
Clinical study of poorly differentiated head and neck squamous cell carcinoma: a prospective cohort study in China
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the 6th most common cancer in the world (1), and the main pathological type of head and neck cancer, accounting for >90% of the total. In recent years, the incidence rate has been gradually increasing, and >500,000 new cases are diagnosed annually in the worldwide (2). According to the degree of differentiation of the tumor cells, SCC can be divided into three subtypes: well, medium and poorly differentiated, with the main subtype being well-differentiated SCC. Although poorly differentiated laryngeal SCC is rare among HNSCC, previous research indicates that survival and prognosis are worse than for the well-differentiated subtype, and the 5-year overall survival (OS) rate is 30% lower. Patients with the poorly differentiated subtype are more likely to have local recurrence or distant metastasis (DS), and this subtype has completely different characteristics to well-differentiated SCC (3). However, there is a general lack of treatment guidelines or consensus on the pathological differentiation of laryngeal SCC, so more clinical study is required to clarify the most favorable treatment options for this pathological subtype with poor prognosis.
The aims of this study were to clarify the current combinations of existing treatment methods, which treatment scheme is most suitable for patients with poorly differentiated HNSCC, and which treatment scheme could provide guidelines for the clinical treatment of patients. From this, it may be possible to improve the formulation of individualized treatment plans for patients with different pathological subtypes. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2630/rc).
Methods
Patient recruitment
This study was a prospective cohort study (clinical trial: ChiCTR-ONC-16009290), mainly for patients with poorly differentiated HNSCC (larynx or hypopharynx), to identify better treatment options for this subtype. Since September 2016, patients who met the following criteria were recruited: (I) primary lesion located in the larynx or hypopharynx; (II) T1–4a, N0–2, M0; (III) poorly differentiated SCC by pathological examination; (IV) newly treated patient (no history of surgery, radiotherapy or chemotherapy); (V) age 18–70 years; (VI) Karnofsky Performance Status >80; (VII) hemogram, heart, lung, liver, kidney and other major organs essentially normal; (VIII) CT, MRI and endoscopy completed before enrollment. The first patient was recruited in September 2016 and the last in October 2020.
Ethics and approvals
This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (No. trecky2015-026/trecky2016-025). All patients were informed of the purpose of the study and signed informed consent before treatment. All information will be stored securely. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Treatment regimen
The flow diagram for this study is shown in Figure 1. According to disease stage, the patients in stage I/II were divided into surgery group, induction chemotherapy (IC) + surgery group, or surgery + adjuvant therapy group. Stage III/IV patients were divided into surgery group, IC + surgery + adjuvant therapy group, or surgery + adjuvant therapy group. In the surgery groups, the patients underwent laser resection or open surgery according to the location of the lesions. Two cycles of docetaxel/cisplatin/5-FU regimen comprised the IC. Postoperative adjuvant therapy was 30–35 doses of radiotherapy, to a total dose of 55–60 Gy. During radiotherapy and chemotherapy, if the patient could not complete the treatment, it was suspended and the patient was discharged from the study. After completion of the treatment plan, the patients were followed up regularly to observe disease progression as local recurrence, lymph node metastasis and DS, and the corresponding treatment was carried out according to existing treatment guidelines.
Statistical analysis
The Chi-squared test and non-parametric test were used to analyze the differences between groups. The Kaplan-Meier method was used to construct the OS rate curve and risk curve. Also, we performed univariate analysis on the factors that may affect local recurrence, DS, lymph node metastasis and survival rate, including gender, age, smoking history, drinking history, lesion location, surgical method, incision margin and comprehensive treatments. The meaningful possible factors are included in the multivariate analysis to obtain the independent influencing factors. SPSS 25.0 (IBM Corp., Armonk, NY, USA) was used, and the significance level was set as P<0.05 double-sided.
Results
Baseline information of the patients
In total, 70 patients were recruited and followed up to October 2021 (median follow-up, 19.5 months). Of them, 34 patients were in stage I/II: 12 were in the Surgery group, 10 in the IC + surgery group, and 12 in the Surgery + adjuvant therapy group. There were 36 patients in stage III/IV, with 12 patients in the Surgery group, 12 in the IC + surgery + adjuvant therapy group, and 12 in the surgery + adjuvant therapy group. We excluded 8 patients (5 patients in stage I/II, 3 patients in stage III/IV) who either did not wish to take part in the study or were intolerant to chemoradiotherapy. Therefore, 62 patients were finally included in the statistical analysis (Table 1). There were 29 patients with stage I/II disease (12 patients in the surgery group, 7 in the IC + surgery group, and 10 in the surgery + adjuvant therapy group), and 33 patients with stage III/IV disease (12 patients in the surgery group, 11 in the IC + surgery + adjuvant therapy group, and 10 in the surgery + adjuvant therapy group). The Chi-squared test and non-parametric test were used to analyze differences in the general clinical characteristics of patients with different disease stages and in the different treatment groups. There were no differences in age, sex, primary location of disease, smoking history, drinking history or surgery among the groups (Table 1). And the differences between different treatment groups are excluded.
Table 1
| Characteristics | Stage I/II (n=29) | Stage III/IV (n=33) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery (n=12) | IC + surgery (n=7) | Surgery + adjuvant therapy (n=10) | P | Surgery (n=12) | IC + surgery + adjuvant therapy (n=11) | Surgery + adjuvant therapy (n=10) | P | ||
| Mean age, years | 62.5 | 57 | 61.5 | 0.210 | 62.5 | 59 | 58 | 0.220 | |
| Sex | 0.130 | 0.406 | |||||||
| Male | 12 | 7 | 8 | 11 | 11 | 10 | |||
| Female | 0 | 0 | 2 | 1 | 0 | 0 | |||
| Site | 0.060 | 0.063 | |||||||
| Larynx | 9 | 2 | 8 | 10 | 4 | 5 | |||
| Hypopharynx | 3 | 5 | 2 | 2 | 7 | 5 | |||
| Smoking | 0.899 | 0.783 | |||||||
| Yes | 6 | 4 | 6 | 8 | 8 | 8 | |||
| No | 6 | 3 | 4 | 4 | 3 | 2 | |||
| Drinking | 0.283 | 0.672 | |||||||
| Yes | 4 | 4 | 2 | 7 | 6 | 4 | |||
| No | 8 | 3 | 8 | 5 | 5 | 6 | |||
| Surgery | 0.142 | — | |||||||
| Laser | 8 | 3 | 5 | 0 | 0 | 0 | |||
| Open | 4 | 4 | 5 | 12 | 11 | 10 | |||
IC, induction chemotherapy.
Prognostic analysis of patients
The Kaplan-Meier univariate method was used to analyze the risk of local recurrence, lymph node metastasis and DS in patients with different stages and in different treatment groups. Among the stage I/II patients, there was no difference in prognosis for the three treatment groups. Among the stage III/IV patients, there were differences in DS for the three treatment groups. Different treatment is an independent factor affecting DS, of which IC + surgery + adjuvant therapy had the best control of DS (Table 2, Figure 2).
Table 2
| Prognosis and survival | Stage I/II (n=29) | Stage III/IV (n=33) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery (n=12) | IC + surgery (n=7) | Surgery + adjuvant therapy (n=10) | P | Surgery (n=12) | IC + surgery + adjuvant therapy (n=11) | Surgery + adjuvant therapy (n=10) | P | ||
| Local recurrence | 0.143 | 0.737 | |||||||
| Yes | 0 | 2 | 1 | 2 | 3 | 3 | |||
| No | 12 | 5 | 9 | 10 | 8 | 7 | |||
| Distant metastasis | 0.069 | 0.013 | |||||||
| Yes | 1 | 0 | 3 | 6 | 2 | 4 | |||
| No | 11 | 7 | 7 | 6 | 9 | 6 | |||
| Lymph node metastasis | – | 0.176 | |||||||
| Yes | 0 | 0 | 0 | 6 | 1 | 2 | |||
| No | 0 | 0 | 0 | 6 | 10 | 8 | |||
| Survival | 0.447 | 0.009 | |||||||
| Yes | 10 | 6 | 10 | 4 | 9 | 4 | |||
| No | 2 | 1 | 0 | 8 | 2 | 6 | |||
IC, induction chemotherapy.
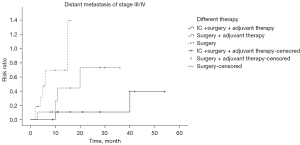
Analysis of patient survival
To analyze the OS and progression-free survival (PFS) of patients with stage I/II and stage III/IV disease, the Kaplan-Meier method was used for univariate analysis of all factors that may be related to OS and PFS. Positive results were then selected for multivariate analysis using the Cox regression method to calculate the survival rate. There was no significant difference in OS or PFS among the treatment groups of stage I/II patients, but the long-term survival benefit in the surgery + adjuvant therapy group was better than the 1-, 2-, and 3-year OS of the other two groups. For the stage III/IV patients, there were significant differences in OS and PFS among the treatment groups: OS: 0.021 [hazard ratio (HR): 2.317, 95% confidence interval (CI): 1.133–4.741]; PFS: 0.020 (HR: 2.392, 95% CI: 1.150–4.973). As shown by the survival curves (Figures 3,4), the survival time of each group decreased in the order of: IC+ surgery + adjuvant therapy > surgery + adjuvant therapy > surgery. Based on the 1-, 2-, and 3-year OS for each group, the IC+ surgery + adjuvant therapy group showed a significant advantage in OS (Table 3).
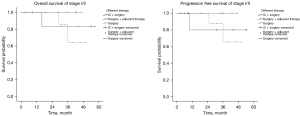
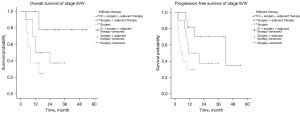
Table 3
| Survival rates | Stage I/II (n=29) | Stage III/IV (n=33) | |||||
|---|---|---|---|---|---|---|---|
| Surgery (n=12) | IC + surgery (n=7) | Surgery + adjuvant therapy (n=10) | Surgery (n=12) | IC + surgery + adjuvant therapy (n=11) | Surgery + adjuvant therapy (n=10) | ||
| 1-year OS | 100% | 83% | 100% | 23% | 78% | 48% | |
| 2-year OS | 85% | 83% | 100% | – | 78% | 32% | |
| 3-year OS | 56% | 83% | 100% | – | 78% | 32% | |
| OS (P) | 0.447 | 0.021 (HR: 2.317, 95% CI: 1.133–4.741) | |||||
| PFS (P) | 0.504 | 0.020 (HR: 2.392, 95% CI: 1.150–4.973) | |||||
IC, induction chemotherapy; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; P, P value of different treatment groups.
Discussion
Because of the significant difference in survival among patients with different degrees of differentiation of SCC, research into poorly differentiated HNSCC is important clinically for improving the prognosis of patients through individualized treatment.
According to the degree of keratinization of tumor cells and the overall similarity between cancerous and normal squamous epithelium, tumors can be divided into three grades: well differentiated, medium differentiated and poorly differentiated. Well-differentiated tumor cells are similar to normal squamous cells, whereas poorly differentiated tumor cells are mainly immature cells with more mitotic figures, fewer interstitial bridges, and fewer or no keratinizing beads. Determination of cytokeratin, p63 and epithelial membrane protein is helpful to determine the pathology and degree of differentiation of laryngeal SCC (4). When the type of disease is confirmed pathologically, further knowledge of the pathological subtype is conducive to formulation of the treatment plan.
Despite multidisciplinary treatment, the 5-year survival rate has not improved significantly in the past 20 years, and is still only 40–50%, even though the quality of life of patients has continuously improved (5,6). Patients with poorly differentiated HNSCC showed significant differences in disease characteristics and survival compared with those with well-differentiated grades. In the past, there were few studies on poorly differentiated squamous cell carcinoma of head and neck. A study on 57 cases of poorly differentiated laryngeal carcinoma in China confirmed that the OS rate decreased with the increase of stage, and concluded that for patients without lymph node metastasis, there was no significant difference in survival between surgery and surgery with postoperative adjuvant radiotherapy (7). But patients with advanced stage should be combined with adjuvant radiotherapy, which is also consistent with the conclusion of tour study (7). However, this study is a retrospective cohort study, which may has a selection bias. Similarly, the study on the population of poorly differentiated oral and oropharyngeal cancer also found that the disease stage, lymph node metastasis and location of disease were the risk factors affecting the survival rate of poorly differentiated tumors, but the study showed that the 5-year OS of patients with different treatment methods was similar and maintained at about 60% (P=0.907) (8). However, the study was also a retrospective analysis.
In our previous study, we found that the rate of positive surgical margins in patients with moderately and poorly differentiated laryngeal cancer (22.2%) was higher than in patients with well-differentiated laryngeal cancer (3.1%), and the difference between groups was statistically significant (P<0.01) (9), which indicates that poorly differentiated laryngeal SCC is highly invasive. We further matched 55 patients with poorly differentiated and 55 patients with well-differentiated glottic SCC (10), and found that the 5-year OS of the well-differentiated group and the poorly differentiated group were 90.2% and 50.9%, respectively, with a difference of nearly 30%. Moreover, the disease-specific survival (DSS) and disease-free survival (DFS) of patients in the well-differentiated group were significantly higher than those in the poorly differentiated group (10). Our center carried out a preliminary analysis of 93 patients with poorly differentiated hypopharyngeal cancer and the results also confirmed that OS (P=0.004), DSS (P=0.024) and DFS (P=0.019) of patients with poorly differentiated cancer were significantly lower than those of the patients with well-differentiated cancer (unpublished data). Therefore, independent study and analysis of this special group with poorly differentiated laryngeal SCC is desirable for the formulation of treatment plans for the different degrees of differentiation.
In 2019, we published a review of past medical records and clinical observations on poorly differentiated laryngeal SCC in the Chinese population (11). In it, we hypothesized that patients with early-stage poorly differentiated cancer should be given relatively active comprehensive treatment of combination radiotherapy and chemotherapy after surgery to improve the control rate and OS of the patients. Patients with advanced stage disease should be given systemic comprehensive treatment such as early intervention as adjuvant chemotherapy. Therefore, according to the different disease stages, we divided the study patients into I/II and III/IV stages, and different treatment groups. We found that for early-stage I/II patients, there was no significant difference in OS among the surgery, IC + surgery, and surgery + adjuvant therapy groups, but the long-term survival benefit in the group with surgery + postoperative adjuvant therapy was better than that of the other two treatment groups, with 3-year OS of 100%, 83% and 56%, respectively. This was consistent with our previous hypothesis.
Earlier a study showed that poorly differentiated disease was an independent factor for good prognosis of radiotherapy in patients with nasopharyngeal carcinoma (12), suggesting that poorly differentiated SCC is more sensitive to radiotherapy than the well-differentiated subtype. In the present study, for patients with advanced stage III/IV cancer, the incidence of DS differed among the treatment groups, and the OS and PFS were also significantly different. The IC + surgery + adjuvant therapy group had the lowest incidence of DS, followed by the surgery + adjuvant therapy group. Based on the 1-, 2-, and 3-year OS of each group, we observed that the IC + surgery + adjuvant therapy group had a significant advantage in OS compared with the other two treatments, which was also consistent with our previous hypothesis and the previous literature (13).
Some scholars have suggested that patients with poorly differentiated laryngeal cancer may be more sensitive to chemotherapy. The possible mechanism is that cytotoxic drugs are most effective in the cells undergoing active division, which is more common in poorly differentiated tumors, suggesting that the degree of tissue differentiation may also be an important factor in determining the sensitivity of chemotherapy in patients with laryngeal cancer. Therefore, for patients with advanced disease, IC should be considered to improve the patient's condition and prognosis (14).
The differentiation grading system is considered to be an important prognostic tool for HNSCC. Some studies on oral cancer (15,16) have reported that poorly differentiated tumors have a higher recurrence rate and shorter survival time than well-differentiated and moderately differentiated tumors, but the prognostic benefits of this classification system are still controversial (17). There are few studies on poorly differentiated SCC of the larynx and pharynx. The results of the prospective trials carried out in this study will assist in determining the treatment of poorly differentiated SCC. Under the current medical model of individualized and hierarchical treatment, clinicians should explore better treatment schemes for poorly differentiated HNSCC to guide individualized precise treatment and comprehensively predict the prognosis of patients.
Conclusions
For patients with early poorly differentiated HNSCC, postoperative radiotherapy-based comprehensive treatment can improve the OS of patients. For patients with advanced disease, early intervention as adjuvant chemotherapy should be performed. Preoperative adjuvant chemotherapy combined with surgery and postoperative adjuvant radiotherapy should be carried out to control DS and improve the OS of patients. However, due to the relatively low incidence rate of poorly differentiated, our study draws the above conclusions based on small sample data. Our recommendations for this treatment model need to be verified by clinical trials using more patients’ data.
Acknowledgments
Funding: The study was supported by National Natural Science Foundation of China (No. 82071032) and National Natural Science Foundation of China (No. 82072997).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2630/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2630/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2630/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (No. trecky2015-026/trecky2016-025). All patients were informed of the purpose of the study and signed informed consent before treatment. All information will be stored securely. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008;83:489-501. [Crossref] [PubMed]
- Wiernik G, Millard PR, Haybittle JL. The predictive value of histological classification into degrees of differentiation of squamous cell carcinoma of the larynx and hypopharynx compared with the survival of patients. Histopathology 1991;19:411-7. [Crossref] [PubMed]
- Fletcher CDM. Diagnostic Histopathology of Tumors. New York: Churchill Livingstone, 1995.
- Patel V, Leethanakul C, Gutkind JS. New approaches to the understanding of the molecular basis of oral cancer. Crit Rev Oral Biol Med 2001;12:55-63. [Crossref] [PubMed]
- Rodrigo JP, Suárez C, Ferlito A, et al. Potential molecular prognostic markers for lymph node metastasis in head and neck squamous cell carcinoma. Acta Otolaryngol 2003;123:100-5. [Crossref] [PubMed]
- Liu WS, Tang PZ, Qi YF, et al. Clinical analysis of 57 patients with poorly differentiated carcinomas of the supraglottic larynx. Zhonghua Er Bi Yan Hou Ke Za Zhi 2004;39:562-5. [PubMed]
- Feng Z, Xu QS, Wang C, et al. Clinicopathological features, management and outcome of patients with poorly-differentiated oral and oropharyngeal squamous cell carcinoma. J Craniomaxillofac Surg 2017;45:1478-85. [Crossref] [PubMed]
- Huang Z, Han D, Wang Q, et al. Security of surgical excision of CO2 laser in the treatment for glottic carcinoma. Chin Arch Otolaryngol Head Neck Surg 2004;11:73-6.
- Chen P, Yu W, Huang J, et al. Matched-pair analysis of survival in patients with poorly differentiated versus well-differentiated glottic squamous cell carcinoma. Oncotarget 2017;8:14770-6. [Crossref] [PubMed]
- Huang ZG. Several issues and prospects of poorly differentiated laryngeal squamous cell carcinoma in Chinese population. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019;54:321-4. [PubMed]
- Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the nasopharynx--an analysis of treatment results in 149 consecutive patients. Acta Oncol 2001;40:801-9. [Crossref] [PubMed]
- Ackall FY, Issa K, Barak I, et al. Survival Outcomes in Sinonasal Poorly Differentiated Squamous Cell Carcinoma. Laryngoscope 2021;131:E1040-8. [Crossref] [PubMed]
- Coca-Pelaz A, Rodrigo JP, Suárez C. Clinicopathologic analysis and predictive factors for distant metastases in patients with head and neck squamous cell carcinomas. Head Neck 2012;34:771-5. [Crossref] [PubMed]
- Rodrigues RM, Bernardo VG, Da Silva SD, et al. How pathological criteria can impact prognosis of tongue and floor of the mouth squamous cell carcinoma. J Appl Oral Sci 2020;28:e20190198. [Crossref] [PubMed]
- Lindenblatt Rde C, Martinez GL, Silva LE, et al. Oral squamous cell carcinoma grading systems--analysis of the best survival predictor. J Oral Pathol Med 2012;41:34-9. [Crossref] [PubMed]
- Boxberg M, Jesinghaus M, Dorfner C, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: proposal for an adjusted grading system. Histopathology 2017;70:1125-37. [Crossref] [PubMed]
(English Language Editor: K. Brown)



