
A study on correlation between preprocedural CT indexes and procedural success rate of transfemoral transcatheter aortic valve replacement with different self-expanding valves (VitaFlow or VenusA-Valve) in patients with pure native aortic regurgitation
Introduction
Aortic valve disease is a common heart valve disease in the general population, and its incidence increases with age (1). Symptomatic severe aortic valve disease has dismal prognosis and interventional therapies are strongly recommended, including surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) (2-4). With the advantages of safety, effectiveness and minimal invasiveness, TAVR has been increasingly performed in broader aortic valve pathologies. The indications of TAVR for patients with aortic stenosis (AS) have gradually expanded in the lower surgical risk population (2-5), and the number of TAVR cases has exceeded that of surgical aortic valve replacement (SAVR) in the United States of America (USA) and Europe. However, the use of TAVR for cases of aortic regurgitation (AR) is less common. In all TAVR procedures in the USA from 2011 to 2019, AR accounted for less than 1.0% (6). However, it is the most effective treatment for high-risk patients with a contraindication for SAVR.
In both AS and AR patients, we must evaluate whether the anatomy is appropriate for TAVR. Computed tomography (CT) has high spatial resolution, providing key information concerning the anatomy for TAVR. Therefore, CT is the preferred imaging tool to assess aortic valve morphology, annular size and shape, extent and distribution of valve and vascular calcification, risk of coronary ostial obstruction, aortic root dimensions, optimal fluoroscopic projections for valve deployment, and feasibility of vascular access. Adverse anatomical findings may suggest higher risk and lower success rate of TAVR (4).
There are few TAVR valves specially designed to treat AR. Previous studies have reported on the “off-label” use of transfemoral self-expanding transcatheter aortic valves (SE-TAVs) to treat patients with pure native AR (PNAR), but the success rate of the procedure was relatively low due to the difficulty in anchoring the SE-TAV (7-9). Therefore, conducting a preprocedural CT evaluation to strictly select patients with an appropriate anatomy is important to improve the procedural success. In mainland China, lacking of “on-label” transfemoral SE-TAVs for AR, the early-generation SE-TAVs are mainly used. There are 2 kinds of SE-TAVs with different shaped frameworks, including the VenusA-Valve (A-shaped) and the VitaFlow valve (non-A-shaped). There was no head-to-head study to compare their performance in TAVR for AS or AR. The aim of this study was to evaluate the relationship between preprocedural CT indexes and the procedural success rate of TAVR to identify which anatomical characteristics are associated with high procedural success. In addition, this study compared the procedural success rates of 2 kinds of SE-TAVs with different shaped frameworks to determine which is more suitable for PNAR. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2588/rc).
Methods
Study design and subjects
A total of 77 patients with symptomatic severe PNAR from Zhongshan Hospital, Fudan University were enrolled in this retrospective comparative study. All enrolled patients had comorbid conditions that would preclude SAVR and underwent transfemoral TAVR using early generation SE-TAVs from October 2018 to December 2020.
The inclusion criteria were as follows: (I) patients had severe PNAR diagnosed by thoracic echocardiography according to the management guidelines of valvular diseases (3,4,10); and (II) patients underwent CT examination prior to TAVR. The exclusion criteria were as follows: (I) patients with failed bioprosthetic surgical heart valves; and (II) patients had a peak aortic valve pressure gradient greater than 20 mmHg measured by echocardiography before TAVR.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University prior to study onset (No. B2020-039). Informed consent was waived for this retrospective study.
Data collection and definitions
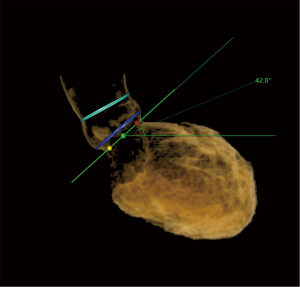
Preprocedural CT scans were quantitatively evaluated using Anythink Structural Heart & Vascular software (Beijing Crealife Technology Co., Ltd., Beijing, China). The circumferences of the aortic annulus (AA), left ventricular outflow tract (LVOT), sinotubular junction (STJ), and ascending aorta (AAO) were measured, as well as the angle of the aortic root, which is the angle between the planar cross section of the AA and the body cross section (Figures 1,2). The average diameter of the plane was defined by dividing the perimeter by 3.14, and the difference between the average diameter of the AAO and the STJ was calculated. Thickening of the leaflets was observed through cross-section (Figure 3).
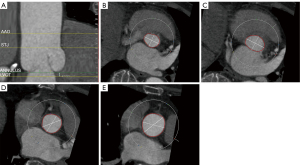
Baseline clinical, laboratory, CT, echocardiographic, and procedural data were collected immediately after TAVR and during hospitalization. Additionally, information regarding TAVR approach, SE-TAV, procedural success rate [successful TAVR assessing by the Valve Academic Research Consortium (VARC-2) standard (11,12)], new pacemaker implant, aortic valve regurgitation after procedure, and new complete left bundle branch block (CLBBB) was recorded. According to the information provided by SE-TAV manufacturers, the diameter of a SE-TAV is defined as the maximum diameter of the framework below the leaflet of the prosthetic valve. For example, the maximum diameter of the framework below the leaflet of the #32 VenusA-Valve is 32 mm, and the maximum diameter of the framework below the leaflet of the #30 VitaFlow is also 32 mm.
Statistical analysis
Statistical analysis was conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation (SD) and were compared using the Student’s t-test or the Mann-Whitney U test. Categorical variables are presented as counts or percentages and were compared using the chi-square or Fisher’s exact test. All tests were 2 sided, and P values <0.05 were considered to indicate statistical significance.
Results
A total of 77 patients with symptomatic PNAR were treated with TAVR, including 47 patients who received a VenusA-Valve and 30 patients who received a Vita-Flow valve. The mean age was 78 years, and 48% of the patients were men. The mean Society of Thoracic Surgeons (STS) risk score was 7.8% in the VenusA-Valve group and 7.6% in the Vita-Flow group. There were no significant differences in baseline characteristics between the two groups. The data of baseline characteristics can be found in Table 1.
Table 1
| Baseline characteristics | Overall (n=77) | VenusA-Valve (n=47) | Vita-Flow (n=30) | P value |
|---|---|---|---|---|
| Age, years | 78.1±12.5 | 78.2±13.1 | 78.0±11.6 | 0.82 |
| Male | 37 (48%) | 23 (49%) | 14 (47%) | 0.73 |
| STS score | 7.7±5.9 | 7.8±5.6 | 7.6±6.3 | 0.32 |
| NYHA functional class III or IV | 52 (68%) | 31 (66%) | 21 (70%) | 0.29 |
| Hypertension | 59 (77%) | 37 (79%) | 22 (73%) | 0.35 |
| Diabetes mellitus | 11 (14%) | 8 (17%) | 3 (10%) | 0.09 |
| Chronic pulmonary disease | 22 (29%) | 15 (32%) | 7 (23%) | 0.12 |
| Peripheral vascular disease | 14 (18%) | 9 (19%) | 5 (17%) | 0.33 |
| Coronary artery disease | 29 (38%) | 19 (40%) | 10 (33%) | 0.36 |
| Prior myocardial infarction | 17 (22%) | 11 (23%) | 6 (20%) | 0.45 |
| CKD (eGFR <30 mL/min) | 10 (13%) | 7 (15%) | 3 (10%) | 0.71 |
| Previous thoracic surgery | 17 (22%) | 10 (21%) | 7 (23%) | 0.13 |
| Echocardiographic findings | ||||
| LVEF, % | 43.9±13.9 | 43.5±14.2 | 44.6±13.5 | 0.31 |
| LVEDD, mm | 59.2±10.2 | 59.8±10.1 | 58.2±10.3 | 0.75 |
| Peak aortic valve gradient <20 mmHg | 77 (100%) | 47 (100%) | 30 (100%) | – |
| Mitral regurgitation, moderate or severe | 26 (34%) | 17 (36%) | 9 (30%) | 0.42 |
Values are mean ± SD or n (%). SD, standard deviation; STS, Society of Thoracic Surgeons; NYHA, New York Heart Association; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter.
The circumferences of the AA, LVOT, and STJ in the failed procedure group were significantly larger than those in the successful procedure group (85.45±6.39 vs. 79.81±8.68, 93.60±12.49 vs. 82.95±11.42, 116.15±21.27 vs. 105.99±16.27, respectively; P<0.05). The rate of leaflet thickening in the failed procedure group was lower than that in the successful procedure group (20.0% vs. 58.1%; P<0.05). However, there were no significant differences in AAO circumference or AAO and STJ average diameter between the failed and successful procedure groups (Table 2).
Table 2
| CT data | Procedural success (n=62) | Procedural failure (n=15) | P value |
|---|---|---|---|
| Annulus circumference (mm) | 79.81±8.68 | 85.45±6.39 | 0.02 |
| LVOT circumference (mm) | 82.95±11.42 | 93.60±12.49 | 0.002 |
| AAO circumference (mm) | 124.97±16.54 | 135.49±30.64 | 0.22 |
| STJ circumference (mm) | 105.99±16.27 | 116.15±21.27 | 0.045 |
| Difference between average diameter of AAO and STJ (mm) | 6.00±3.06 | 6.16±5.03 | 0.88 |
| Leaflet thickening (yes) | 36 (58.1%) | 3 (20.0%) | 0.02 |
| Angle (°) | 53.77±10.71 | 57.87±12.07 | 0.20 |
Values are mean ± SD or n (%). SD, standard deviation; LVOT, left ventricular outflow tract; AAO, ascending aorta; STJ, sinotubular junction.
All patients were treated with early generation SE-TAVs, including a VitaFlow valve (30, 39.0%) and a VenusA-Valve (47, 61.0%). According to the preprocedural CT examination, the rate of leaflet thickening in the Vita-Flow group was significantly higher than that in the VenusA-Valve group (80.0% vs. 31.9%; P<0.01). There was no significant difference in the other CT quantitative indexes between the 2 groups, including the circumference of the AA, LVOT, STJ, and AAO, the difference between the average diameter of the AA and STJ, and the angle of the aortic root (Table 3). Based on the VARC-2 criteria, 62 cases (80.5%) were successful in total, 28 in the Vita-Flow group and 34 in the VenusA-Valve group. The procedural success rate of the Vita-Flow group was significantly higher than that of the VenusA-Valve group (93.3% vs. 72.3%; P<0.05), as the Vita-Flow group was associated with a lower incidence of second valve implantation (6.7% vs. 27.7%; P<0.05). There was no significant difference in new permanent pacemaker implantation, new CLBBB, and moderate or severe AR between the 2 groups (Table 4).
Table 3
| CT data | Overall (n=77) | VenusA-Valve (n=47) | VitaFlow (n=30) | P value |
|---|---|---|---|---|
| Annulus circumference (mm) | 80.91±8.55 | 81.66±8.92 | 79.74±7.95 | 0.34 |
| LVOT circumference (mm) | 85.03±12.30 | 85.90±11.82 | 83.66±13.11 | 0.44 |
| AAO circumference (mm) | 127.02±20.30 | 128.20±20.94 | 125.22±19.48 | 0.54 |
| STJ circumference (mm) | 107.97±17.67 | 109.70±18.11 | 105.26±16.90 | 0.28 |
| Difference between average diameter of AAO and STJ (mm) | 18.95±10.97 | 18.28±12.12 | 19.96±9.03 | 0.52 |
| Leaflet thickening (yes) | 39 (50.6%) | 15 (31.9%) | 24 (80.0%) | <0.01 |
| Angle | 54.57±11.02 | 54.64±11.03 | 54.47±11.21 | 0.95 |
Values are mean ± SD or n (%). SD, standard deviation; LVOT, left ventricular outflow tract; AAO, ascending aorta; STJ, sinotubular junction.
Table 4
| Procedural characteristics and outcomes | Overall (n=77) | VenusA-Valve (n=47) | VitaFlow (n=30) | P value |
|---|---|---|---|---|
| General anesthesia | 47 (100%) | 30 (100%) | – | |
| Access route | ||||
| Transfemoral | 77 (100%) | 47 (100%) | 30 (100%) | – |
| Procedure-related death | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Conversion to conventional surgery | 1 (1.3%) | 1 (2.1%) | 0 (0%) | 1 |
| Coronary obstruction | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Aortic root injury | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Successful access | 77 (100%) | 47 (100%) | 30 (100%) | – |
| Procedural success | 62 (80.5%) | 34 (72.3%) | 28 (93.3%) | 0.048 |
| Need for second valve implantation | 15 (19.5%) | 13 (27.7%) | 2 (6.7%) | 0.048 |
| Moderate or severe AR | 2 (2.6%) | 2 (4.3%) | 0 (0.0%) | 0.682 |
| New pacemaker implantation | 9 (11.7%) | 6 (12.8%) | 3 (10.0%) | 0.996 |
| New CLBBB | 30 (39.0%) | 19 (40.4%) | 11 (36.7%) | 0.742 |
Values are mean ± SD or n (%). SD, standard deviation; AR, aortic valve regurgitation; CLBBB, completed left bundle branch block.
All TAVR procedures were performed via the femoral artery. In the study population, procedure-related death, conversion to conventional surgery, coronary obstruction, aortic root injury, and successful access was observed in 0 (0%), 1 (1.3%), 0 (0%), 0 (0%), and 77 (100%) patients, respectively (Table 4).
Discussion
In this retrospective comparative study, we investigated CT evaluation of aortic root anatomy before transfemoral TAVR with SE-TAV in patients with PNAR. The major findings of the study were as follows: (I) in the cohort, the overall procedural success rate of TAVR using early generation SE-TAVs for PNAR was 80.5%; (II) aortic roots with smaller AAs, LVOTs, and STJs and leaflet thickening were associated with improved procedural success rates; (III) non-A-shaped SE-TAVs, such as the Vita-Flow valve, might be the better choice when treating patients with PNAR, with lower rates of second valve implantation; (IV) leaflet thickening played an important role in procedural success.
AR is the most common aortic valve lesion requiring surgery or transcatheter intervention (13), and its prevalence is rising rapidly as a consequence of the ageing population (14). Degenerative tricuspid and bicuspid AR are the main etiologies for AR in high-income countries, while rheumatic AR is the most common cause globally (1,13). Guidelines (2-4,10) for severe AR recommend surgery in symptomatic patients regardless of left ventricle (LV) function (Class I). Surgery is also recommended in asymptomatic patients with left ventricular end systolic dimension (LVESD) >50 mm or LVESD >25 mm/m2 body surface area (BSA; in patients with a small body size) or resting left ventricular ejection fraction (LVEF) ≤50% with the surgery recommendation in these cases upgraded from Class IIa to Class I. In addition, surgery may be considered in asymptomatic patients with LVESD >20 mm/m2 BSA (especially in patients with a small body size) or resting LVEF ≤55%, if the surgery is low risk (Class IIb). Therefore, the guidelines now recommend surgery in a wider range of early stage cases.
It is well known that SAVR is the best therapy for patients with PNAR. However, data from the Euro Heart Survey on Valvular Heart Disease showed that only 20% of patients with severe AR and a LVEF between 30% and 50% underwent SAVR, and less than 5% of patients with a LVEF less than 30% underwent SAVR (15). In the aging population, more patients with PNAR have multiple comorbidities, which results in a high STS risk score for the operation and conservative treatment. In our study, the patients with severe PNAR had an average STS of 7.7±5.9 and comorbid conditions that would preclude SAVR. However, when left untreated, these patients face an annual mortality risk of 20% (4,15). Therefore, TAVR is an effective therapeutic option for patients with severe AR (16).
Historically, TAVR has been contraindicated for PNAR due to lack of a calcified anchoring zone for valve deployment and the subsequent increased risk of valve migration and paravalvular regurgitation. Most transcatheter devices in the current market are designed for treating calcified AS with an anchoring zoon to hold them in place. However, as a result of technological developments, TAVR is now used in experienced centers for selected patients with AR who are ineligible for SAVR (4). For TAVR valves via the femoral artery, the procedural success rate in patients with PNAR is only around 70%, and these valves can only be used in strictly selected patients with an appropriate anatomy (7,17-20). In China, the early generation of self-expanding valves are currently used in most TAVR procedures. Thus, in our study, we observed the performance of early generation SE-TAVs in the TAVR procedure for treating PNAR. The overall procedural success rate was 80.5%, which was better than that reported in previous research data. This improved success rate may be related to accumulated procedure experience and the strict selection of patients with an appropriate anatomy. In 15 patients, the position of the prosthetic valve was too deep, which resulted in more than moderate perivalvular leakage. After valve-in-valve implantation, perivalvular leakage decreased to less than mild in 13 patients. But 1 patient still had moderate perivalvular leakage. Another 1 patient had severe perivalvular leakage, and intraoperative transesophageal echocardiography indicated that the opening of the anterior mitral valve was affected by the prosthetic valve. This patient was transferred to surgery.
In our study, the improved procedural success rate of early generation SE-TAVs relied on evaluation of the anatomical characteristics of the aortic root by preprocedural CT imaging. Our analysis of preprocedural CT imaging suggested that PNAR might rely on AA, LVOT, and STJ size and leaflet thickening to provide the anchoring force of the prosthetic valve. The patients in the successful procedure group had smaller AAs, LVOTs, and STJs than the patients in the failed group. In patients with PNAR, a smaller AA and LVOT is beneficial as it provides the anchoring force for the SE-TAV. In addition, a smaller STJ circumference may provide an anchoring for the “crown” design of the prosthetic valve and avoid it slipping down. Moreover, the rate of leaflet thickening in the successful procedure group was significantly higher than that in the failed procedure group. This suggests that the procedure is more likely succeed with leaflet thickening, perhaps due to the much greater friction provided by thickening leaflets tightly wrapping the prosthetic valve framework.
In our study, we found that the procedural success rate in the Vita-Flow group was significantly higher than that in the VenusA-Valve group. This may be due to the different shaped frameworks of the 2 kinds of SE-TAVs. The VenusA-Valve has an A-shaped framework, while the VitaFlow valve has a non-A-shaped framework. The non-A-shaped framework may put more pressure on the surrounding tissue and cause more friction between the thickening native valve and the prosthetic valve frame. This suggests that SE-TAV with non-A-shaped framework may be more suitable for the treatment of PNAR.
Recently, the new generation valves (such as J-Valve and JenaValve) indicated for AR have demonstrated satisfying TAVR clinical outcomes in patients with PNAR. The success rate of the procedure has increased to over 90% (21-24). However, recent studies have shown no significant difference in mortality between patients treated with an “off-label” valve and those treated with an “on-label” valve (7,17). A reason for this may be that the “on-label” valve replacements are all performed via transapical access, which may reduce the clinical benefits. Previous knowledge of treating AS has suggested that the clinical benefits of transfemoral TAVR are better than those of transapical TAVR. Thus, “on-label” valves are now developing transfemoral versions. The transfemoral J-Valve has obtained acceptable early first-in-human results, and the transfemoral JenaValve has achieved CE marking and joined FDA’s Breakthrough Device Program (25,26). Our study results suggested that transfemoral TAVR using an “off-label” valve with a non-A-shaped framework could achieve a similar procedural success rate in well-selected patients.
Our study had several limitations. Firstly, device selection was not randomized but was at the operator’s discretion and experience, which may have affected the results. Secondly, evaluating the procedural success of a specific treatment using a retrospective study can lead to weaker conclusions than using a randomized trial due to confounding factors and the small sample size. Lastly, this was a single-center study.
Conclusions
Patients with severe PNAR with smaller AAs, LVOTs, and STJs and leaflet thickening might have a higher success rate in transfemoral TAVR using a self-expanding valve. The SE-TAV with a non-A-shaped framework might be a better choice for improved procedural outcomes.
Acknowledgments
The authors wish to thank the Zhongshan Hospital, Fudan University for providing the cases.
Funding: This study was supported by the National Key R&D Program of China (No. 2020YFC 2008100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2588/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2588/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2588/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University prior to study onset (No. B2020-039). Informed consent was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Writing Committee Members. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25-e197. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021;60:727-800. [Crossref] [PubMed]
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706-15. [Crossref] [PubMed]
- Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:2492-516. [Crossref] [PubMed]
- Rawasia WF, Khan MS, Usman MS, et al. Safety and efficacy of transcatheter aortic valve replacement for native aortic valve regurgitation: A systematic review and meta-analysis. Catheter Cardiovasc Interv 2019;93:345-53. [Crossref] [PubMed]
- Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc Interv 2017;10:1048-56. [Crossref] [PubMed]
- Jiang J, Liu X, He Y, et al. Transcatheter Aortic Valve Replacement for Pure Native Aortic Valve Regurgitation: A Systematic Review. Cardiology 2018;141:132-40. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- De Backer O, Pilgrim T, Simonato M, et al. Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am J Cardiol 2018;122:1028-35. [Crossref] [PubMed]
- Iung B, Delgado V, Rosenhek R, et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019;140:1156-69. [Crossref] [PubMed]
- Yadgir S, Johnson CO, Aboyans V, et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990-2017. Circulation 2020;141:1670-80. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Stachon P, Kaier K, Heidt T, et al. Nationwide outcomes of aortic valve replacement for pure aortic regurgitation in Germany 2008-2015. Catheter Cardiovasc Interv 2020;95:810-6. [Crossref] [PubMed]
- Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol 2017;70:2752-63. [Crossref] [PubMed]
- Huded CP, Allen KB, Chhatriwalla AK. Counterpoint: challenges and limitations of transcatheter aortic valve implantation for aortic regurgitation. Heart 2021;107:1942-5. [Crossref] [PubMed]
- Haddad A, Arwani R, Altayar O, et al. Transcatheter aortic valve replacement in patients with pure native aortic valve regurgitation: A systematic review and meta-analysis. Clin Cardiol 2019;42:159-66. [Crossref] [PubMed]
- Takagi H, Hari Y, Kawai N, et al. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ 2020;29:729-41. [Crossref] [PubMed]
- Wernly B, Eder S, Navarese EP, et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation: "on-label" versus "off-label" use of TAVR devices. Clin Res Cardiol 2019;108:921-30. [Crossref] [PubMed]
- Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7:1168-74. [Crossref] [PubMed]
- Shi J, Wei L, Chen Y, et al. Transcatheter Aortic Valve Implantation With J-Valve: 2-Year Outcomes From a Multicenter Study. Ann Thorac Surg 2021;111:1530-6. [Crossref] [PubMed]
- Urena M, Himbert D, Ohlmann P, et al. Transcatheter Aortic Valve Replacement to Treat Pure Aortic Regurgitation on Noncalcified Native Valves. J Am Coll Cardiol 2016;68:1705-6. [Crossref] [PubMed]
- Hensey M, Murdoch DJ, Sathananthan J, et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: the J-Valve transfemoral system. EuroIntervention 2019;14:e1553-5. [Crossref] [PubMed]
- Ng VG, Khalique OK, Nazif T, et al. Treatment of Acute Aortic Insufficiency With a Dedicated Device. JACC Case Rep 2021;3:645-9. [Crossref] [PubMed]



