
WX-0593 combined with an epithelial growth factor receptor (EGFR) monoclonal antibody in the treatment of xenograft tumors carrying triple EGFR mutations
Introduction
A driver mutation on epithelial growth factor receptor (EGFR) is one of the most common oncogene mutations in non-small cell lung cancer (NSCLC), especially lung adenocarcinoma among Asian female patients (1,2). The studies showed significantly higher incidence of EGFR mutation that in NSCLC patients with adenocarcinomas, advanced stage, female, non-smoker, stages Ia–IIIa. Mutation (3,4). An analysis based on large-scale data from China showed that advanced NSCLC patients with EGFR mutations occur in approximately 35% of Asian patients, and 60% of patients with adenocarcinoma (5). Subtypes of EGFR mutations, such as exon 19 deletions and exon 21 L858R substitutions, etc., have been widely detected in NSCLC patients and show an impressive response to tyrosine kinase inhibitors (TKIs) targeting EGFR (6). There is a potent therapeutic efficacy of EGFR-TKIs against tumors with mutant EGFR, which has largely changed the treatment of NSCLC, becoming the most successful paradigm of targeted therapy (7-9). However, even with this outstanding therapeutic efficacy, resistance will always develop in tumors after treatment with EGFR-TKIs for a certain period of time (10,11). Among all of the resistance mechanisms identified, secondary mutations are the most common and are well known in clinical practice (12,13). The secondary mutation of T790M on EGFR is the most common mechanism mediating resistance to first-generation TKIs such as erlotinib and gefitinib. A recently developed third-generation EGFR-TKI (osimertinib) successfully overcame EGFR-TKI resistance driven by T790M (14-16), but recent studies have identified another resistance mutation on EGFR, C797S, that occurs after long-term treatment with osimertinib (17-20). Therefore, more effective targeted therapy is strongly sought for tumors that carry mutant EGFR with the secondary mutations T790M and C797S and are resistant to all of the current EGFR-TKIs.
A monoclonal antibody targeting EGFR has been applied as a later line of treatment for tumors with mutant EGFR that have established resistance to EGFR-TKIs (21-23). However, its therapeutic efficacy has always been modest and associated with some therapeutic side effects. Echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (ALK) is another targetable oncokinase that drives the development and progression of cancer; its fusion is commonly detected in NSCLC. Several TKIs have shown potent inhibition of tumor growth driven by ALK activation and have been approved for the clinical treatment of NSCLC with ALK rearrangement (24,25). ALK-TKIs have shown a certain therapeutic efficacy against tumors with an EGFR mutation and have been explored as an alternative treatment for tumors that have developed resistance against EGFR-TKIs (26-28). WX-0593 is a newly developed ALK inhibitor that has shown potent therapeutic efficacy in patients with lung cancer driven by ALK rearrangement, in preclinical studies the result have showed that it can effectively inhibited the activity of both wild type and resistant mutants of ALK in vitro and strong antitumor activity in a crizotinib-resistant in vivo (29,30); however, its effect on EGFR-mutant tumors with secondary mutations of T790M and C797S is unclear. Also, whether there is any synergistic effect between WX-0593 as an ALK inhibitor and a monoclonal antibody targeting EGFR in the treatment of tumors with mutant EGFR/T790M/C797S is yet to be clarified.
Utilizing two xenograft tumor models driven by mutant EGFR/T790M/C797S (NCI-H1975 and Ba/F3), we tested the therapeutic effect of WX-0593 and two EGFR monoclonal antibodies (QL1203 and Vectibix) as single agents and in combination in these tumor models. Compared with previous study the pharmacokinetics and safety profiles were also explored in this study. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2780/rc).
Methods
Cell culture
Five commercially available EGFR triple-mutant cell lines (Ba/F3 (EGFR L858R/T790M/C797S), Ba/F3 (EGFR Del19/T790M/C797S), NCI-H1975 (EGFR L858R/T790M/C797S), H1975 (EGFR Del19/T790M/C797S), and PC9 (EGFR Del19/T790M/C797S)) were used in the present study (Table S1). All cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (Gibco), 1% penicillin-streptomycin, and 10 µg/mL blasticidin, in a humidified incubator under 5% CO2 at 37 ℃.
Drug preparation
Three drugs were used in the present study: one ALK inhibitor (WX-0593) and two recombinant monoclonal antibodies targeting EGFR (QL1203 and Vectibix). WX-0593 was provided by Qilu Pharmaceutical Co., Ltd. (Lot no. T9001L51Ns) as a powder and kept away from light at 2–8 ℃. QL1203 (Lot no. 201905001KJB) and Vectibix (Lot no. 1100612/1092837) were obtained from Qilu Pharmaceutical Co., Ltd. and Amgen, and as a solution at a concentration of 20 mg/mL and kept away from light at 2–8 ℃. For the in vitro experiments, WX-0593 was dissolved in DMSO at a concentration of 2.0 mM. For in vivo administration, WX-0593 was first dissolved in alcohol and then mixed with PEG-40 castor oil at a ratio of 1:1, after which the mixture (5%) was diluted in 0.9% sodium chloride (95%) to obtain a working solution of 7.5 mg/mL. Both QL1203 and Vectibix were diluted in 0.9% sodium chloride to achieve a working concentration of 0.5 mg/mL. All drugs were prepared prior to use.
Drug-combination treatment in vitro
Ba/F3 (EGFR L858R/T790M/C797S) and Ba/F3 (EGFR Del19/T790M/C797S) were seeded in 96-well plates at a density of 5×104 cells/mL, with 50 µL/well. To each well was added 50 µL of 9 different concentrations of WX-0593 (6.25–1,600 nM in 2-fold serial dilutions) or Vectibix (0.011–2.67 nM in 2-fold serial dilutions) or WX-0593 combination with Vectibix (ratio of 600:1 with 1600 nM and 2.67 nM the highest concentrations in 2-fold serial dilutions). NCI-H1975 (EGFR L858R/T790M/C797S) and PC9 (EGFR Del19/T790M/C797S) were seeded in 96-well plates at a density of 4×104 cells/mL, with 75 µL/well. To each well was added 25 µL of 5 different concentrations of WX-0593 (125–2,000 nM in 2-fold serial dilutions) in combination with 25 µL of 8 different concentrations of Vectibix (0.0128–1,000 µg/mL in 2-fold serial dilutions). Cells were continuously cultured for 72 h, and cell viability was detected using the Cell Titer-Glo luminescent cell viability assay (Promega, USA) according to the manufacturer’s protocol. A synergistic effect was evaluated using the combination index (CI), which is the fractional sum of the inhibitory concentrations of the two drugs and calculated as CI = (D)1/(Dx)1 + (D)2/(Dx)2. (Dx)1 and (Dx)2 represented the concentrations of each drug alone to exert x% effect, and (D)1 and (D)2 were the concentrations of the drugs in combination to elicit the same effect.
Tumor growth: dose-effect studies
To develop the xenograft tumor model, tumor cells resuspended in 1:1 Matrigel/phosphate-buffered saline were subcutaneously injected into the right flank fat pads of nude mice (6–8-week-old female BALB/c nude mice, weighing ~18–22 g; purchased from Zhejiang Weitong Lihua Laboratory Animal Technology Co., Ltd.). A total of 1×105 early T-cell precursor tumor cells in a volume of 100 µL were injected into the right fat pad of each mouse. Tumor growth was monitored by measuring the tumor size with digital calipers every 3 days. The greatest longitudinal diameter (length) and the greatest transverse diameter (width) were measured to determine the tumor volume: 0.50 × length × width2.
Treatment was initiated when the average tumor volume reached 185 mm3. The mice were randomized into different groups (n=6) before treatment to ensure the average tumor volume was comparable across groups. The mice were treated with WX-0593, QL1203, and Vectibix as single agents or in combination. WX-0593 was administered by daily gavage at two dosages (25 or 75 mg/kg). Both QL1203 and Vectibix were administered as a weekly intravenous injection at a dosage of 0.1 mg/mouse. Two parameters [the tumor growth inhibition rate (TGI) and the relative tumor proliferation rate (T/C)] were used to evaluate the efficacy of each therapeutic regimen. TGI (%) was calculated as: [1 – (Tn – T0)/(Vn – V0)] × 100 (Tn, tumor volume of the treatment group at the nth day of treatment; T0, tumor volume of the treatment group right before the initiation of treatment; Vn, tumor volume of the control group at the nth day of treatment; V0, tumor volume of the control group at a certain time point after treatment). The relative tumor volume (RTV) was calculated as Vt/V0 (Vt, tumor volume at a certain time point after treatment; V0, tumor volume right before the initiation of treatment). T/C was calculated as T/C (%) = TRTV/CRTV × 100 (TRTV, RTV in the treatment group; CRTV, RTV in the control group).
Tumor xenograft model and monitoring of side effects
The mice were housed in sterile cages (3 per cage) in IVC (individually ventilated) laminar flow hoods housed in specific pathogen-free animal rooms with temperature 20–26 ℃, relative humidity 40–70% and a 12:12-h day–night light cycle. They had free access to autoclaved water and commercial mouse food. Baseline information included the number of animals per cage, sex, strain, date of receipt, dosing regimen, experiment number, group, and the date the experiment commenced. The health status of each mouse was monitored daily. Specifically, we monitored their appearance, physical activity, and water consumption by close observation. The body weight of each mouse was measured daily with electronic scales. Any abnormality was recorded. The mice were humanely killed by CO2 inhalation when the tumor volume met humane endpoints described in the Institutional Animal Care and Use Committee protocols (i.e., 20-mm diameter) or upon severe health deterioration. The formulation and any modification of this experimental protocol have been evaluated and approved by the laboratory animal management and use Committee (IACUC) of Wuxi Apptec (Shanghai) Co., Ltd. (No. ON01-003-2019v1.0). The use and welfare of laboratory animals shall comply with the provisions of the International Committee for the evaluation and Accreditation of laboratory animals (AAALAC).
Pharmacokinetic analysis
Blood samples were obtained from each mouse at several time points after the last dosage of drug for pharmacokinetic analysis: 1, 3, 8, 24, 72, 120, and 168 after the last dosage of drug for each treatment group; 72 and 168 h after the last dosage for the control group. The serum concentrations of each drug at the different time points were measured by Qilu Pharmacy Co., Ltd.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v8 or SPSS 17.0. Experiments with multiple comparisons of multiple data sets were analyzed by one-way analysis of variance before the Student-Newman-Keuls test or after Bonferroni’s test for samples with a normal distribution, or by the Kruskal-Wallis test for samples with a non-normal distribution. Comparisons between groups were performed using the unpaired Student’s t-test for parameters with a normal distribution and the Wilcoxon rank sum test for parameters with a non-normal distribution. A P value <0.05 was considered statistically significant.
Results
Synergistic effect of WX-0593 and Vectibix on the proliferation of EGFR triple-mutant cell lines
First, we investigated the effect of WX-0593 in combination with Vectibix on the proliferation of the four EGFR triple-mutant cell lines. WX-0593 + Vectibix exhibited an obvious synergistic effect on the inhibition of cell proliferation of the Ba/F3 (EGFR L858R/T790M/C797S) and Ba/F3 (EGFR Del19/T790M/C797S) cell lines (Figure 1). For Ba/F3 (EGFR L858R/T790M/C797S), the CI was 0.39, 0.35, 0.31, and 0.27 when the proliferation inhibition was 30%, 50%, 75%, and 90% respectively (Figure 1A). The CI was 0.36, 0.29, 0.27, and 0.28 for proliferation inhibition of 30%, 50%, 75%, and 90%, respectively in Ba/F3 (EGFR Del19/T790M/C797S) (Figure 1B). WX-0593 + Vectibix showed little synergistic effect on the inhibition of cell proliferation of NCI-H1975 (EGFR L858R/T790M/C797S) and PC9 (EGFR Del19/T790M/C797S) (Figure 1C,1D).
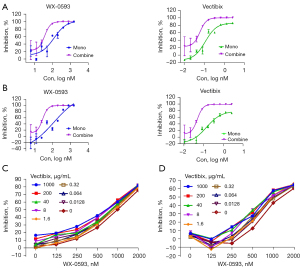
Therapeutic efficacy and safety of drugs in the H1975 (EGFR Del19/T790M/C797S) model
In the subcutaneous xenograft model using H1975 cells (EGFR Del19/T790M/C797S), we tested the therapeutic efficacy and safety profiles of WX-0593, QL1203, Vectibix, and their combination. The in vivo experiment was terminated on the 21st day of treatment, when the median tumor volume of the vehicle group reached 1,683 mm3 (Figure 2A). The tumor growth curves of the different groups are shown in Figure 2A, and the detailed data of the tumor volume and therapeutic parameters at the endpoint (21st day of treatment) of the different groups are summarized in Table 1. WX-0593 was tested at two dosages (25 mg/kg, OD; 75 mg/kg, OD). Neither WX-0593 (25 mg/kg, OD), QL1203 (0.1 mg/mouse, QW), nor Vectibix (0.1 mg/mouse, QW) as a single agent exhibited significant inhibition of tumor growth (Figure 2B and Table S1). The combination of WX-0593 (25 mg/kg, OD) with QL1203 (0.1 mg/mouse, QW) or Vectibix (0.1 mg/mouse, QW) showed a modest effect in controlling tumor growth (Table S1). WX-0593 (75 mg/kg, OD) as a single agent showed significant inhibition of tumor growth, which was further enhanced after its combination with QL1203 (0.1 mg/mouse, QW) or Vectibix (0.1 mg/mouse, QW) (Figure 2C). Specifically, the combination of WX-0593 (75 mg/kg, OD) with QL1203 or Vectibix was significantly better than all of the other treatment groups in the control of tumor growth (Figure 2B,2C and Table S1). In addition, no significant difference was observed between Vectibix + WX-0593 and QL1203 + WX-0593.
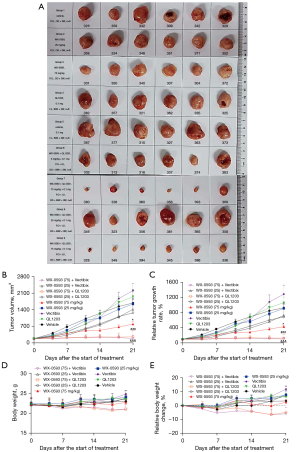
Table 1
| Parameter | QL1203 (0.1 mg/mouse) | Vectibix (0.1 mg/mouse) | WX-0593 (25 mg/kg) + QL1203 | WX-0593 (75 mg/kg) + QL1203 | WX-0593 (25 mg/kg) + Vectibix | WX-0593 (75 mg/kg) + Vectibix |
|---|---|---|---|---|---|---|
| No. of samples | 6 | 6 | 6 | 6 | 6 | 6 |
| HL_Lambda_z (h) | 40.5 | 26.0 | 61.1 | 194 | 52.2 | 143 |
| Tmax | 1 | 1 | 1 | 1 | 1 | 1 |
| Cmax (µg/mL) | 77.6 | 64.5 | 96.6 | 175 | 91.7 | 181 |
| AUClast (h·µg/mL) | 3174 | 2204 | 7015 | 16,766 | 5864 | 16,385 |
| Cl_obs (mL/h/k) | 1.49 | 2.24 | 0.611 | 0.139 | 0.754 | 0.167 |
| Vss_obs (mL/kg) | 81.4 | 88.3 | 52.4 | 37.9 | 58.0 | 34.8 |
AUC, area under the curve; Cmax, peak concentration; CI, combination index.
To monitor the side effects of the different therapeutic regimens, the body weights of the mice were routinely monitored during the treatment and compared across the different groups (Table S2). The body weights of the mice in the groups receiving WX-0593 (75 mg/kg, OD) in combination with QL1203 (0.1 mg/mouse, QW) or Vectibix (0.1 mg/mouse, QW) decreased slowly with treatment, with a reduced average body weight at the endpoint (compared with the body weight when the treatment was first initiated). For WX-0593 + QL1203, the body weight was reduced by an average of 1.65 mg; and for WX-0593 + QL1203, by an average of 1.21 mg. No obvious body weight reduction was observed for the other treatment groups (Figure 2D,2E). All of the mice were otherwise healthy, without any recognizable abnormalities in appearance or physical activity during the treatment. No treatment-related deaths occurred.
Therapeutic efficacy and safety of drugs in the Ba/F3 (EGFR L858R/T790M/C797S) model
We also tested WX-0593, QL1203, Vectibix, and their combination in another xenograft model (Ba/F3 cells with the EGFR mutations of L858R, T790M, and C797S) to further validate their therapeutic efficacy and safety profiles. As the results derived from the H1975 (EGFR Del19/T790M/C797S) model showed no therapeutic effect for WX-0593 at a dosage of 25 mg/kg QD, we only tested WX-0593 at a dosage of 75 mg/kg QD in the Ba/F3 (EGFR L858R/T790M/C797S) model. The tumor growth curves and relative tumor growth rates of the different groups are shown in Figure 3A,3B. Therapeutic parameters such as median tumor volume, TGI (%), T/C, etc. at two time points (10th and 14th days of treatment) are summarized in Tables S3,S4, respectively. The median tumor volume of the vehicle group reached 449 mm3 on the 10th day of treatment. All single agents exhibited significant inhibition of tumor growth by the 10th day of treatment (Table S3). The combination of WX-0593 + QL1203 or Vectibix achieved impressive therapeutic efficacy (Figure 3). The therapeutic efficacy of all treatment groups was more prominent on the 14th day of treatment (Table S4). Six tumors (75%) in the WX-0593 + QL1203 group and five (62.5%) in the WX-0593 + Vectibix group partially regressed, and one tumor (12.5%) in the WX-0593 + QL1203 and two tumors (25%) in the WX-0593 + Vectibix group completely regressed (Table S4).
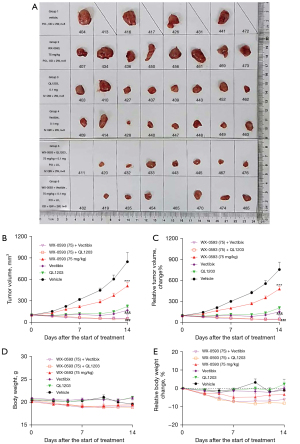
The safety profiles in the Ba/F3 model were similar to those in the H1975 (EGFR Del19/T790M/C797S) model. The body weights of each individual mouse at different time points are summarized in Table S5 and the body weight of each individual mouse during treatment in the Ba/F3 (EGFR-L858R/T790M/C797S) model were shown in Table S6. The body weights and relative body weight change in all treatment groups were stable throughout the treatment and showed no significant difference compared with the vehicle-treated group (Figure 3D,3E). No abnormality in appearance or physical activity was observed in any of the treatment groups during the treatment. No treatment-related deaths occurred.
Pharmacokinetic properties of the different treatments
The pharmacokinetic properties of the various drugs were evaluated by assessing the blood concentration of the drugs at different time points after administration of the last dosage. As shown in Table 1, the peak concentrations (Cmax) and the area under the concentration time curve (AUC0–168h) of QL1203 after intravenous administration of 0.1 mg/mouse were different under the three conditions: QL1203 as a single agent, QL1203 + WX-0593 (25 mg/kg), and QL1203 + WX-0593 (75 mg/kg). Similarly, the pharmacokinetic properties of Vectibix were also altered in combination with WX-0593 or not (Table 1).
Discussion
In the present study, we evaluated the synergistic inhibitory effect of WX-0593 and EGFR monoclonal antibody Vectibix on the proliferation of four EGFR triple-mutant cell lines carrying both mutant EGFR and resistance mutations (T790M and C797S). We also systematically evaluated the therapeutic efficacy of WX-0593 and EGFR monoclonal antibodies (QL1203 and Vectibix) as single agents or in combination for tumors with triple EGFR mutations using two xenograft tumor models (H1975 (EGFR Del19/T790M/C797S) and Ba/F3 (EGFR L858R/T790M/C797S)), following the ARRIVE guidelines to the use of experimental animals and rigorously recording the data in the laboratory (27,28). We found that WX-0593 + Vectibix showed a strong synergistic inhibition on the proliferation of two EGFR triple-mutant Ba/F3 cell lines (EGFR L858R/T790M/C797S and Del19/T790M/C797S), but a weak synergistic inhibitory effect on the proliferation of NCI-H1975 (EGFR L858R/T790M/C797S) and PC9 (EGFR Del19/T790M/C797S). We also found that treatment with the monoclonal antibody as a single agent showed marginal inhibition on tumor growth. WX-0593 exhibited a modest therapeutic efficacy at a dose rate of 75 mg/kg (daily), but the effect was enhanced when combined with QL1203 or Vectibix. No significant side effects were observed during treatment, even among the mice receiving the combination treatment. Pharmacokinetic analysis confirmed the enhancement and prolongation of the blood concentrations of the drugs. Our findings revealed that WX-0593 in combination with an EGFR monoclonal antibody might be a promising therapeutic strategy for EGFR-mutated tumors that are resistant to EGFR-TKIs due to the existence of resistance mutations (T790M and C797S).
The development of resistance mutations is the most troublesome issue in targeted therapy. With the T790M mutation mediating resistance to first- and second-generation EGFR-TKIs (15,16) and the C797S mutation driving the resistance to third-generation EGFR-TKIs (20), the coexistence of the mutations T790M and C797S is untreatable by EGFR-TKIs right now. Many ongoing studies have sought to tackle this issue by developing fourth-generation EGFR-TKIs that can overcome the resistance driven by T790M and C797S (31). Some mutant-selective allosteric inhibitors such as EAI-001/045 and JBJ-04-125-02 have shown potent therapeutic effects against tumors carrying EGFR L858R/T790M/C797S mutations, but they failed to inhibit those with EGFR Del19/T790M/C797S mutations (32-34). Another recently discovered inhibitor, CH7233163, is effective against tumors harboring EGFR Del19/T790M/C797S mutations, but its therapeutic efficacy against EGFR L858R/T790M/C797S mutations is modest (13). Novel TKIs that can overcome EGFR/T790M/C797S mutations, regardless of the mutation subtype, have yet to be identified. Alternative strategies must be explored for the treatment of tumors with such triple mutations.
EGFR antibodies inhibit EGFR activation and have been used as the next line of treatment after EGFR-TKIs and chemotherapy (35-37). The chemotherapy effect showed no significant difference from the EGFR gene changes. The platinum-based chemotherapy in EGFR mutation positive patients had a curative effect that was better than that of patients with a negative status (38). However, the therapeutic efficacy of EGFR antibodies is only modest for tumors driven by mutant EGFR, whose activation largely relies on autophosphorylation rather than the binding of epithelial growth factor (39-41). Consistent with previous findings, the EGFR antibodies proved to be ineffective or only marginally effective for the treatment of tumors harboring EGFR/T790M/C797S triple mutations in our study. Recent studies have started to explore the therapeutic potential of EGFR antibodies combined with other TKIs that originally targeted the activation of other oncogenes. One of the most promising treatments is the combination of brigatinib with anti-EGFR antibody in the treatment of EGFR-mutated NSCLC that is resistant to osimertinib (21). Brigatinib is an ALK inhibitor that also exhibits a certain inhibition of EGFR (31). Its combination with cetuximab as a monoclonal antibody targeting EGFR has shown outstanding therapeutic efficacy for EGFR/T790M/C797S triple-mutated NSCLC in both preclinical and clinical studies (21,23,42). EGFR and ALK, both of which are tyrosine kinases, share some commonality in phosphorylation sites and downstream pathways; thus, their inhibitors target both of these kinases. WX-0593 is a TKI that primarily targets ALK and also has been shown to inhibit EGFR in vitro (43). As its mode of action is different from that of conventional EGFR-TKIs, it can bypass the resistance mediated by mutations such as T790M (43). Consistent with the in vitro findings, our study demonstrated that WX-0593 can also significantly inhibit tumor growth; however, a relatively higher concentration is needed. Of note, WX-0593 alone is not sufficient to achieve potent inhibition of tumor growth, indicating that WX-0593 incompletely inhibits EGFR activation. Similar to the combination of brigatinib and cetuximab, the preclinical study showed that the combination therapy of brigatinib with anti-EGFR antibody in EGFR-mutated against triple-mutation in vitro and in vivo is an effective measure, supplementation with an anti-EGFR antibody as in our study also significantly enhanced the therapeutic efficacy of WX-0593, At present, such studies are not fully carried out, but these preclinical experimental data could indicated that the new therapeutic modality may become a promising treatment (21). Specifically, the therapeutic efficacy of the combination was tested using two tumor models driven by distinct subtypes with triple mutations, including EGFR Del19/T790M/C797S mutations and EGFR L858R/T790M/C797S mutations., Our findings indicated the therapeutic efficacy of WX-0593 or its combination with anti-EGFR-antibody is not restricted by the subtype of EGFR mutations. The strength of this research portfolio is it may provide a new treatment idea for the clinical patients without effective therapeutic drugs for EGFR triple-mutant NSCLC resistant to osimertinib.
To better understand the mechanism by which the synergetic effect was achieved by the combination of WX-0593 and an anti-EGFR-antibody, the pharmacokinetic properties of the different treatment combinations were evaluated in this study. Our findings demonstrated that the maximal blood concentration of the drugs was significantly increased when they were administered in combination. Also, the reduction of the drug concentration in the blood over time was also significantly slowed when the drugs were used in combination. The mutual effects of the drugs when administered together could be attributed to their competition for the same metabolic pathway in vivo (44). The interaction between WX-0593 and the anti-EGFR antibody in terms of the pharmacokinetic process could be one of the reasons for their synergetic effect on tumor growth. Another possible explanation could be the distinct mode of action of these two types of drugs. On the one hand, WX-0593 as a TKI can block the binding site of ALK to kinase, thus preventing its autoactivation. On the other hand, anti-EGFR antibody inhibits activation of EGFR through blocking the binding of EGFR with its ligand, EGF. Their combination exerts enhanced inhibition of the downstream pathways of EGFR and thus inhibits tumor growth more potently.
Targeting EGFR with recombinant antibodies has been associated with a high incidence of adverse events compared with EGFR-TKIs due to the nonspecific targeting of all EGFR sites (45,46). In addition, WX-0593 as a single agent for the treatment of ALK-positive or ROS1-positive NSCLC has been found to induce some treatment-associated adverse events, including hypercholesterolemia, hypertension, mild liver damage, etc. (29). The combination of WX-0593 with QL1203 or Vectibix was associated with a high maximal blood concentration and prolonged retention of this drug in the circulatory system, which makes the therapeutic safety of the combination treatment an even bigger concern. Our study showed that the body weights of the mice receiving the drug combination were mildly reduced after the treatment, but all of the mice were otherwise healthy and did not exhibit any other abnormalities. Our findings indicated an acceptable safety profile of WX-0593 combined with QL1203 or Vectibix, which improves the potential of clinical application.
Even with these promising findings, the limitations of the present study must be objectively addressed. First, this was a preclinical study carried out on xenograft tumor models that aimed to provide a proof of concept that WX-0593 combined with QL1203 or Vectibix could be a promising treatment for tumors harboring EGFR/T790M/C797S triple mutations. The clinical significance of these findings needs to be confirmed in by further clinical studies. Second, the xenograft model may not fully recapitulate the biological characteristics of clinical tumors with EGFR/T790M/C797S triple mutations. One of the xenograft models was developed using the Baf3 cell line, which is a malignant cell line derived from murine B cells and may not be well represent the characteristics of solid tumors, especially lung cancer (47). Last but not the least, the evaluation of safety profiles using animal models is associated with some intrinsic limitations. Some subtle abnormalities and human-specific side effects would not be observed in mice. The therapeutic dosage for humans is also different from that of mice, which could also cause inconsistencies in the safety profiles between humans and animal models. Besides, several studies indicate that monitoring the changes in levels of serum tumor makers such as Serum carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA21-1) and squamous-cell carcinoma-related antigen (SCC-Ag) might be relevant for the prognosis of advanced NSCLC patients (48,49). Developing new therapies is a good medical strategy, but it is also important for prognostic judgments in order for patients to benefit more from clinical.
To conclude, our study demonstrated the safety and efficacy of WX-0593 in combination with QL1203 or Vectibix for the treatment of EGFR-mutated tumors that are resistant to EGFR-TKIs due to the existence of resistance mutations (T790M, C797S). Our study provides a proof of concept that the combination of WX-0593 with an anti-EGFR antibody could be a promising therapeutic strategy for tumors harboring EGFR/T790M/C797S triple mutations; however, no corresponding clinical trials have been carried out, these findings need to be validated by further clinical studies.
Acknowledgments
Funding: This research was supported by Qilu Pharmaceutical Co., Ltd.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2780/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2780/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2780/coif). All authors report that they are employees of Qilu Pharmaceutical Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license granted by Wuxi Apptec (Shanghai) Co., Ltd. (No. ON01-003-2019v1.0). In compliance with International Committee for the evaluation and Accreditation of laboratory animals (AAALAC).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi Y, Li J, Zhang S, et al. Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology - Mainland China Subset Analysis of the PIONEER study. PLoS One 2015;10:e0143515. [Crossref] [PubMed]
- Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer 2019;137:113-22. [Crossref] [PubMed]
- Zhou J, Mo W, Zhao J, et al. Clinicopathological features associated with EGFR gene mutation in non-small cell lung cancer patients. Zhonghua Yi Xue Za Zhi 2014;94:2332-6. [PubMed]
- Li Z, Zhang LJ, Wang WP, et al. Correlation between EGFR gene mutation and high copy number and their association with the clinicopathological features in Chinese patients with non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2011;33:666-70. [PubMed]
- Pi C, Xu CR, Zhang MF, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: Analysis based on large-scale data from China. Thorac Cancer 2018;9:814-9. [Crossref] [PubMed]
- Castellanos E, Feld E, Horn L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:612-23. [Crossref] [PubMed]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 2006;12:5268-72. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Baek JH, Sun JM, Min YJ, et al. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: a retrospective analysis in Korea. Lung Cancer 2015;87:148-54. [Crossref] [PubMed]
- Yoneda K, Imanishi N, Ichiki Y, et al. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J UOEH 2019;41:153-63. [Crossref] [PubMed]
- Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. [Crossref] [PubMed]
- Guo Y, Song J, Wang Y, et al. Concurrent Genetic Alterations and Other Biomarkers Predict Treatment Efficacy of EGFR-TKIs in EGFR-Mutant Non-Small Cell Lung Cancer: A Review. Front Oncol 2020;10:610923. [Crossref] [PubMed]
- Kashima K, Kawauchi H, Tanimura H, et al. CH7233163 Overcomes Osimertinib-Resistant EGFR-Del19/T790M/C797S Mutation. Mol Cancer Ther 2020;19:2288-97. [Crossref] [PubMed]
- Remon J, Steuer CE, Ramalingam SS, et al. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol 2018;29:i20-7. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol 2017;35:1288-96. [Crossref] [PubMed]
- Mu Y, Hao X, Xing P, et al. Acquired resistance to osimertinib in patients with non-small-cell lung cancer: mechanisms and clinical outcomes. J Cancer Res Clin Oncol 2020;146:2427-33. [Crossref] [PubMed]
- Wang F, Diao XY, Zhang X, et al. Identification of genetic alterations associated with primary resistance to EGFR-TKIs in advanced non-small-cell lung cancer patients with EGFR sensitive mutations. Cancer Commun (Lond) 2019;39:7. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 2017;8:14768. [Crossref] [PubMed]
- Yaeger R, Kotani D, Mondaca S, et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin Cancer Res 2019;25:7089-97. [Crossref] [PubMed]
- Wang Y, Yang N, Zhang Y, et al. Effective Treatment of Lung Adenocarcinoma Harboring EGFR-Activating Mutation, T790M, and cis-C797S Triple Mutations by Brigatinib and Cetuximab Combination Therapy. J Thorac Oncol 2020;15:1369-75. [Crossref] [PubMed]
- Heigener DF, Reck M. Crizotinib. Recent Results Cancer Res 2018;211:57-65. [Crossref] [PubMed]
- Li Y, Su S, Cai G, et al. Responses to crizotinib and chemotherapy in patients with lung adenocarcinoma harboring a concomitant EGFR mutation and ALK gene rearrangement: A case report and review of the literature. Mol Clin Oncol 2017;7:173-82. [Crossref] [PubMed]
- Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun 2019;10:3991. [Crossref] [PubMed]
- van Veggel B, de Langen AJ, Hashemi S, et al. Crizotinib treatment for patients with EGFR mutation positive NSCLC that acquire cMET amplification after EGFR TKI therapy results in short-lived and heterogeneous responses. Lung Cancer 2018;124:130-4. [Crossref] [PubMed]
- Wang Y, Tian P, Xia L, et al. The clinical efficacy of combinatorial therapy of EGFR-TKI and crizotinib in overcoming MET amplification-mediated resistance from prior EGFR-TKI therapy. Lung Cancer 2020;146:165-73. [Crossref] [PubMed]
- Shi YK, Fang J, Zhang S, et al. Safety and efficacy of WX-0593 in ALK-positive or ROS1-positive non-small cell lung cancer. Ann Oncol 2019;30:v607-v608. [Crossref]
- Liu X, Zhang L, Wan H, et al. Discovery and preclinical evaluations of WX-0593, a novel ALK inhibitor targeting crizotinib-resistant mutations. Bioorg Med Chem Lett 2022;66:128730. [Crossref] [PubMed]
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. [Crossref] [PubMed]
- Zhou Z, Zhao Y, Shen S, et al. Durable Clinical Response of Lung Adenocarcinoma Harboring EGFR 19Del/T790M/in trans-C797S to Combination Therapy of First- and Third-Generation EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2019;14:e157-9. [Crossref] [PubMed]
- To C, Jang J, Chen T, et al. Single and Dual Targeting of Mutant EGFR with an Allosteric Inhibitor. Cancer Discov 2019;9:926-43. [Crossref] [PubMed]
- Li Q, Zhang T, Li S, et al. Discovery of Potent and Noncovalent Reversible EGFR Kinase Inhibitors of EGFRL858R/T790M/C797S. ACS Med Chem Lett 2019;10:869-73. [Crossref] [PubMed]
- Mazzarella L, Guida A, Curigliano G. Cetuximab for treating non-small cell lung cancer. Expert Opin Biol Ther 2018;18:483-93. [Crossref] [PubMed]
- Wang Y, Wang H, Jiang Y, et al. A randomized phase III study of combining erlotinib with bevacizumab and panitumumab versus erlotinib alone as second-line therapy for Chinese patients with non-small-cell lung cancer. Biomed Pharmacother 2017;89:875-9. [Crossref] [PubMed]
- Vlahovic G, Meadows KL, Hatch AJ, et al. A Phase I Trial of the IGF-1R Antibody Ganitumab (AMG 479) in Combination with Everolimus (RAD001) and Panitumumab in Patients with Advanced Cancer. Oncologist 2018;23:782-90. [Crossref] [PubMed]
- Wang Y, Ma X, Wei Y, et al. Effect of platinum-based chemotherapy on EGFR gene mutation status in lung adenocarcinoma. Medicine (Baltimore) 2018;97:e9602. [Crossref] [PubMed]
- Zhao Y, Liu J, Cai X, et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: systematic review and network meta-analysis. BMJ 2019;367:l5460. [Crossref] [PubMed]
- Yun J, Lee SH, Kim SY, et al. Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET Bispecific Antibody, in Diverse Models of EGFR Exon 20 Insertion-Driven NSCLC. Cancer Discov 2020;10:1194-209. [Crossref] [PubMed]
- Hoyle M, Crathorne L, Peters J, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess 2013;17:1-237. [Crossref] [PubMed]
- Wang X, Zhou L, Yin JC, et al. Lung Adenocarcinoma Harboring EGFR 19del/C797S/T790M Triple Mutations Responds to Brigatinib and Anti-EGFR Antibody Combination Therapy. J Thorac Oncol 2019;14:e85-8. [Crossref] [PubMed]
- Xile L, Ding CZ, Mao W, et al. Abstract 4794: Preclinical evaluation of WX-0593, a potent orally active ALK inhibitor. Cancer Res 2018;78:4794. [Crossref]
- Toshimoto K, Tomoda Y, Chiba K, et al. Analysis of the Change in the Blood Concentration-Time Profile Caused by Complex Drug-Drug Interactions in the Liver Considering the Enterohepatic Circulation: Examining Whether the Inhibition Constants for Uptake, Metabolism, and Biliary Excretion Can be Recovered by the Analyses Using Physiologically Based Pharmacokinetic Modeling. J Pharm Sci 2017;106:2727-38. [Crossref] [PubMed]
- Kumai T, Oikawa K, Aoki N, et al. Assessment of the change in cetuximab-induced antibody-dependent cellular cytotoxicity activity of natural killer cells by steroid. Head Neck 2016;38:410-6. [Crossref] [PubMed]
- Funakoshi T, Suzuki M, Tamura K. Infectious complications in cancer patients treated with anti-EGFR monoclonal antibodies cetuximab and panitumumab: a systematic review and meta-analysis. Cancer Treat Rev 2014;40:1221-9. [Crossref] [PubMed]
- Lord M, Arvidsson G, Wasik AM, et al. Impact of Sox11 over-expression in Ba/F3 cells. Haematologica 2018;103:e594-7. [Crossref] [PubMed]
- Zhang ZH, Han YW, Liang H, et al. Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung cancer. Cancer Med 2015;4:1633-8. [Crossref] [PubMed]
- Ma R, Xu H, Wu J, et al. Identification of serum proteins and multivariate models for diagnosis and therapeutic monitoring of lung cancer. Oncotarget 2017;8:18901-13. [Crossref] [PubMed]
(English Language Editor: K. Brown)


