additionally, likely attributed to the recanalization of the nidus and the recruitment of neo-collateral feeders from adjacent feeding arteries after embolization (31,32), we have observed a relatively higher volume of intraoperative blood loss in MO group as well as significantly longer microsurgery duration.
The benefit of improved neurological prognosis in multimodality treatment has been discussed in the existing literature. Kocer et al. reported morbidities of 6-month NDs to be 4.5% in bAVMs treated with multimodal treatments (33). In our study, NDs at 6 months occurred in 5.3% of cases, conforming to the result of the previous study. The declining morbidity of NDs was suggested to be associated with the advantages in microsurgeries brought by the prior endovascular embolization on the following aspects: (I) decreasing the intraoperative blood loss and operating time by occluding the blood supply to the nidus, with lowering the SM grade of bAVMs, and thus reducing the risk of hemorrhage, particularly for large or high-flow bAVMs (28,34-36); (II) eliminating the bAVMs in difficult regions which gains more time for neurosurgeons to operate (34); (III) embolizing feeding arteries that are inaccessible in the subsequent resection (e.g., deep perforators) or flow-related aneurysms (6,27,37). The single-staged HO also resulted in better neurological outcomes than the multi-staged. It suggested that HO occupied more potential in the protection of neurological function.
Complete obliteration is the ultimate goal of bAVM treatments. As reported in our study, the obliteration rates were achieved in 96.7% in 3 months and 98.5% in 6 months in total, which were similar to the outcomes reported in the literature of microsurgery without embolization (≈96%) (28). There were differences in patient selection between multimodal treatments and microsurgery. The former approach provided the potential of total resection for complex bAVMs, while the microsurgical resection is appropriate for lesions with SM grade I–III (20). In this study, the average diameter of bAVMs treated with MO/HO is >3 cm. Therefore, the advantage of multimodal treatments is significant for medium-high grade bAVMs. However, the treatment planning is different between HO and MO. Although the embolization facilitates the subsequent resection in the HO modality, the outcomes before microsurgery commonly remain uncertain. Thus, the capacity requirements are increased for the operators. For MO modality, the process of embolization tends to be more aggressive, which may explain the higher rate of hemorrhage after embolization. The treatment strategy of bAVMs requires well-designed plans made by a multidisciplinary team.
There are limitations to our study. First, the sample size of our study was limited, which reduced the efficiency of case-control matching analysis. Second, variables (e.g., coexisting aneurysm or arteriovenous fistula, diffusiveness of bAVMs nidus) could not be entirely enrolled in the case-control matching, which might result in potential bias of baselines. Third, a propensity score matching (PSM) analysis would be a better solution to nonrandomized clinical trials. However, PSM had been attempted and made available cases much fewer. Fourth, patterns of minor and major hemorrhagic complications should be distinguished. Fifth, a longer-term angiographic follow-up was not available, and the recurrent and residual bAVMs could not be detected.
Conclusions
HO for bAVMs resection utilizing pre-operative embolization and microsurgery in a single setting is an effective setup to treat complex bAVMs that need multi-modal management. Compared to MO, the unique workflow advantages in HO reduced the perioperative risk by avoidance of repeat anesthesia, as well as obliteration of interval hemorrhagic risk by eliminating embolization-resection interval. With careful planning and selection of patients, HO may offer reduced surgical risk from less intraoperative blood loss and shorter operative duration compared to MO. The obliteration rate and resulting clinical outcomes are overall similar between patients undergoing HO and MO.
Acknowledgments
We thank Junsheng Li and Long Ma for their assistance in manuscript preparation. The abstract of the study was presented at 2021 American Association of Neurological Surgeons (AANS) Annual Scientific Meeting, August 21, 2021.
Funding: This study was supported by National Key Technologies Research and Development Program of China (2016YFC1301800), Beijing Science and Technology Supporting Plan (D161100003816005), Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150501), Project of China Postdoctoral Science Foundation (2019M660921), and Science Foundation for Postdoctorate Research of Beijing (2017-ZZ-123).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-811/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-811/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-811/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-811/coif). All authors report that this study was supported by National Key Technologies Research and Development Program of China (2016YFC1301800), Beijing Science and Technology Supporting Plan (D161100003816005), Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150501), Project of China Postdoctoral Science Foundation (2019M660921), and Science Foundation for Postdoctorate Research of Beijing (2017-ZZ-123). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted following the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Beijing Tiantan Hospital (No. KY2016-034-02). Written consent was provided by all participants or their legal representatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedlander RM. Arteriovenous malformations of the brain. N Engl J Med 2007;356:2704-12. [Crossref] [PubMed]
- Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med 2018;378:250-61. [Crossref] [PubMed]
- Kim H, Su H, Weinsheimer S, et al. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl 2011;111:83-92. [Crossref] [PubMed]
- Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg 1987;67:17-28. [Crossref] [PubMed]
- Rodríguez-Boto G, Gutiérrez-González R, Gil A, et al. Combined staged therapy of complex arteriovenous malformations: initial experience. Acta Neurol Scand 2013;127:260-7. [Crossref] [PubMed]
- Flores BC, Klinger DR, Rickert KL, et al. Management of intracranial aneurysms associated with arteriovenous malformations. Neurosurg Focus 2014;37:E11. [Crossref] [PubMed]
- Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017;48:e200-24. [Crossref] [PubMed]
- Iwama T, Yoshimura K, Keller E, et al. Emergency craniotomy for intraparenchymal massive hematoma after embolization of supratentorial arteriovenous malformations. Neurosurgery 2003;53:1251-8; discussion 1258-60. [Crossref] [PubMed]
- Vinuela F, Duckwiler G, Guglielmi G. Contribution of interventional neuroradiology in the therapeutic management of brain arteriovenous malformations. J Stroke Cerebrovasc Dis 1997;6:268-71. [Crossref] [PubMed]
- Walcott BP, Gerrard JL, Nogueira RG, et al. Microsurgical retrieval of an endovascular microcatheter trapped during Onyx embolization of a cerebral arteriovenous malformation. J Neurointerv Surg 2011;3:77-9. [Crossref] [PubMed]
- Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke 2005;36:2431-5. [Crossref] [PubMed]
- Reitz M, Schmidt NO, Vukovic Z, et al. How to deal with incompletely treated AVMs: experience of 67 cases and review of the literature. Acta Neurochir Suppl 2011;112:123-9. [Crossref] [PubMed]
- Wang M, Qiu H, Cao Y, et al. One-staged in situ embolization combined with surgical resection for eloquence protection of AVM: technical note. Neurosurg Rev 2019;42:783-90. [Crossref] [PubMed]
- Murayama Y, Arakawa H, Ishibashi T, et al. Combined surgical and endovascular treatment of complex cerebrovascular diseases in the hybrid operating room. J Neurointerv Surg 2013;5:489-93. [Crossref] [PubMed]
- Chen Y, Li R, Ma L, et al. Single-Stage Combined Embolization and Resection for Spetzler-Martin Grade III/IV/V Arteriovenous Malformations: A Single-Center Experience and Literature Review. Front Neurol 2020;11:570198. [Crossref] [PubMed]
- Pal S, Nicholson F, Boet R, et al. Multimodality treatment of intracranial arteriovenous malformations in South Island, New Zealand. J Clin Neurosci 2020;73:74-9. [Crossref] [PubMed]
- Wang M, Jiao Y, Cao Y, et al. Surgical management of complex brain arteriovenous malformations with hybrid operating technique: study protocol of a prospective registry and a pragmatic clinical trial. BMC Neurol 2019;19:75. [Crossref] [PubMed]
- Ogilvy CS, Stieg PE, Awad I, et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation 2001;103:2644-57. [Crossref] [PubMed]
- Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871-80. [Crossref] [PubMed]
- Wang M, Jiao Y, Zeng C, et al. Chinese Cerebrovascular Neurosurgery Society and Chinese Interventional & Hybrid Operation Society, of Chinese Stroke Association Clinical Practice Guidelines for Management of Brain Arteriovenous Malformations in Eloquent Areas. Front Neurol 2021;12:651663. [Crossref] [PubMed]
- Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 2008;50:589-97. [Crossref] [PubMed]
- Potts MB, Zumofen DW, Raz E, et al. Curing arteriovenous malformations using embolization. Neurosurg Focus 2014;37:E19. [Crossref] [PubMed]
- Martin NA, Khanna R, Doberstein C, et al. Therapeutic embolization of arteriovenous malformations: the case for and against. Clin Neurosurg 2000;46:295-318. [PubMed]
- Young WL, Kader A, Ornstein E, et al. Cerebral hyperemia after arteriovenous malformation resection is related to "breakthrough" complications but not to feeding artery pressure. The Columbia University Arteriovenous Malformation Study Project. Neurosurgery 1996;38:1085-93; discussion 1093-5. [Crossref] [PubMed]
- Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg 1993;78:60-9. [Crossref] [PubMed]
- Pasqualin A, Zampieri P, Nicolato A, et al. Surgery after embolization of cerebral arterio-venous malformation: experience of 123 cases. Acta Neurochir Suppl 2014;119:105-11. [Crossref] [PubMed]
- Wang A, Mandigo GK, Feldstein NA, et al. Curative treatment for low-grade arteriovenous malformations. J Neurointerv Surg 2020;12:48-54. [Crossref] [PubMed]
- van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 2011;306:2011-9. [Crossref] [PubMed]
- Luksik AS, Law J, Yang W, et al. Assessing the Role of Preoperative Embolization in the Surgical Management of Cerebral Arteriovenous Malformations. World Neurosurg 2017;104:430-41. [Crossref] [PubMed]
- Lv X, Wu Z, Li Y, et al. Hemorrhage risk after partial endovascular NBCA and ONYX embolization for brain arteriovenous malformation. Neurol Res 2012;34:552-6. [Crossref] [PubMed]
- Zaki Ghali MG, Kan P, Britz GW. Curative Embolization of Arteriovenous Malformations. World Neurosurg 2019;129:467-86. [Crossref] [PubMed]
- Fournier D, TerBrugge KG, Willinsky R, et al. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg 1991;75:228-33. [Crossref] [PubMed]
- Kocer N, Kandemirli SG, Dashti R, et al. Single-stage planning for total cure of grade III-V brain arteriovenous malformations by embolization alone or in combination with microsurgical resection. Neuroradiology 2019;61:195-205. [Crossref] [PubMed]
- Natarajan SK, Ghodke B, Britz GW, et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery 2008;62:1213-25; discussion 1225-6. [PubMed]
- Consoli A, Scarpini G, Rosi A, et al. Endovascular treatment of unruptured and ruptured brain arteriovenous malformations with Onyx18: a monocentric series of 84 patients. J Neurointerv Surg 2014;6:600-6. [Crossref] [PubMed]
- Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 2011;115:78-88. [Crossref] [PubMed]
- Elhammady MS, Gaynor BG, Peterson EC, et al. Microsurgery of Arteriovenous Malformations. In: Winn HR, editor. Youmans & Winn Neurological Surgery. 7th ed. Philadelphia, PA: Elsevier, 2017:3505.


