
Clinical efficacy of endoscopic antireflux mucosectomy vs. Stretta radiofrequency in the treatment of gastroesophageal reflux disease: a retrospective, single-center cohort study
Introduction
Gastroesophageal reflux disease (GERD) refers to a series of symptoms and/or complications caused by the reflux of gastric contents, which seriously affects patients’ quality of life (1-4). It has been reported that the incidence rate in Western countries is 10–20% and has risen to 10.5% in recent years in Asia (2,5). Clinically, the main treatment of GERD is oral proton pump inhibitors (PPIs). Most patients’ symptoms are relieved, but 10–40% of GERD patients have a poor response to PPIs (6-8). With the advancement of endoscopic technology, endoscopic antireflux therapies continue to emerge, supplementing or replacing traditional drugs and surgical treatment as the main treatment for refractory GERD (9). Currently, antireflux mucosectomy (ARMS) and Stretta radiofrequency (SRF) are the most commonly used endoscopic antireflux therapies. The mechanisms of action of ARMS and SRF differ, and the clinical efficacies reported also vary. However, to date, no comparison study has been reported on the clinical efficacies of ARMS and SRF. In this study, the efficacies of ARMS and SRF were compared in GERD patients for whom PPIs were ineffective or not tolerated, with the goal of providing more appropriate treatment for these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2071/rc).
Methods
Subjects
This was a retrospective, single-center cohort study conducted in the Department of Gastroenterology of the Strategic Support Force Medical Center (Beijing, China) between January 2020 and May 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Strategic Support Force Medical Center (Beijing, China) (No. K201906). Informed consent was taken from all the patients.
Inclusion criteria
The inclusion criteria were: (I) GERD diagnosed by clinical symptoms, 24-hour esophageal pH monitoring (DeMeester score >14.72), and gastroscopy (1); (II) PPI-dependent or refractory GERD; (III) gastroesophageal flap valve (GEFV) Hill grade (10) II or III, or grade IV with hiatal hernia <2 cm; and (IV) age between 18 and 80 years.
Exclusion criteria
The exclusion criteria were: (I) pregnancy; (II) achalasia or other primary esophageal motility disorders; (III) severe heart, liver, or kidney dysfunction; (IV) previous esophagogastric surgery; and (V) coagulation disorders.
Treatment and procedure
All procedures were performed by 1 skilled endoscopist. Fasting and water deprivation were performed for 8 hours before the procedure. The procedure was performed under general anesthesia with endotracheal intubation and vital monitoring. Patients were placed in the left lateral decubitus position.
ARMS (11,12): (I) submucosal injection: submucosal injection (glycerol fructose + methylene blue mixture) was performed along the outside of the marking points until the mucosa was fully raised. (II) Endoscopic mucosal resection (EMR): the transparent cap was mounted onto the distal tip of the endoscope, and the snare was inserted into the cap through the sheath. The high-frequency electric snare was used to remove 2/3–3/4 of the mucosa around the cardia and the lesser curvature of the stomach, while 1/4–1/3 of the mucosa of the greater curvature was retained. The resection length was 3 cm (1 cm in the esophagus and 2 cm in the cardia), and the incision was crescent shaped (Figure 1). During resection, heat biopsy forceps were used for hemostasis.
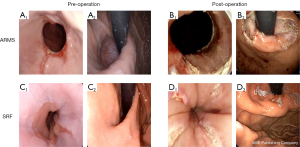
SRF (13): first, upper gastrointestinal endoscopy was performed to confirm the location and depth of the squamo-columnar junction (SCJ). The endoscope was then removed, and the Stretta catheter was positioned 1 cm above the SCJ according to the distance previously determined. After appropriate inflation of the balloon, 4 needle electrodes were deployed, each delivering RF energy for 60 seconds. The needles were then withdrawn, the balloon was deflated, and the catheter was rotated 45 degrees. The treatment site covered an area 1 cm above the SCJ and 0.5 cm below the SCJ, with a total of 4 planes spaced 0.5 cm apart. After we advanced the catheter into the fundus of the stomach and inflated the balloon to 25 and 22 mL, we delivered the first treatment cycle at this level. At the completion of the first treatment cycle, we retracted the needles, advanced the catheter into the stomach, rotated it 30 degrees to the right, extended the needles, then repeated the cycle again 30 degrees to the left. After completion of the procedure and catheter removal, the diagnostic endoscopy procedure was repeated to verify that there were no complications (Figure 1).
Postoperative treatment
The patients fasted for 48 hours after the procedure, after which their diet was restored gradually. Intravenous PPI was given for 48 hours, and the patients were observed for bleeding, perforation, infection, and other complications. The patient continued the previous antisecretory regimen for 2 months after the procedure.
Follow-up
The gastroesophageal reflux disease questionnaire (GERDQ) (14) score and gastroesophageal reflux disease health-related quality of life (GERD-HRQL) (15) score before the operation and the GERDQ score, GERD-HRQL score, PPI withdrawal and PPI reduction of the patients 6 months after the operation were collected.
Statistical analysis
SPSS 21.0 statistical software was used to process the data. The measurement data with a normal distribution are expressed as mean ± standard deviation (SD). The mean of the two groups was compared using Student’s t-test. Measurement data with a nonnormal distribution are expressed as M (P25, P75). The Wilcoxon rank sum test was used to compare the two groups. Count data are expressed as the number of cases and percentages, and they were compared between groups by the χ2 test or Fisher’s exact test. Differences were considered significant when P<0.05.
Results
Patient characteristics
The data of 69 GERD patients who received ARMS or SRF treatment were collected, including 40 male and 29 female patients with ages ranging from 19 to 78 (55.16±11.256). Thirty-nine and 30 patients received ARMS and SRF treatment, respectively. There was no significant difference in sex, age, body mass index (BMI), course of GERD, or GEFV grade between the two groups (P>0.05) (Table 1).
Table 1
| Subgroup | ARMS group | SRF group | Statistic | P value |
|---|---|---|---|---|
| N | 39 | 30 | ||
| Sex, M/F | 22/17 | 18/12 | χ2=0.090 | 0.765 |
| Age (years), mean ± SD | 57.21±12.881 | 52.04±9.502 | t=1.640 | 0.106 |
| BMI (kg/m2), mean ± SD | 23.59±1.73 | 23.78±1.31 | t=−0.515 | 0.608 |
| Course of GERD (years), M (P25, P75) | 6.0 (3.7, 7.2) | 4.6 (3.0, 6.1) | Z=−1.600 | 0.110 |
| GEFV grade, II/III/IV | 12/15/12 | 8/11/11 | χ2=0.750 | 0.386 |
Differences were considered significant when P<0.05. ARMS, antireflux mucosectomy; SRF, Stretta radiofrequency; M, male; F, female; SD, standard deviation; BMI, body mass index; GERD, gastroesophageal reflux disease; GEFV, gastroesophageal flap valve.
Clinical efficacy
At the 6-month follow-up, both therapies were effective as evaluated by the GERDQ score and GERD-HRQL score. The GERDQ scores dropped from 10.46±4.148 to 5.05±3.699 in the ARMS group and from 9.73±4.510 to 5.57±2.431 in the SRF group (P=0.000). The GERD-HRQL scores dropped from 23.49±6.871 to 13.05±5.858 in the ARMS group and from 21.27±6.275 to 13.17±6.165 in the SRF group (P=0.000). There was no significant difference in the GERDQ score (5.05±3.699 vs. 5.57±2.431, P=0.489) or GERD-HRQL score (13.05±5.858 vs. 13.17±6.165, P=0.937) between the ARMS group and SRF group at the 6-month follow-up (Table 2).
Table 2
| Subgroup | ARMS group | SRF group | Statistic | P value |
|---|---|---|---|---|
| N | 39 | 30 | ||
| GERDQ score, mean ± SD | ||||
| Before | 10.46±4.148 | 9.73±4.510 | t=0.688 | 0.494 |
| After | 5.05±3.699 | 5.57±2.431 | t=−0.696 | 0.489 |
| Statistic | t=5.989 | t=4.458 | ||
| P value | 0.000 | 0.000 | ||
| GERD-HRQL score, mean ± SD | ||||
| Before | 23.49±6.871 | 21.27±6.275 | t=1.398 | 0.167 |
| After | 13.05±5.858 | 13.17±6.165 | t=−0.079 | 0.937 |
| Statistic | t=9.605 | t=6.861 | ||
| P value | 0.000 | 0.000 |
Differences were considered significant when P<0.05. GERDQ, gastroesophageal reflux disease questionnaire; GERD-HRQL, gastroesophageal reflux disease health-related quality of life; ARMS, antireflux mucosectomy; SRF, Stretta radiofrequency; SD, standard deviation.
At the 6-month follow-up, in the ARMS group, 28 patients had stopped using PPIs, 7 had decreased their PPI dosage, and 4 had no improvement. In the SRF group, 19 had stopped PPI use, 6 had decreased their PPI dosage, and 5 had no improvement. No difference was found between the ARMS group and SRF group in the symptom improvement rate [35/39 (89.7%) vs. 25/30 (83.3%), P=0.836], PPI withdrawal rate [28/39 (71.8%) vs. 19/30 (63.3%), P=0.455], or PPI reduction rate [7/39 (17.9%) vs. 6/30 (20.0%), P=0.829]. In the ARMS group, 7/12 GEFV grade II patients had stopped using PPIs, 12/15 GEFV grade III patients had stopped PPI use, and 9/12 GEFV grade IV patients had stopped PPI use. In the SRF group, 7/8 GEFV grade II patients, 9/11 GEFV grade III patients, and 3/11 GEFV grade IV patients had stopped using PPIs. There was no significant difference in PPI withdrawal rate between treatments among the GEFV grade II [7/12 (58.3%) vs. 7/8 (87.5%), P=0.163] or GEFV grade III patients [12/15 (80.0%) vs. 9/11 (81.8%), P=0.907]. The PPI withdrawal rate of the GEFV grade IV patients in the ARMS group was significantly higher than that in the SRF group [9/12 (75.0%) vs. 3/11 (27.3%), P=0.022] (Table 3).
Table 3
| Subgroup | ARMS group | SRF group | Statistic | P value |
|---|---|---|---|---|
| N | 39 | 30 | ||
| Symptom improvement rate, n (%) | 35 (89.7) | 25 (83.3) | χ2=0.043 | 0.836 |
| PPI withdrawal rate, n (%) | 28 (71.8) | 19 (63.3) | χ2=0.559 | 0.455 |
| GEFV II | 7 (58.3) | 7 (87.5) | χ2=1.944 | 0.163 |
| GEFV III | 12 (80.0) | 9 (81.8) | χ2=0.014 | 0.907 |
| GEFV IV | 9 (75.0) | 3 (27.3) | χ2=5.239 | 0.022 |
| PPI reduction rate, n (%) | 7 (17.9) | 6 (20.0) | χ2=0.047 | 0.829 |
Differences were considered significant when P<0.05. PPI, proton pump inhibitor; ARMS, antireflux mucosectomy; SRF, Stretta radiofrequency; GEFV, gastroesophageal flap valve.
Adverse events
The ARMS and Stretta operations were successful in all patients, with no severe adverse events occurring during the procedures. One patient in the ARMS group experienced dysphagia 1 month after the operation, and the symptoms were relieved after endoscopic balloon dilatation. No adverse events occurred in the SRF group in the 6-month follow-up period.
Discussion
ARMS is an endoscopic therapy for the treatment of GERD recently developed on the basis of mature endoscopic technologies such as endoscopic submucosal dissection (ESD), EMR, and ligation (16-19). The SRF procedure involves inserting a radiofrequency catheter into the esophagus, piercing the lower esophageal sphincter and cardiac muscle layer with a radiofrequency therapeutic instrument electrode, and burning the gastroesophageal junction at multiple points on multiple surfaces to increase the pressure at the lower end of the esophagus and reduce tissue compliance to achieve an antireflux effect (20-22). Compared with endoscopic injection therapy, transoral incisionless fundoplication (TIF), and medial ultrasonic surgical endostapler (MUSE) treatment, ARMS and SRF are simple, do not leave any foreign objects in the body, and complications are less likely (6,12,23). The procedures increase cardiac contraction and lower esophageal sphincter pressure in different ways, thus reducing the occurrence of reflux events. At present, a number of studies have confirmed the effectiveness of ARMS and SRF in the treatment of GERD. Inoue et al. (16) performed ARMS on 10 patients with GERD, including 8 patients with crescent mucosal resection and 2 patients with circumferential resection. The heartburn score decreased from 2.7 points before operation to 0.3 points after operation, and the reflux score decreased from 2.5 points before operation to 0.3 points after operation. Yoo et al. (11) treated 33 patients with GERD by endoscopic ARMS. The results showed that after 6 months, 63% of patients stopped using PPIs, 30% of patients reduced the dose of PPIs, the score of the GERDQ decreased markedly from 11.0 to 6.0, and 2 patients received balloon dilatation without other serious adverse reactions. Dughera et al. (24) followed up 69 GERD patients who underwent SRF therapy for 2 years. A total of 56 patients completed the follow-up, of which the heartburn score, GERD score, and quality of life were significantly improved 24 and 48 months after operation compared with before treatment. Forty-eight months after treatment, 41 patients completely stopped using PPIs. Kalapala et al. (25) divided patients into SRF and PPI treatment groups, and the results showed that the scores of acid reflux, heartburn, chest pain, and cough in the SRF treatment group were significantly improved after 3 months of treatment, and the degree of improvement in symptom scores was significantly better than that in the PPI treatment group. Sixty percent of the patients stopped using PPIs after SRF treatment, and 80% of the patients who received SRF treatment were satisfied with the treatment, while only 30% of the patients in the PPI group were satisfied with the treatment. The difference was statistically significant. Our study reached the same conclusions. The GERDQ score and GERD-HRQL score of patients after ARMS and SRF treatments were significantly lower than those before operation, so both treatments were effective.
No studies have compared ARMS and SRF specifically. The artificial ulcer formed after the ARMS procedure is large, and the healing time is long. It has been reported that the complete healing rate of artificial ulcers 8 weeks after ESD was less than 90%, scar tissue induced by mucosal repair had not completely formed during follow-up 2–3 months after ESD, and the cardiac tightening effect was not complete, resulting in an unsatisfactory antireflux effect (26). Therefore, in our study, we followed up patients for 6 months after ARMS. We found that there was no significant difference in the symptom improvement rate, GERDQ score, GERD-HRQL score, PPI withdrawal rate, or PPI reduction rate between the ARMS and SRF groups, suggesting that the curative effects of the 2 procedures were similar 6 months after operation. In this study, there were 4 patients in the ARMS group and 5 patients in the SRF group whose acid reflux and heartburn symptoms were not significantly improved, and thus they continued treatment with the original PPI dose. We considered these failures to be related to the patients’ obesity, poor lifestyle habits, gastric emptying dysfunction, or psychological factors.
In this study, we also found that there was no significant difference in the PPI withdrawal rate between the 2 procedures among GEFV grade II and GEFV grade III patients. However, for GEFV grade IV patients, 9 of the 12 patients in the ARMS group stopped using PPIs, while only 3 of the 11 patients in the SRF group stopped PPI use. That is, ARMS was significantly more effective than SRF for GEFV grade IV patients. There were 2 possible reasons for this. First, in GEFV grade IV patients, the hiatal hernia increases markedly, and the cardiac relaxation is obvious. SRF treatment mainly treats these conditions through a thermal effect. Heat energy is released at multiple points by radiofrequency treatment needles, resulting in local esophageal mucosal edema, muscle tissue destruction and regeneration, and collagen tissue contraction and reconstruction to increase the thickness and pressure of the lower esophageal sphincter and enhance the antireflux barrier (20-22). The advantage of this approach is that the radiofrequency energy acts evenly on the treatment area, which is suitable for GEFV grade II and III patients, but its disadvantage is that the effect is gentle and it rarely completely corrects the severely relaxed lower esophageal sphincter of GEFV grade IV. ARMS, by contrast, can strongly contract the severely relaxed lower esophageal sphincter by removing the mucosa on the gastroesophageal junction and via scar formation. Second, the key point of ARMS in the treatment of GERD is to produce benign stenosis through the formation of scar tissue so that it can form an antireflux barrier after operation, while at the same time not causing severe esophageal stenosis that interferes with eating. In our procedures, we selected 2/3 circumferential resections for GEFV grade II and III patients and 3/4 for GEFV grade IV patients. Sixty-eight of 69 patients did not have postoperative eating obstruction. One GEFV grade II patient who had received 2/3 circumferential mucosa resection developed esophageal stenosis 1 month after operation and recovered after endoscopic dilation. That patient was found to have a scar constitution based on medical history. Therefore, the advantage of ARMS over SRF is that the scope of mucosal resection can be determined according to the patient’s GEFV grade and hiatal hernia size. At present, there is no unified standard for the scope of surgical resection. For GEFV grade IV patients, we found that the effective rate of 3/4 circumferential mucosa resection was high; for GEFV grade II and III patients, the stenosis rate of 2/3 circumferential mucosa resection was low. We will continue to analyze the scope of resection to effectively treat reflux and prevent esophageal stenosis in future studies. We concluded that the effects of ARMS and SRF were equivalent for GEFV grade II and III patients, but for GEFV grade IV patients with hiatal hernia <2 cm, ARMS was preferable.
In clinical work, we found that the expenses of SRF were significantly higher than those of ARMS. The high cost of SRF is due to the use of micro-RF catheters imported from the United States. ARMS does not require costly add-on devices. There was no significant difference in duration between the 2 procedures. Both methods are performed under gastroscopy and are thus minimally invasive. In most cases, the procedure can be completed within 30–40 minutes, with little surgical trauma and few adverse reactions.
A limitation of this study is that it was a single-center, uncontrolled, nonrandomized study with a small sample size. However, the results were promising. This study preliminarily explored the antireflux efficacy and safety of ARMS and SRF in the treatment of GERD. We also confirmed the value of adaptive surgical procedures for different GEFV-grade patients. As there are still many uncertainties about the long-term effects and postoperative recurrence rate of the 2 procedures, a multicenter, randomized controlled trial with a larger sample and long-term follow-up is required to reach a conclusion regarding the clinical efficacy of ARMS and SRF.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2071/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2071/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2071/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Strategic Support Force Medical Center (Beijing, China) (No. K201906). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. [Crossref] [PubMed]
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Gawron AJ, French DD, Pandolfino JE, et al. Economic evaluations of gastroesophageal reflux disease medical management. Pharmacoeconomics 2014;32:745-58. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. [Crossref] [PubMed]
- Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018;67:430-40. [Crossref] [PubMed]
- El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther 2010;32:720-37. [Crossref] [PubMed]
- Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012;61:1340-54. [Crossref] [PubMed]
- Hershcovici T, Fass R. Step-by-step management of refractory gastresophageal reflux disease. Dis Esophagus 2013;26:27-36. [Crossref] [PubMed]
- Rodríguez de Santiago E, Albéniz E, Estremera-Arevalo F, et al. Endoscopic anti-reflux therapy for gastroesophageal reflux disease. World J Gastroenterol 2021;27:6601-14. [Crossref] [PubMed]
- Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 1996;44:541-7. [Crossref] [PubMed]
- Yoo IK, Ko WJ, Kim HS, et al. Anti-reflux mucosectomy using a cap-assisted endoscopic mucosal resection method for refractory gastroesophageal disease: a prospective feasibility study. Surg Endosc 2020;34:1124-31. [Crossref] [PubMed]
- Hedberg HM, Kuchta K, Ujiki MB. First Experience with Banded Anti-reflux Mucosectomy (ARMS) for GERD: Feasibility, Safety, and Technique (with Video). J Gastrointest Surg 2019;23:1274-8. [Crossref] [PubMed]
- He S, Xu F, Xiong X, et al. Stretta procedure versus proton pump inhibitors for the treatment of nonerosive reflux disease: A 6-month follow-up. Medicine (Baltimore) 2020;99:e18610. [Crossref] [PubMed]
- Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009;30:1030-8. [Crossref] [PubMed]
- Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 2007;20:130-4. [Crossref] [PubMed]
- Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol 2014;27:346-51. [PubMed]
- Monino L, Gonzalez JM, Vitton V, et al. Antireflux mucosectomy band in treatment of refractory gastroesophageal reflux disease: a pilot study for safety, feasibility and symptom control. Endosc Int Open 2020;8:E147-54. [Crossref] [PubMed]
- Debourdeau A, Vitton V, Monino L, et al. Antireflux Mucosectomy Band (ARM-b) in Treatment of Refractory Gastroesophageal Reflux Disease After Bariatric Surgery. Obes Surg 2020;30:4654-8. [Crossref] [PubMed]
- Sumi K, Inoue H, Kobayashi Y, et al. Endoscopic treatment of proton pump inhibitor-refractory gastroesophageal reflux disease with anti-reflux mucosectomy: Experience of 109 cases. Dig Endosc 2021;33:347-54. [Crossref] [PubMed]
- Utley DS. The Stretta procedure: device, technique, and pre-clinical study data. Gastrointest Endosc Clin N Am 2003;13:135-45. [Crossref] [PubMed]
- Utley DS, Kim M, Vierra MA, et al. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: a porcine model. Gastrointest Endosc 2000;52:81-6. [Crossref] [PubMed]
- Franciosa M, Triadafilopoulos G, Mashimo H. Stretta Radiofrequency Treatment for GERD: A Safe and Effective Modality. Gastroenterol Res Pract 2013;2013:783815. [Crossref] [PubMed]
- Brar TS, Draganov PV, Yang D. Endoluminal Therapy for Gastroesophageal Reflux Disease: In Between the Pill and the Knife? Dig Dis Sci 2017;62:16-25. [Crossref] [PubMed]
- Dughera L, Navino M, Cassolino P, et al. Long-Term Results of Radiofrequency Energy Delivery for the Treatment of GERD: Results of a Prospective 48-Month Study. Diagn Ther Endosc 2011;2011:507157. [Crossref] [PubMed]
- Kalapala R, Shah H, Nabi Z, et al. Treatment of gastroesophageal reflux disease using radiofrequency ablation (Stretta procedure): An interim analysis of a randomized trial. Indian J Gastroenterol 2017;36:337-42. [Crossref] [PubMed]
- Lee SH, Lee CK, Chung IK, et al. Optimal duration of proton pump inhibitor in the treatment of endoscopic submucosal dissection-induced ulcers: a retrospective analysis and prospective validation study. Dig Dis Sci 2012;57:429-34. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


