
Correlation of the characteristics of symptomatic intracranial atherosclerotic plaques with stroke types and risk of stroke recurrence: a cohort study
Introduction
Intracranial atherosclerosis is a progressive pathological process that causes progressive luminal stenosis and insufficient brain perfusion (1). Symptomatic intracranial atherosclerotic stenosis (sICAS), including acute ischemic stroke (AIS) and transient ischemic attack (TIA), occurs in as much as 46.6% of the Chinese populations and is related to the high risk of stroke recurrence (2). The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial showed that although patients with severe sICAS received active medication treatment, 15% of them still had a recurrent stroke. Subgroup analysis showed that AIS patients had a higher risk of recurrence than TIA patients (3-5), indicating that AIS and TIA patients should be subject to stratified management in clinics. Previous studies have shown that hypertension, diabetes, smoking, dyslipidemia, and vulnerable plaque are the risk factors of sICAS (6,7). However, the differences of these risk factors in AIS and TIA and in anterior circulation ischemic symptom (ACiS) and posterior circulation ischemic symptom (PCiS) are still unclear. Early identification of the differences in risk factors is critical for developing targeted and standardized treatment measures and strategies.
High-resolution vessel wall imaging (HR-VWI) is currently an important diagnostic technique for assessing atherosclerosis. It can not only accurately assess the degree of vascular luminal stenosis but also provide the size and composition information of plaques with extremely high repeatability (8,9). Recurrent strokes tend to be more severe and have a worse prognosis than primary strokes, Early identification of patients at high risk of recurrent stroke is important. In previous studies, intracranial atherosclerotic plaque (ICAP) features based on HR-VWI, including positive remodeling, intraplaque hemorrhage, and plaque enhancement, have been shown to be correlated with the recurrence of stroke (10-14), although conclusions regarding the specific correlations have remained controversial. The characteristics of intracranial arterial culprit plaques based on HR-VWI can provide a basis for differential diagnosis of clinical subtypes of sICAS patients and stratification of risk of stroke recurrence.
In this study, we focused on the relationship between plaque characteristics and clinical subtype of sICAS patients undergoing HR-VWI examination and followed up the patients to assess the differences in clinical risk factors and ICAP characteristics between patients with and without recurrent stroke, with the hope to identify novel intervention targets to develop more standardized secondary prevention strategies for ischemic stroke and TIA to reduce stroke recurrence. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2586/rc).
Methods
Participants
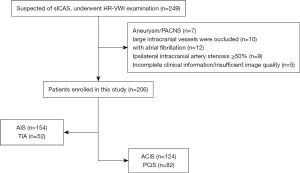
This was a single-center prospective cohort study. A total of 249 consecutive inpatients of the Department of Neurology of the Second Affiliated Hospital of Nantong University from February 2020 to December 2020 who were hospitalized with suspected sICAS, including TIA or AIS, were recruited to the study. Patients underwent one or more types of tests to determine the cause of ischemic events. The tests included carotid ultrasound, echocardiography, electrocardiogram, computed tomography (CT), CT angiography, magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA). If these tests classified the patient as having a large atherosclerotic stroke of trial of ORG 10172 in acute stroke treatment (TOAST) type or indicated that the ischemic event was caused by intracranial atherosclerosis, HR-VWI tests were performed within one week. Patients were enrolled if they met the following criteria: (I) ischemic events occurred in the anterior or posterior circulation areas within 2 weeks; and (II) age >18 years old. Patients were excluded if they had any of the following: (I) non-atherosclerotic vascular disease, such as primary angiitis of the central nervous system (PACNS) or intracranial aneurysm (n=7); (II) ipsilateral extracranial artery stenosis ≥50%, and intracranial large vascular occlusion (n=19); (III) potential cardiogenic embolism, such as atrial fibrillation (n=12); and (IV) incomplete clinical information or poor imaging quality (n=5) (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All research protocols were approved by the Ethics Committee of the Second Affiliated Hospital of Nantong University (No. 2016YXJS010) and conducted following the relevant guidelines and regulations. Written informed consent was provided by patients.
Clinical index collection
The demographic data, atherosclerosis risk factors, blood pressure at admission, and laboratory test results during hospitalization were collected of all participants. Blood pressure was measured twice in the brachial artery of seated participants using an automatic electronic device after 5 minutes of rest. The mean of the two measurements was used as the final reading. Hypertension was defined as having a systolic blood pressure (SBP) >140 mmHg, a diastolic blood pressure (DBP) >90 mmHg, or a history of hypertension with antihypertensive therapy. Diabetes mellitus was defined as a fasting glucose level exceeding 126 mg/dL, random glucose level exceeding 200 mg/dL, a glycated hemoglobin (HbA1c) level exceeding 6.5%, and/or a medical or self-reported history of diabetes or treatment with oral antidiabetic drugs or insulin. Dyslipidemia was defined by serum triglyceride level exceeding 150 mg/dL, high-density lipoprotein (HDL) cholesterol level below 40 mg/dL, or a medical history of dyslipidemia. Smoking history was defined as past or current smoking. All patients were initiated with aspirin and clopidogrel dual antibody therapy in accordance with the guidelines within 24 hours after admission (15), had good compliance, and were able to adhere to regular medication during follow-up. The follow-up time was 3, 6, 9, and 12 months after discharge, and the follow-up method was outpatient or telephone follow-up, the time of recurrence was recorded as the month between the time of recurrence and the last discharge from hospital. Recurrence of stroke was defined as the occurrence of a new acute infarct in the same blood vessel supply area revealed by diffusion weighted imaging (DWI). When there was no imaging examination for the suspected recurrence event, whether the follow-up was based on the duration of the new neurological function defect or not, 24 hours was the threshold to determine the occurrence of outcome events; recurrent TIA (duration of neurological impairment <24 hours) was not considered a result event (16).
HR-VWI image acquisition
The HR-VWI images were acquired using the Siemens Verio 3.0 T MR imaging system (Siemens, Erlangen, Germany). In addition to the conventional MRI and MRA scanning sequence, 3-dimensional T1 sampling perfection with application optimized contrasts using different flip angle evolution (3D-T1-SPACE) sequence was scanned before and after injecting the contrast agent with the following parameters: repetition time (TR) =700 ms, echo time (TE) =12 ms, field of view (FOV) =200×200×40 mm3, voxel size = 0.8×0.8×0.7 mm3, and scanning time = 7 min 16 s. Gadoteric acid was used as the contrast agent at a dose of 0.1 mmol/kg, and the enhanced images were collected at 8 minutes after injection. The scanning range included the complete presentation of the intracranial segment of the internal carotid artery, M1 and M2 segments of the middle cerebral artery, A1 and A2 segments of the anterior cerebral artery, P1 and P2 segments of the posterior cerebral artery, V4 segment of the vertebral artery, and the basilar artery.
Image analysis and evaluation
All images were analyzed by two neuroradiologists with 5 years and 15 years of experience, respectively, and in the case of disagreement, a consensus was reached after consultation with the third neuroradiologis who had 20 years of experience. The quality of each image was scored with 1 for poor quality, 2 for acceptable, and 3 for good. Only images with a score of 2 or above were used in the study. Images were processed using the Multimodality Workplace Siemens Erlangen workstation. The location of the vascular stenosis was determined on the obtained time-of-flight (TOF)-MRA image, and the location of the ICAP was then determined using the 3D-T1-SPACE sequence in combination with the sagittal, coronal, and axial images, that is, the location of eccentric stenosis in the lumen or the site with locally thickened vascular wall. The two evaluators jointly determined the culprit plaque based on the clinical manifestations. When multiple plaques existed in the same vascular area, the one that caused the most severe stenosis was defined as the culprit plaque.
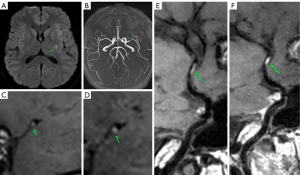
The degree of luminal stenosis was evaluated according to the Warfarin-Aspirin Symptomatic Intracranial Disease Study (17). The plaque burden was defined as 1 minus the ratio of the remaining luminal area to the vascular wall area ×100% (18). The remodeling index was defined as the ratio of the vascular wall area at the most severe stenosis to the reference vascular wall area. The remodeling index of ≥1.05, 0.95–1.05, and ≤0.95 was considered as positive remodeling, no remodeling, and negative remodeling, respectively. The increase of vascular wall thickness involving ≥50% of arterial wall circumference was defined as centripetal, otherwise as eccentric. Plaque T1 weighted imaging (T1WI) signal intensity greater than 150% of the adjacent muscle signal intensity was defined as hyperintense on T1WI. A plaque signal intensity after enhancement less than the signal intensity of the pituitary stalk was defined as a mild enhancement. The enhancement degree equal to or greater than the signal intensity of the pituitary stalk was defined as a significant enhancement. Case presentation is shown in Figure 2.
Statistical analysis
The software SPSS 21.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis of the data. Kolmogorov-Smirnov test was used to evaluate whether the data conformed to normal distribution. In single factor analysis, median (interquartile spacing) was used to represent trend variables of skewness distribution, and Mann-Whitney U test was performed. The variables consistent with the normal distribution trend were represented by mean ± standard deviation, and the t-test was performed. Counting data were expressed as frequency (percentage) and chi-square test was performed. Multivariate analysis was performed using a binary logistic regression model with variables that were P value <0.1 in univariate analyses to identify potential associated factors for sICAS clinical subtypes. Similarly, all variables with a P value <0.1 in the univariate analysis were included in Cox regression analysis, for further screening of risk factors related to recurrent stroke. All reported P values were two-tailed, P<0.05 was considered statistically significant.
Results
Comparison of clinical data and HR-VWI based qualitative and quantitative indicators between TIA group and AIS group
A total of 206 patients were enrolled, including 141 males (68.0%). There were 52 patients (25.2%) in the TIA group and 154 patients (74.8%) in the AIS group. The rate of prior statin use in the TIA group was higher than that in the AIS group (χ2=9.646, P=0.009), and the serum apolipoprotein A/B value was higher than that in the AIS group (t=−2.865, P=0.006). The serum LDL level in the AIS group was higher than that in the TIA group (t=−2.424, P=0.016), and the difference was statistically significant. Compared with participants in the TIA group, those in the AIS group had smaller lumen area in culprit plaque (t=2.539, P=0.013), and greater stenosis degree (t=−3.317, P<0.001), the difference was statistically significant. There was no significant difference between the TIA group and AIS group in terms of culprit plaque thickness, plaque burden, significant plaque enhancement, centripetal distribution, positive remodeling, and T1WI high signal incidence. Binary logistic regression analysis showed that male gender [odds ratio (OR) =5.575, 95% confidence interval (CI): 2.120 to 14.658] was an independent risk factor for AIS, and history of statin use (OR =0.309, 95% CI: 0.113 to 0.843) and serum apolipoprotein A/B (OR =0.363, 95% CI: 0.139 to 0.948) were protective factors for AIS. The clinical and imaging data of the two groups are shown in Table 1.
Table 1
| Index | TIA (n=52) | AIS (n=154) | t/Z/χ2 | P value | Binary logistic regression | |
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | |||||
| Age, years, mean ± SD | 63.61±1.84 | 63.97±0.93 | −0.407 | 0.870 | 1.016 (0.981, 1.054) | 0.372 |
| Male, n (%) | 29 (55.8) | 111 (72.1) | 7.779 | 0.023 | 5.575 (2.120, 14.658) | <0.001 |
| Smoking history, n (%) | 11 (21.1) | 43 (27.5) | 0.245 | 0.588 | ||
| History of hypertension, n (%) | 25 (48.1) | 87 (56.5) | 1.110 | 0.335 | ||
| History of diabetes, n (%) | 14 (26.9) | 53 (34.4) | 0.270 | 0.499 | ||
| Hyperlipidemia, n (%) | 10 (19.2) | 41 (26.6) | 1.140 | 0.354 | ||
| Previous use of statins, n (%) | 15 (28.8) | 19 (12.3) | 9.646 | 0.009 | 0.309 (0.113, 0.843) | 0.022 |
| Apolipoprotein A/B, mean ± SD | 1.74±0.15 | 1.40±0.05 | −2.865 | 0.006 | 0.363 (0.139, 0.948) | 0.039 |
| LDL, mmol/L, mean ± SD | 2.25±0.17 | 2.60±0.10 | −2.424 | 0.016 | 1.625 (0.767, 3.442) | 0.205 |
| Lipoprotein phospholipase A2, ng/ml, median (IQR) | 290.50 (195.00, 482.25) | 317.00 (245.00, 487.50) | −2.151 | 0.054 | 1.000 (0.998, 1.003)) | 0.947 |
| Homocysteine, µmol/L, median (IQR) | 11.00 (9.03, 14.45) | 12.20 (10.20, 15.55) | −1.368 | 0.194 | ||
| Plaque thickness, mm, mean ± SD | 1.64±0.08 | 1.52±0.03 | 1.963 | 0.155 | ||
| Luminal area at the plaque, mm2, mean ± SD | 5.21±0.46 | 3.06±0.18 | 2.539 | 0.013 | 0.876 (0.727, 1.054) | 0.161 |
| Degree of stenosis, mean ± SD | 0.43±0.02 | 0.53±0.07 | −3.317 | <0.001 | 6.187 (0.241, 158.625) | 0.271 |
| Plaque burden, mean ± SD | 0.71±0.01 | 0.73±0.01 | −0.938 | 0.287 | ||
| Significant enhancement, n (%) | 22 (42.3) | 78 (50.6) | 0.853 | 0.337 | ||
| Centripetal distribution, n (%) | 13(25.0) | 53 (34.4) | 1.583 | 0.233 | ||
| Positive remodeling, n (%) | 32 (61.5) | 90 (58.4) | 5.438 | 0.746 | ||
| Hyperintense on T1WI, n (%) | 13 (25.0) | 50 (32.5) | 0.527 | 0.385 | ||
T1WI, T1 weighted imaging; HR-VWI, high-resolution vessel wall imaging; LDL, low-density lipoprotein; TIA, transient ischemic attack; AIS, acute ischemic stroke; OR, odds ratio; CI, confidence interval; SD, standard deviation; IQR, interquartile range.
Comparison of clinical data and qualitative and quantitative indicators based on HR-VWI between ACiS and PCiS
In this group, there were 124 cases (60.2%) of ACiS, and 82 cases (59.8%) of PCiS. Patients with PCiS had a higher history of diabetes (χ2=7.456, P=0.010) and hyperlipidemia (χ2=4.509, P=0.048) than those with ACiS. Binary logistic regression analysis showed that diabetes history (OR =2.137, 95% CI: 1.168 to 3.912) was an independent risk factor for PCiS. Posterior circulation ICAP tended to have larger plaque thickness (t=−4.205, P<0.001) and lumen area (Z=−4.127, P<0.001), the difference was statistically significant, while the degree of lumen stenosis was not statistically significant. The incidence of ICAP significant enhancement (χ2=9.681, P=0.002), positive remodeling (χ2=5.661, P=0.020), and hyperintensity on T1WI (χ2=16.472, P=0.003) in posterior circulation was higher than that in anterior circulation, and the difference was statistically significant (Table 2).
Table 2
| Index | ACiS (n=124) | PCiS (n=82) | t/Z/χ2 | P value | Binary logistic regression | |
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | |||||
| Age, years, mean ± SD | 64.28±11.83 | 63.29±12.26 | 0.579 | 0.563 | 0.994 (0.970, 1.019) | 0.798 |
| Male, n (%) | 84 (68.3) | 56 (67.5) | 0.015 | 1.000 | 0.922 (0.497, 1.711) | 0.652 |
| Smoking history, n (%) | 32 (26.2) | 22 (26.5) | 0.022 | 1.000 | ||
| History of hypertension, n (%) | 65 (52.8) | 47 (56.6) | 0.286 | 0.669 | ||
| History of diabetes, n (%) | 31 (25.2) | 36 (43.4) | 7.456 | 0.010 | 2.137 (1.168, 3.912) | 0.014 |
| Hyperlipidemia, n (%) | 24 (19.5) | 27 (32.5) | 4.509 | 0.048 | 1.780 (0.918, 3.453) | 0.088 |
| Apolipoprotein A/B, mean ± SD | 1.48±0.66 | 1.40±0.51 | 0.956 | 0.340 | ||
| LDL, mmol/L, mean ± SD | 2.70±1.30 | 2.45±0.96 | 1.417 | 1.158 | ||
| Lipoprotein phospholipase A2, ng/ml, median (IQR) | 313 (245, 482) | 348 (237, 513) | −0.182 | 0.856 | ||
| Homocysteine, µmol/L, median (IQR) | 12.95 (10.70, 15.95) | 11.60 (10.15, 15.35) | 1.357 | 0.175 | ||
| TIA, n (%) | 25 (20.3) | 27 (32.5) | 3.912 | 0.052 | ||
| Plaque thickness, mm, mean ± SD | 1.45±0.03 | 1.71±0.06 | −4.205 | <0.001 | ||
| Luminal area at the plaque, mm2, median (IQR) | 3.30 (2.13, 4.70) | 4.70 (2.95, 6.70) | −4.127 | <0.001 | ||
| Degree of stenosis, mean ± SD | 0.46±0.01 | 0.45±0.01 | 0.957 | 0.328 | ||
| Plaque burden, mean ± SD | 0.72±0.01 | 0.74±0.01 | −1.655 | 0.161 | ||
| Significant enhancement, n (%) | 49 (39.5) | 51 (62.2) | 9.681 | 0.002 | ||
| Centripetal distribution, n (%) | 34 (27.6) | 32 (38.6) | 2.710 | 0.128 | ||
| Positive remodeling, n (%) | 65 (52.4) | 57 (69.5) | 5.661 | 0.020 | ||
| Hyperintense on T1WI, n (%) | 28 (22.6) | 35 (42.7) | 16.472 | 0.003 | ||
ACiS, anterior circulation ischemic symptom; PCiS, posterior circulation ischemic symptom; T1WI, T1 weighted imaging; HR-VWI, high-resolution vessel wall imaging; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TIA, transient ischemic attack; AIS, acute ischemic stroke; OR, odds ratio; CI, confidence interval; SD, standard deviation; IQR, interquartile range.
Comparison of clinical data and qualitative and quantitative indicators based on HR-VWI between recurrent and non-recurrent groups
All participants were followed up at 3, 6, 9, and 12 months in outpatient or telephone visits, and 24 patients (11.6%) had recurrent stroke within 12 months. Groups were divided according to whether there was recurrence and comparisons were made between groups. Univariate analysis showed that the serum LDL level (t=6.808, P=0.047) and the rate of hyperintensity on T1WI (χ2=13.035, P=0.001) in the recurrence group were higher than those in the non-recurrence group, and the differences were statistically significant. There was no significant difference in smoking history, hypertension history, diabetes history, hyperlipidemia, and other clinical risk factors between the recurrence group and the non-recurrence group. In the recurrence group, the culprit plaque thickness and plaque burden were larger, and the lumen area at the plaque was smaller, but the differences were not statistically significant. The proportion of participants with PCiS presenting with AIS and the recurrence rate of stroke were higher than those of participants with ACiS, but the difference was not statistically significant. Cox regression analysis showed that hyperintensity on T1WI in culprit plaque [hazard ratio (HR) =3.798, 95% CI: 1.433 to 10.062] was an independent risk factor for recurrent stroke (Table 3).
Table 3
| Index | Non-recurrence group (n=182) | Recurrence group (n=24) | t/Z/χ2 | P value | Cox regression | |
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | |||||
| Age, years, mean ± SD | 63.84±12.08 | 64.17±11.47 | −0.125 | 0.900 | 1.107 (0.976, 1.060) | 0.419 |
| Male, n (%) | 126 (69.2) | 14 (58.3) | 1.156 | 0.352 | 0.742 (0.274, 2.009) | 0.557 |
| Smoking history, n (%) | 46 (25.3) | 8 (33.3) | 0.459 | 0.459 | ||
| History of hypertension, n (%) | 98 (53.8) | 14 (58.3) | 0.133 | 0.716 | ||
| History of diabetes, n (%) | 58 (31.9) | 9 (37.5) | 0.306 | 0.644 | ||
| Hyperlipidemia, n (%) | 44 (24.2) | 7 (29.2) | 0.284 | 0.618 | ||
| Apolipoprotein A/B, mean ± SD | 1.44±0.59 | 1.34±0.39 | 0.753 | 0.452 | ||
| LDL, mmol/L, mean ± SD | 2.54±0.92 | 3.09±2.42 | 6.808 | 0.047 | 1.319 (0.916, 1.898) | 0.136 |
| Lipoprotein phospholipase A2, ng/ml, median (IQR) | 317 (245, 486) | 424 (225, 500) | −0.144 | 0.885 | ||
| Homocysteine, µmol/L, median (IQR) | 12.70 (10.50, 15.90) | 11.25 (9.78, 15.32) | −0.949 | 0.343 | ||
| AIS, n (%) | 135 (74.2) | 19 (79.2) | 0.280 | 0.803 | ||
| Pcis, n (%) | 72 (39.6) | 11 (45.8) | 0.347 | 0.659 | ||
| Plaque thickness, mm, mean ± SD | 1.55±0.42 | 1.59±0.41 | −0.517 | 0.645 | ||
| Luminal area at the plaque, mm2, mean ± SD | 4.36±0.21 | 3.60±0.34 | 1.338 | 0.062 | 0.997 (0.807, 1.231) | 0.976 |
| Degree of stenosis, mean ± SD | 0.47±0.03 | 0.52±0.06 | −0.266 | 0.771 | ||
| Plaque burden, mean ± SD | 0.72±0.01 | 0.75±0.02 | −1.172 | 0.237 | ||
| Significant enhancement, n (%) | 87 (47.8) | 12 (50.0) | 0.041 | 0.839 | ||
| Centripetal distribution, n (%) | 56 (30.8) | 10 (41.7) | 1.156 | 0.352 | ||
| Positive remodeling, n (%) | 110 (60.4) | 12 (50.0) | 0.957 | 0.379 | ||
| Hyperintense on T1WI, n (%) | 48 (28.0) | 15 (50.0) | 13.035 | 0.001 | 3.798 (1.433, 10.062) | 0.007 |
ACiS, anterior circulation ischemic symptom; PCiS, posterior circulation ischemic symptom; T1WI, T1 weighted imaging; HR-VWI, high-resolution vessel wall imaging; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TIA, transient ischemic attack; AIS, acute ischemic stroke; OR, odds ratio; CI, confidence interval; SD, standard deviation; IQR, interquartile range.
Discussion
In this study, we included 206 patients with symptomatic ICAP and collected their clinical data and HR-VWI image information. Tarnutzer et al. (19) found that the misdiagnosis rate of cerebrovascular disease events during the first visit to the emergency department was approximately 9%. Patients with mild symptoms and non-specific or transient symptoms were found to be at higher risk of misdiagnosis, and were often misdiagnosed with TIA. Therefore, they were subjected to delayed treatments or had a poor prognosis. All these observations suggest that there might be some shortcomings to the clinical management of patients based only on the results of DWI examinations. We diagnosed the patients based on their clinically localized symptoms and HR-VWI. The results showed that AIS patients had higher diastolic blood pressure and blood lipid level than TIA patients. Moreover, HR-VWI showed that AIS patients had higher stenosis degrees and smaller remaining luminal areas at the plaque than TIA patients. Feng et al. (16) showed that patients with sICAS caused by a mixed mechanism of arterial-arterial embolism and low perfusion distal to stenosis were more likely to have a history of dyslipidemia and hypertension, which is consistent with our results. Dyslipidemia might increase the risk of vulnerable plaque rupture and arterial-arterial embolism (20). The luminal stenosis caused by ICAP was negatively correlated with the distal perfusion status, and the blood flow acceleration caused by the reduction of the remaining luminal area could also increase the risk of ICAP rupture (21,22). Therefore, we speculated that the degree of luminal stenosis and the remaining luminal area might be related to the stroke type of sICAS. We also found that previous use of statins had a certain protective effect on the occurrence of AIS, consistent with the results of Chung et al. (23).
Through a 6-month follow-up of the participants, we found that the baseline HR-VWI images of patients with recurrent stroke were more likely to have hyperintensity on T1WI. Hyperintensity on T1WI often represents intraplaque hemorrhage, which is currently a more recognized feature of vulnerable plaque and is significantly related to the downstream ischemic events (24,25). A recent meta-analysis of 1,542 patients and a total of 1,750 plaques showed that plaque enhancement, positive remodeling, hyperintensity on T1WI, and surface irregularities were significantly related to the downstream ischemic events. In all subgroups, plaque enhancement was significantly associated with downstream ischemic events (26). However, in this study, no significant difference was found in enhancement degree between patients with and without recurrent stroke. This discrepancy might be because the enhancement degree in this study was defined as classification data rather than quantitative data, leading to the loss of significance. Moreover, patients were followed up for 6 months, which a is relatively short follow-up duration.
Edlow et al. (27) found that the incidence of DWI-negative AIS was 6.8%, and five times higher in patients with PCiS than in patients with ACiS. This suggests that the pathogenesis might be different between patients with PCiS and ACiS. Our study included 82 patients with PCiS. After comparing the clinical data and culprit plaque conditions of patients with PCiS, we found that more patients with PCiS had a history of diabetes. Diabetes mellitus is an independent risk factor for posterior circulation ischemia. It is worth mentioning that the plaque thickness and the lumen area at the plaque of the posterior circulation is larger than that of the anterior circulation, which may be because the diameter of the vertebrobasilar artery is usually larger than that of the anterior circulation artery. The incidence of culprit plaque significant enhancement, positive remodeling, and hyperintensity on T1WI in posterior circulation was higher than that in anterior circulation. Studies have shown that poor blood sugar control might play an important role in the occurrence and development of plaques in patients with AIS or TIA. Compared with non-diabetic patients, diabetic patients had a significantly higher incidence of plaque enhancement, longer maximum plaque length, greater maximum plaque wall thickness, and increased luminal stenosis degree (28-30). Therefore, we speculated that the appearance of the vulnerable characteristics of the posterior circulation culprit plaque might be related to the metabolic factors of secondary diabetes. In the future, we will focus on the role of glucose fluctuation in the occurrence and development of ICAP in diabetic patients.
This study had certain limitations. First, the sample size was relatively small, especially the number of patients with recurrent stroke, which may have led to certain statistical bias. Second, the follow-up time was relatively short; these patients need to be followed up for a longer period in the future. Third, ICAP features based on HR-MRI may not reflect the true characteristics of intracranial plaques because of its limited resolution. In addition, some data were manually measured, which might have introduced certain errors.
Conclusions
Gender, previous statin use, and serum apolipoprotein A/B values are independently associated with sICAS clinical subtypes. The incidence of vulnerable plaques in posterior circulation is higher than that in anterior circulation, which may be related to metabolic factors secondary to diabetes. Hyperintensity on T1WI in culprit plaque is an independent risk factor for recurrence of stroke within 1 year, and stroke recurrence is not associated with anterior and posterior circulation or sICAS clinical subtypes.
Acknowledgments
Funding: This study was funded by grants from the Nantong Municipal Science and Technology Plan Project (Nos. MS12020041, MS12021101, and HS2019002) and Nantong Municipal Health Commission Youth Project (No. MB2020001).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2586/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2586/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2586/coif).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All research protocols were approved by the Ethics Committee of the Second Affiliated Hospital of Nantong University (No. 2016YXJS010) and conducted following the relevant guidelines and regulations. Written informed consent was provided by patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang Y, Meng R, Liu G, et al. Intracranial atherosclerotic disease. Neurobiol Dis 2019;124:118-32. [Crossref] [PubMed]
- Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014;45:663-9. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]
- Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333-41. [Crossref] [PubMed]
- Lutsep HL, Barnwell SL, Larsen DT, et al. Outcome in patients previously on antithrombotic therapy in the SAMMPRIS trial: subgroup analysis. Stroke 2015;46:775-9. [Crossref] [PubMed]
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106-14. [Crossref] [PubMed]
- Skarpathiotakis M, Mandell DM, Swartz RH, et al. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol 2013;34:299-304. [Crossref] [PubMed]
- Wang Y, Liu X, Wu X, et al. Culprit intracranial plaque without substantial stenosis in acute ischemic stroke on vessel wall MRI: A systematic review. Atherosclerosis 2019;287:112-21. [Crossref] [PubMed]
- Kim HJ, Choi EH, Chung JW, et al. Luminal and Wall Changes in Intracranial Arterial Lesions for Predicting Stroke Occurrence. Stroke 2020;51:2495-504. [Crossref] [PubMed]
- Kassem M, Florea A, Mottaghy FM, et al. Magnetic resonance imaging of carotid plaques: current status and clinical perspectives. Ann Transl Med 2020;8:1266. [Crossref] [PubMed]
- Kim JM, Jung KH, Sohn CH, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke 2016;11:171-9. [Crossref] [PubMed]
- Ran Y, Wang Y, Zhu M, et al. Higher Plaque Burden of Middle Cerebral Artery Is Associated With Recurrent Ischemic Stroke: A Quantitative Magnetic Resonance Imaging Study. Stroke 2020;51:659-62. [Crossref] [PubMed]
- Song X, Zhao X, Liebeskind DS, et al. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology 2020;62:1123-31. [Crossref] [PubMed]
- Donners SJA, Toorop RJ, de Kleijn DPV, et al. A narrative review of plaque and brain imaging biomarkers for stroke risk stratification in patients with atherosclerotic carotid artery disease. Ann Transl Med 2021;9:1260. [Crossref] [PubMed]
- Jung SJ, Kim BJ, Kim CK, et al. Antiplatelet regimens after ischemic stroke or transient ischemic attack: a systematic review and updated network meta-analysis. Ann Transl Med 2022;10:245. [Crossref] [PubMed]
- Feng X, Chan KL, Lan L, et al. Stroke Mechanisms in Symptomatic Intracranial Atherosclerotic Disease: Classification and Clinical Implications. Stroke 2019;50:2692-9. [Crossref] [PubMed]
- Teng Z, Peng W, Zhan Q, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol 2016;26:2206-14. [Crossref] [PubMed]
- Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643-6. [PubMed]
- Tarnutzer AA, Lee SH, Robinson KA, et al. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: A meta-analysis. Neurology 2017;88:1468-77. [Crossref] [PubMed]
- Kojima S, Kojima S, Maruyoshi H, et al. Hypercholesterolemia and hypoadiponectinemia are associated with necrotic core-rich coronary plaque. Int J Cardiol 2011;147:371-6. [Crossref] [PubMed]
- Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke 2014;16:8-17. [Crossref] [PubMed]
- Kwak BR, Bäck M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 2014;35:3013-20, 3020a-3020d.
- Chung JW, Hwang J, Lee MJ, et al. Previous Statin Use and High-Resolution Magnetic Resonance Imaging Characteristics of Intracranial Atherosclerotic Plaque: The Intensive Statin Treatment in Acute Ischemic Stroke Patients With Intracranial Atherosclerosis Study. Stroke 2016;47:1789-96. [Crossref] [PubMed]
- Li J, Li D, Yang D, et al. Co-existing cerebrovascular atherosclerosis predicts subsequent vascular event: a multi-contrast cardiovascular magnetic resonance imaging study. J Cardiovasc Magn Reson 2020;22:4. [Crossref] [PubMed]
- Schindler A, Schinner R, Altaf N, et al. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging 2020;13:395-406. [Crossref] [PubMed]
- Song JW, Pavlou A, Xiao J, et al. Vessel Wall Magnetic Resonance Imaging Biomarkers of Symptomatic Intracranial Atherosclerosis: A Meta-Analysis. Stroke 2021;52:193-202. [Crossref] [PubMed]
- Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI-negative acute ischemic stroke: A meta-analysis. Neurology 2017;89:256-62. [Crossref] [PubMed]
- Huang J, Jiao S, Song Y, et al. Association between type 2 diabetes mellitus, especially recently uncontrolled glycemia, and intracranial plaque characteristics: A high-resolution magnetic resonance imaging study. J Diabetes Investig 2020;11:1278-84. [Crossref] [PubMed]
- Lindenholz A, van der Kolk AG, van der Schaaf IC, et al. Intracranial Atherosclerosis Assessed with 7-T MRI: Evaluation of Patients with Ischemic Stroke or Transient Ischemic Attack. Radiology 2020;295:162-70. [Crossref] [PubMed]
- Xu Z, Li M, Lyu J, et al. Different risk factors in identical features of intracranial atherosclerosis plaques in the posterior and anterior circulation in high-resolution MRI. Ther Adv Neurol Disord 2020;13:1756286420909991. [Crossref] [PubMed]
(English Language Editor: J. Jones)


