
A case report of the metagenomics next-generation sequencing for early detection of central nervous system mucormycosis with successful rescue in patient with recurrent chronic lymphocytic leukemia
Introduction
Relevant studies have shown that the annual incidence of mucormycosis is about 3.3 cases per 100,000 hospital admissions (1). The incidence of central nervous system (CNS) mucormycosis infection accounted for less than 0.04% of immunocompromised patients with central infection, which is extremely rare and fatal, with a mortality rate of 80% (2-4). Contemporary datas show that the incidence of CNS mucormycosis is increasing (5). Thus, clinicians should be aware of the potential infections in immuno-impaired patients. The diagnosis of mucormycosis depends on histopathology and identification of microorganisms in tissue culture (6). In emergency situations, gold standard diagnostic methods in practical clinical application have limited value in improving prognosis. Cornely’s study showed that rapid diagnosis and treatment of mucormycosis is critical for patient survival, with the risk of death doubling within a week due to delayed diagnosis and treatment (3).
Herein, we report a case in which a recurrent chronic lymphocytic leukemia (CLL) patient with CNS mucormycosis was successfully treated. Most patients with CLL have been shown to have defective humoral and cell-mediated immunity, and is more evident in progressive phase (7). When new clinical problems arise in CLL patients, it is important to identify multiple factors. Firstly, we need to rule out disease progression, and identify the presence of other conditions, such as invasive fungal infections that occur during ibrutinib treatment (8). Nervous system infections are uncommon, but they will develop insidiously during the application of ibrutinib and progress rapidly. In the face of difficulty obtaining pathological results, evidence of mucormycosis can be obtained using pathogenic microorganism DNA/RNA high-throughput genetic sequencing (PMseq) of cerebrospinal fluid (CSF).
In the case reported here, in response to the pathological findings, we gave the patient amphotericin B cholesterol sulfate complex and posaconazole immediately for anti-infection, and interventional surgical treatment was performed to save the patient’s life. Furthermore, CLL was kept in an ideal remission state by applying the next-generation Bruton tyrosine kinase (BTK) inhibitor zanubrutinib. For CNS mucormycosis infection, we need the early introduction of effective drugs (like liposomal amphotericin B), appropriate surgical intervention, thus we are able to save patients’ lives without delay. This case demonstrates successful treatment of hematologic malignancy complicated with CNS mucormycosis infection, which is extremely deadly. We can obtain the risk factors associated with invasive disease in patients receiving ibrutinib therapy through this case. And we also use new methods for rapid diagnosis of the disease, which was difficult to diagnose in the past, directly improving the prognosis of the patient. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2533/rc).
Case presentation
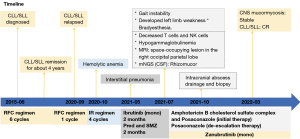
The patient was a 52-year-old man with progressive lymph node enlargement and onset of fatigue. An ultrasound showed enlargement of the superficial lymph nodes. The maximum diameter of the left cervical lymph node was 4 cm. The spleen size was 80 mm × 211 mm × 91 mm. He was diagnosed with CLL/small lymphocytic leukemia (SLL) after left cervical lymph node biopsy in June 2015. Flow immunophenotype of the peripheral blood showed CD79b (dim), CD20 (dim), CD5 (+), CD10 (−), CD23 (+), FMC7 (−), CD200 (+), CD43 (+), and immunoglobulin light chain restricted expression. Fluorescence in situ hybridization (FISH) showed ATM (−), RB1 (−), CSP12 (−), D13S25 (−), and TP53 (−). The results of NGS of the genome showed mutated IGHV, SF3B1, and MYC. Assessment of the disease stage showed that it was Rai stage IIb and Binet stage B. The CLL International Prognostic Index score was 3 points, indicating moderate risk. At that time, the rituximab plus fludarabine and cyclophosphamide (RFC) regimen with standard doses [rituximab 500 mg/m2 d0 + fludarabine 25 mg/m2 d1–d3 + cyclophosphamide (CTX) 250 mg/m2 d1–d3] was administered as treatment for a total of 6 cycles. The disease state was later evaluated as complete remission, and the patient was regularly followed up in the outpatient department for 4 years.
In September 2020, the patient had left cervical lymph node enlargement again, and the lymph nodes doubled in size within a month. Lymph node biopsy was led to the pathological diagnosis of CLL, and recurrence was thus considered. The RFC regimen was administered again. Due to the presence of hemolytic anemia during the treatment with fludarabine, the drug effect was considered, so the treatment program was changed to ibrutinib + rituximab (IR) (ibrutinib 420 mg qd plus rituximab 500 mg/m2 d1, 28 d/cycle) in October 2020. After 4 cycles of the IR regimen, rituximab was terminated due to interstitial pneumonia. Prednisone and sulfamethoxazole (SMZ) maintenance therapy was followed for about 2 months, as recommended by a pulmonologist. The patient sustained remission with continued ibrutinib monotherapy, with the dose of 420 mg once daily. In mid-July 2021, the patient developed gait instability without obvious causes but did not have fever, disturbance of consciousness, headache, dizziness, purulent nasal discharge, nausea, vomiting, joint swelling or pain, deviated mouth opening, or drooling. He did not give much attention to this new symptom and no diagnosis or treatment was sought. In late July, he developed a feeling of fullness in the head originating in the occipital region and had a fever that reached 38 ℃. However, the patient was not accompanied by any other uncomfortable symptoms. Complete blood count after admission showed that the white blood cell count was 5.92×109/L (3.97×109–9.15×109/L) and the neutrophil cell count was 4.76×109/L (2.00×109–7.00×109/L). The (1,3)-beta-d-glucan test and the galactomannan test were both unremarkable, and procalcitonin was <0.02 ng/mL. The lymphocyte subsets of the patient were decreased T cells and natural killer (NK) cells; CD4/CD8: 0.2 (1.0–2.5), the CD4 absolute count was 90/µL (384–1,346/µL), CD4/CD45RA: 0.6% (15.0–25.0%), and the patient presented with hypogammaglobulinemia, immunoglobulin G (IgG): 7.62 g/L (8.6–17.4 g/L).
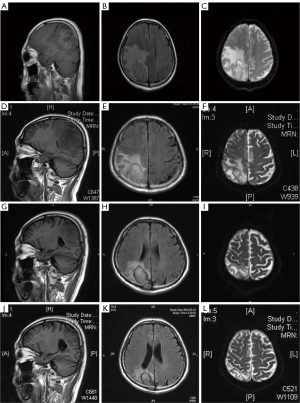
The cranial magnetic resonance imaging (MRI) non-contrast scan results showed a space-occupying lesion in the right occipital parietal lobe with substantial edema and ischemic foci in the left frontal lobe and bilateral centrum semiovale. The contrast-enhanced cranial MR results (3 August 2021) revealed a space-occupying lesion in the right occipital parietal lobe with large edema. Based on the patient’s medical history and imaging, it was suspected that the lesion was a metastatic tumor (Figure 1). Mannitol, glycerol fructose, and furosemide dehydration along with intracranial pressure reduction were administered for treatment, but the symptoms became further aggravated. Specifically, the symptom of gait instability deteriorated. The patient developed left limb weakness and brady esthesia. The results of lumbar puncture on 6 August 2021 showed that the intracranial pressure was higher than 300 cmH2O (80–180 cmH2O). The CSF analysis showed that the CSF was clear and colorless, Pandy’s test was 1+, red blood cell count was 1×106/L, and white blood cell count was <1.0×106/L. The CSF biochemistry showed the following: glucose level: 4.0 mmol/L (2.2–3.9 mmol/L), chloride level: 108 mmol/L (118–132 mmol/L), and protein level: 1,733 mg/L (<500 mg/L).
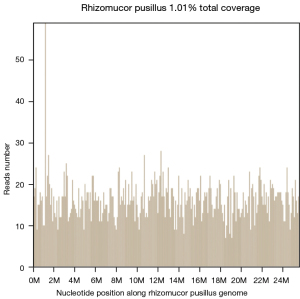
The fungal fluorescent staining smear showed that no fungi was detected in the direct smear. No acid-fast bacilli were detected in the acid-fast bacillus smear. The PMseq report for CSF, which can achieve a broader range of pathogen detection by metagenomic next-generation sequencing (mNGS) (9) suggested the presence of Rhizomucor (number of detected sequences: 3,842) and Rhizomucor pusillus (number of detected sequences: 3,771). Sample sequencing data and theoretical sensitivity to the pathogen showed that the total number of detected sequences was 43,797,028 and that the theoretical sensitivity (copies/mL) to the fungi (100 MB) was 1.00E+00 (Figure 2). Combined with these results, we considered that the patient had CNS mucormycosis.
During this period, the symptoms of the patient were continuously aggravated, and he developed drowsiness and decreased muscle strength in the left arm and leg. Amphotericin B cholesterol sulfate complex combined with posaconazole treatment was started on 11 August 2021 (the dose of the amphotericin B cholesterol sulfate complex was gradually increased to 3 mg/kg/d; the initial dose of posaconazole was 600 mg and was later reduced to 300 mg qd for descending step therapy). On the second day of treatment, the patient regained consciousness and experienced defervescence of fevers. The muscle strength of his left side also continuously improved. The muscle strength recovered to grade 4 after approximately 1 week of medication and completely returned to normal after approximately 2 weeks. Examination of the disease lesion on 31 August by cranial MR showed that it had improved (Figure 1). Later, the patient’s symptoms improved, his mental state improved, and his muscle strength completely returned to normal. Therefore, the treatment for CNS mucormycosis was effective.
To further clear the lesion, on 14 October 2021, intracranial abscess drainage and biopsy were performed under intravenous general anesthesia. The conclusion of pathology was that the right parietal region staining results were consistent with mucormycosis. Biopsy did not yield any evidence of lymphoma. The special staining results showed both periodie acid Schiff and silver stain were positive. Examination using contrast-enhanced cranial MRI on 15 November 2021 showed that the lesion had reduced (Figure 1). Currently, de-escalation therapy with oral posaconazole is underway. A re-examination of the head MRI in March 2022 showed improvement in the infected lesions (Figure 1).
The patient had moderate-risk CLL combined with hemolytic anemia. Zanubrutinib has greater selectivity in relative inhibition of BTK, and its safety and efficacy have been confirmed (10). Therefore, zanubrutinib was chosen for controlling the primary hematologic tumor and was applied together with triazole drugs. The oral dose of zanubrutinib was adjusted to 80 mg bid. As of this writing, the hematologic tumor is stable. On November 18, CLL was evaluated; hematology suggested peripheral blood measurable residual disease was negative and the disease was currently in complete remission. Figure 3 shows the diagnosis and treatment administered in this case.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Discussion among physicians from Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine at Shanghai, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine
We conducted intradisciplinary and multidisciplinary consultation of this case. As CNS mucormycosis is rare and deadly, early diagnosis and treatment are important for improving the prognosis of patients. Patients with recurrent CLL drenching after multiple line treatments, they are usually immunocompromised. During the application of BTK inhibitors, we need to monitor immune function and associated clinical symptoms, which will help us recognize underlying fungal and other related infections. For symptomatic, highly suspected patients with infection, new diagnostic methods can be used to identify them, appropriately with early drug intervention. Although prophylactic fungal therapy is not routinely recommended during ibrutinib treatment in CLL patients, we need to monitor indicators of immune function in these patients. If the patient is complicated with hypogammaglobulinemia, whose acquired humoral immunodeficiency should be corrected and improved. However, the underlying causes and new methods for early diagnosis and treatment should be further investigated.
Department of Epidemiology
Rhizopus is a thermophilic fungus of the order Trichoderma (2). Humans can be infected with trichoderma and rhizopus. Rare infections are caused by Trichoderma lhamia and Absidia (reclassified as Lichtheimia, Saksenaea, and Apophysomyces) (11-14). Mucormycosis infection is a relatively rare cause of human infection, with an overall mortality of approximately 62% (2), of which mucormycosis infection of the CNS is rare and fatal (15). In China, the understanding of mucor infection is somewhat limited, especially the central mucor infection, its related mortality has not been reported.
An international epidemiological survey running from January 2010 to January 2017 showed that in addition to diabetes, hematologic tumors were the second most common factors for mucormycosis infection, accounting for 32%. Especially when patients receive tumor chemotherapy and immunotherapy, the incidence of immunosuppression will be higher (16).
Our patient was a refractory CLL treated with ibrutinib. The peripheral blood cell analysis of the patient at the time of infection showed that he did not have neutropenia. The lymphocyte subsets of the patient were decreased T cells and NK cells, and he also had hypogammaglobulinemia, which displayed high-risk factors of mucormycosis infection. After controlled mucormycosis infection, the patient was given intravenous immunoglobulins (IVIG) at 1 g/kg per month (for 1 week) to correct the humoral immunity.
Department of Infectious Diseases
There are many host factors that may contribute to invasive mycosis, among which therapy-related immunosuppression that identifies B cells is also a common cause, such as the BTK inhibitor ibrutinib (8). Previous cases of mucormycosis infection during BTK inhibitor use have shown that infection can involve various sites (Table 1) (17-23). Common infection sites are the lungs and skin. The site of infection reported in our case is a rare infection of the CNS.
Table 1
| Clinical features | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Age, years | 52 | 72 | 71 | 72 | 70 | 68 | 67 |
| Gender | Female | Male | Male | Male | Male | Male | Male |
| Malignancy | CLL | CLL | CLL | CLL | CLL | CLL | CLL |
| Methods of diagnosis | Biopsy | Biopsy | Autopsy | Biopsy | Autopsy | Biopsy | Biopsy |
| Site of mucormycosis involvement | Renal | Cutaneous | Brain, lung, kidney, pancreas | Thyroid | Liver | Cutaneous | Lung |
| Outcome | Survival | Survival | Died | Survival | Died | Survival | Died |
| Active malignancy treatment | Ibrutinib | Ibrutinib | Ibrutinib | Ibrutinib | Ibrutinib | Ibrutinib | Ibrutinib |
CLL, chronic lymphocytic leukemia.
The onset of CNS mucormycosis is usually insidious. Once related infection symptoms are present, the disease is invasive and life-threatening. Therefore, early diagnosis is beneficial to improve prognosis. Fungal culture and tissue biopsy are the gold standards for the diagnosis of mucormycosis. The global guideline for the diagnosis of mucormycosis published by the European Confederation of Medical Mycology (ECMM) (3) in 2019 describes the diagnosis of mucormycosis using a combination of tissue culture, molecular technology, and in situ hybridization as well as species identification and drug susceptibility testing. The recommended methods for genus and species identification are ITS sequencing and matrix-assisted laser desorption ionization time of flight (MALDI-TOF). These two types of genus and species identification have certain limitations. Especially for high-risk clinical patients, these methods have low diagnostic rates and are time-consuming (24,25). Fehr et al. (17) reported the case of a 71-year-old patient with CLL who developed CNS mucormycosis and died rapidly during treatment with irutinib. It was finally identified as mucormycosis by polymerase chain reaction (PCR) after autopsy, which delayed medical treatment (17). The PCR is a hypothesis-driven molecular testing method that can involve numerous individual tests for specifically targeted organisms but may still miss a rare pathogen or use primers containing mismatches to the microbial strain involved, which decreases the sensitivity of detection. However, mNGS is an unbiased diagnostic approach that has the potential to detect nearly any organism, which could lead to a dramatic paradigm shift in microbial diagnostic testing (26).
In our case, we sent the CSF of the current patient for PMseq. The report was available after 48 hours and showed the presence of Rhizomucor pusillus, which provided a basis for early medication. The consensus is that CSF mNGS is more available than traditional gold standard methods. At the same time, it has advantages in the identification of meningitis pathogens, especially in species identification, and is recommended for the diagnosis of infectious CNS diseases (27).
Department of Neurology
The disease presentation of our patient was CNS infection. The onset was insidious, but the progression was rapid. Hence, rapid diagnosis and treatment were required to improve his prognosis. Although DNA sequencing that can directly localize the infected region in the tissue is a promising approach, the method has high requirements for laboratory equipment (28). In clinical practice, sometimes there are difficulties obtaining histopathological tissue. At this time, CSF mNGS is recommended as the second-line detection method.
The reporting of the use of NGS in CNS infections is currently mainly in the form of case reports, there are few large-scale studies to refer to. In previous studies on pathogen NGS in CSF, the diagnostic rate was not high, partly because only acellular virus or acellular microbial nucleic acid could be detected in CSF supernatant, and the detection rate was low (29-33). Therefore, CSF should be correctly used as sequencing specimens in clinic to improve the detection rate (34). A study by Miller et al. (35) showed that NGS had a sensitivity of 73%, specificity of 99%, positive predictive value of 81%, and negative predictive value of 99% compared to traditional clinical laboratory results.
Our case was successfully diagnosed by NGS, for which relevant international reports are sporadic. The efficacy and clinical value need to be further explored. Clinical trials with larger sample sizes are still needed to verify the efficacy of NGS for fungal infections of the CNS, and the results should be interpreted and verified in close combination with patient clinical manifestations and laboratory test results.
Determining the etiology of fungal infections can be expedited through a combination of methods such as NGS to achieve an early diagnosis, start effective and systemic antifungal treatment sooner, and treat the underlying diseases. Early and effective disease control can improve the prognosis. During treatment, we can monitor the condition of disease control through the number of sequences found in the CSF. The titer reduction can also reflect the disease condition to a certain extent. Due to the high sensitivity of metagenomics, metagenomic testing results can show the status of disease control and may guide drug withdrawal after the clinical symptoms of the patient improve and imaging results become negative. If the patient requires long-term application of immunosuppressants or the disease progression necessitates further treatment such as cell-mediated immunity, metagenomics can be applied to monitor the activity of mucormycosis.
Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- For patients with recurrent and refractory CLL, which methods can best monitor their immune function during treatment?
Goyal Gaurav: Typically, only through complete blood count (CBC) differential and sometimes checking serum immunoglobulins. However, it hardly changes management.
Fabiana Perna & Manuel Espinoza-Gutarra: Cellular immune function is best monitored through standard CBC, as neutropenia and lymphopenia have been associated with an increased risk of infection, particularly in patients receiving traditional chemotherapy (36). Regarding humoral immunity, low immunoglobulin levels, specifically IgG have been associated with infection risk (37). - Are there any means to prevent latent fungal infection in patients with recurrent and refractory CLL during treatment?
Goyal Gaurav: Currently, it is not recommended to use prophylactic antifungals unless there is neutropenia.
Fabiana Perna & Manuel Espinoza-Gutarra: Currently guidelines do not recommend prophylactic use of antimicrobials or antifungals in CLL patients undergoing treatment, with a few exceptions: patients with prior history of significant infection requiring Intravenous antibiotics and an IgG level <500 mg/dL have decreased rates of bacterial infections when treated with intravenous IVIG to a target level of 500–700 mg/dL, this data has sometimes been extrapolated to fungal infections; however, no trial has specifically studied this (38). Prophylactic antifungals are recommended when patients receive alemtuzumab-based treatment; however, given newer therapeutic options in CLL, this drug is rarely, if ever, used (39). Finally, the use of myeloid-derived growth factors (G-CSF, GM-CSF) in neutropenic CLL patients has been shown to decrease the rate of infections, particularly for regimens where the risk of neutropenia is greater or equal to 20% (40). - How long should the treatment of CNS mucormycosis be maintained? Could mNGS be used to monitor subsequent mucormycosis activity in patients and determine when to stop?
Goyal Gaurav: This question would be best addressed to infectious disease. I have never seen mNGS used at our center before.
Fabiana Perna & Manuel Espinoza-Gutarra: There are no clear guidelines on when to stop antifungal therapy in this setting, a generally accepted approach is to begin to de-escalate therapy once clinical recovery and radiographic resolution of infection takes place, which often takes several weeks (41). There is currently no evidence that mNGS can be used to determine duration of therapy in invasive fungal infections, although its usefulness is certainly intriguing and should be investigated in clinical trials. - If the patient needs further treatment for CLL progression, should antifungal therapy be used prophylactically?
Goyal Gaurav: That would be an open question, and would have to be discussed with infectious disease. There is no clear recommendation on this.
Fabiana Perna & Manuel Espinoza-Gutarra: Given the life-threatening nature of invasive fungal CNS infections and the potential for recurrence, even at later stages of treatment, long term prophylactic therapy with a mold active agent is recommended (42), particularly in patients with a chronic condition that will require long-term treatment such as CLL.
Acknowledgments
The authors appreciate the academic support from the AME Hematology Collaborative Group.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 82070227 and Antrag M-0377).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2533/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2533/coif). FP received grants/contracts from Lonza, Leukemia Research foundation, Indiana University School of Medicine (payments were made to her institution), and received consulting fees from NGMBio. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pagano L, Valentini CG, Posteraro B, et al. Zygomycosis in Italy: a survey of FIMUA-ECMM. J Chemother 2009;21:322-9. [Crossref] [PubMed]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53. [Crossref] [PubMed]
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019;19:e405-21. [Crossref] [PubMed]
- Sonneville R, Magalhaes E, Meyfroidt G. Central nervous system infections in immunocompromised patients. Curr Opin Crit Care 2017;23:128-33. [Crossref] [PubMed]
- Candoni A, Klimko N, Busca A, et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019;62:252-60. [Crossref] [PubMed]
- Siegal T, Levin N. Infectious, metabolic, and endocrine complications. Handb Clin Neurol 2012;105:825-51. [Crossref] [PubMed]
- Palma M, Mulder TA, Österborg A. BTK Inhibitors in Chronic Lymphocytic Leukemia: Biological Activity and Immune Effects. Front Immunol 2021;12:686768. [Crossref] [PubMed]
- Donnelly JP, Chen SC, Kauffman CARevision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium, et al. Clin Infect Dis 2020;71:1367-76. [Crossref] [PubMed]
- The Editorial Committe of Chinese Journal of Infectious Diseases. Clinical practice expert consensus for the application of metagenomic next generation sequencing. Chin J Infect Dis 2020;38:681-9.
- Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol 2020;16:517-23. [Crossref] [PubMed]
- Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis 2012;54:S8-15. [Crossref] [PubMed]
- Spellberg B, Walsh TJ, Kontoyiannis DP, et al. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis 2009;48:1743-51. [Crossref] [PubMed]
- Hibbett DS, Binder M, Bischoff JF, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res 2007;111:509-47. [Crossref] [PubMed]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53. [Crossref] [PubMed]
- Riley TT, Muzny CA, Swiatlo E, et al. Breaking the Mold: A Review of Mucormycosis and Current Pharmacological Treatment Options. Ann Pharmacother 2016;50:747-57. [Crossref] [PubMed]
- Paul SR, Gable PS. Mucormycosis as the Elusive Cause of an Aortic Thrombus and Tissue-Obliterating Abscess. Case Rep Hematol 2019;2019:4842150. [Crossref] [PubMed]
- Fehr M, Cathomas G, Graber A, et al. Multi-fungal sepsis and mucormycosis of the central nervous system in a patient treated with ibrutinib, a case report and review of the literature. Med Mycol Case Rep 2020;27:14-6. [Crossref] [PubMed]
- Pouvaret A, Guery R, Montillet M, et al. Concurrent cerebral aspergillosis and abdominal mucormycosis during ibrutinib therapy for chronic lymphocytic leukaemia. Clin Microbiol Infect 2019;25:771-3. [Crossref] [PubMed]
- Sittig KR, Laageide LG, Akhtar Z, et al. Cutaneous mucormycosis in a chronic lymphocytic leukemia patient on ibrutinib. IDCases 2021;24:e01120. [Crossref] [PubMed]
- Mascarella MA, Schweitzer L, Alreefi M, et al. The infectious thyroid nodule: a case report of mucormycosis associated with ibrutinib therapy. J Otolaryngol Head Neck Surg 2019;48:49. [Crossref] [PubMed]
- Grossi O, Pineau S, Sadot-Lebouvier S, et al. Disseminated mucormycosis due to Lichtheimia corymbifera during ibrutinib treatment for relapsed chronic lymphocytic leukaemia: a case report. Clin Microbiol Infect 2019;25:261-3. [Crossref] [PubMed]
- Stein MK, Karri S, Reynolds J, et al. Cutaneous Mucormycosis Following a Bullous Pemphigoid Flare in a Chronic Lymphocytic Leukemia Patient on Ibrutinib. World J Oncol 2018;9:62-5. [Crossref] [PubMed]
- Kreiniz N, Bejar J, Polliack A, et al. Severe pneumonia associated with ibrutinib monotherapy for CLL and lymphoma. Hematol Oncol 2018;36:349-54. [Crossref] [PubMed]
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54:S23-34. [Crossref] [PubMed]
- Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20:5-26. [Crossref] [PubMed]
- Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol 2019;14:319-38. [Crossref] [PubMed]
- Xing XW, Zhang JT, Ma YB, et al. Metagenomic Next-Generation Sequencing for Diagnosis of Infectious Encephalitis and Meningitis: A Large, Prospective Case Series of 213 Patients. Front Cell Infect Microbiol 2020;10:88. [Crossref] [PubMed]
- Iwen PC, Freifeld AG, Sigler L, et al. Molecular identification of Rhizomucor pusillus as a cause of sinus-orbital zygomycosis in a patient with acute myelogenous leukemia. J Clin Microbiol 2005;43:5819-21. [Crossref] [PubMed]
- Ambrose HE, Granerod J, Clewley JP, et al. Diagnostic strategy used to establish etiologies of encephalitis in a prospective cohort of patients in England. J Clin Microbiol 2011;49:3576-83. [Crossref] [PubMed]
- Tan le V. Identification of a new cyclovirus in cerebrospinal fluid of patients with acute central nervous system infections. mBio 2013;4:e00231-13. [Crossref] [PubMed]
- Phan TG, Mori D, Deng X, et al. Small circular single stranded DNA viral genomes in unexplained cases of human encephalitis, diarrhea, and in untreated sewage. Virology 2015;482:98-104. [Crossref] [PubMed]
- Kawada J, Okuno Y, Torii Y, et al. Identification of Viruses in Cases of Pediatric Acute Encephalitis and Encephalopathy Using Next-Generation Sequencing. Sci Rep 2016;6:33452. [Crossref] [PubMed]
- Salzberg SL, Breitwieser FP, Kumar A, et al. Next-generation sequencing in neuropathologic diagnosis of infections of the nervous system. Neurol Neuroimmunol Neuroinflamm 2016;3:e251. [Crossref] [PubMed]
- Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect 2018;76:225-40. [Crossref] [PubMed]
- Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res 2019;29:831-42. [Crossref] [PubMed]
- Tsiodras S, Samonis G, Keating MJ, et al. Infection and immunity in chronic lymphocytic leukemia. Mayo Clin Proc 2000;75:1039-54. [Crossref] [PubMed]
- Molica S, Musto P, Chiurazzi F, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica 1996;81:121-6. [PubMed]
- Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N Engl J Med 1988;319:902-7. [Crossref] [PubMed]
- Roux C, Thyss A, Gari-Toussaint M. Prostatic and renal aspergillosis due to Aspergillus fumigatus in a patient receiving alemtuzumab for chronic lymphocytic leukaemia. J Mycol Med 2013;23:270-3. [Crossref] [PubMed]
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 2011;52:e56-93. [Crossref] [PubMed]
- Pagano L, Cornely OA, Busca A, et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: a report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013;98:e127-30. [Crossref] [PubMed]
- Kontoyiannis DP. Antifungal prophylaxis in hematopoietic stem cell transplant recipients: the unfinished tale of imperfect success. Bone Marrow Transplant 2011;46:165-73. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


