Molecular mechanism of betulin palliative therapy for chronic obstructive pulmonary disease (COPD) based on P2X7 receptor target of gated ion channel
Introduction
It has recently been revealed that chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death throughout the world. COPD is characterized by prolonged course of disease, high recurrence rate, and extensive population coverage, causing heavy social and economic burden (1). The latest data show that the number of COPD patients over 40 years of age in China has exceeded 100 million, accounting for 13.7% of the total population. One of the major risk factors for COPD is smoking, with 80–90% of COPD patients either smoking or having smoked in the past (2). Currently, clinical treatment of COPD is mainly drug-based, and glucocorticoids such as dexamethasone (DEX) are commonly used (3). However, after long-term application, the body develops tolerance to these drugs, and patients are prone to a large number of side effects, including osteoporosis, hypertension, obesity, cataracts, and fractures, which are the main causes of clinical treatment failure and key technical obstacles. On the other hand, COPD diagnosis and treatment guidelines recommend that patients with advanced COPD should receive palliative care as soon as possible under the best disease guidance (4). Study has shown that palliative care can improve the quality of life and symptom burden of COPD patients, and has a positive intervention effect (5). However, the current demand for palliative care services for COPD patients has not been met, and only a small number of severely ill COPD patients have received professional palliative care (6). Therefore, identification of the key molecular pathway of the occurrence and development of COPD, selection of effective treatment drugs with high efficiency and low toxicity, and inhibition of the development of tolerance are vitally important for overcoming this worldwide problem.
Betula platyphylla is a species of birch tree from the Betulaceae family and is mainly found in northeast China, north China, southwest China, and other areas. Its bark has complex chemical composition and diverse pharmacological effects, including alleviating heat and dampness, eliminating phlegm, relieving cough, detumescence, detoxification, and treating chronic bronchitis (7). Betulin, a triterpenoid extracted from birch bark, is present in a wide range of natural sources. The content of betulin in birch bark is as high as 30% of the dry weight. Betulin, which is considered to be anti-allergenic, anti-inflammatory, and anti-viral, is used for sterilization, relieving asthma, and ultraviolet protection. It is also widely used in food, medicine, the chemical industry, and other fields. It is a very valuable natural material and can be obtained from abundant plant resources. A previous study reported that the level of inflammatory factors in alveolar lavage fluid of COPD mice administered betulin was significantly reduced. This research demonstrated that betulin was able to decrease the inflammatory response of COPD mice, thereby playing a role in the treatment of COPD (8). At present, there are still few studies on betulin treatment for COPD, and its therapeutic mechanism is not yet clear. In this study, betulyl alcohol was hypothesized to be an effective treatment for COPD, and it was proposed for the first time that the P2X7/nuclear transcription factor κB (NF-κB) and P2X7/mitogen-activated protein kinase (MAPK) signaling pathways play an important role in the development and progression of COPD disease, and it was intended to demonstrate by molecular biology that betulyl alcohol improves COPD mainly by mediating airway inflammation mediated by P2X7 receptor. To this end, we carried out relevant experiments to investigate the role and mechanism of betulin in the treatment of COPD. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2629/rc).
Methods
In vivo COPD experiment in mice
Construction of COPD mouse model
Fifty Sprague Dawley (SD) mice (200–220 g) were numbered 1–50 and randomly divided into 5 groups by drawing lots: blank control group, model group, 0.5 mg/kg DEX group, 20 mg/kg betulin group, and 40 mg/kg betulin group. Except for the control group, mice in the cigarette smoke exposure and drug intervention groups were exposed to smoke in a smoke exposure device once a day at a concentration of about 5% volume/volume (v/v), 1 hour/day, for 12 weeks. From the second week of modeling, intragastric administration was used, and alterations in body weight of the mice were continuously monitored during the experiment. In order to reduce potential bias, each step was performed by 5 co-authors, each of whom was responsible for a group of mice. The animal experiment was approved by The First Affiliated Hospital of Zhengzhou University (No. 2022-KY-0472), in compliance with Chinese guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Measurement of pulmonary function in mice
After 12 weeks, all mice were anesthetized with 3% chloral hydrate. The mice were fixed in a supine position, and the skin at the neck was cut in the middle to expose the trachea. The infusion scalp needle (about 3 mm in diameter) was cut into a shuttle shape at 1 end and fixed along the endotracheal intubation. The other end was connected with a T-pipe, which was connected to a spirometer and PowerLab multichannel physiological tester (provided by ADInstruments Shanghai Trading Co., Shanghai, China). Maximal voluntary ventilation (MVV), inspiratory resistance (RI), expiratory resistance (RE), and lung compliance (CL) were measured. The forced expiratory volume in 0.3 seconds (FEV 0.3) was measured by external pressure method.
Detection of inflammatory cells in bronchoalveolar lavage fluid (BALF)
Blood was taken from the common carotid artery of the mice, and after standing for 1 hour, the blood was centrifuged at 3,500 r/min for 15 minutes, and the serum was kept at −80 ℃ for later use. The neck was cut open to expose the trachea and both lungs. A transverse incision was made at the lower part of the trachea, and a No. 12 needle (the tip is ground flat) was inserted into the trachea incision and fixed. After the right main bronchus was clipped, 1 mL of ice-cold phosphate-buffered saline (PBS) was slowly injected into the left lung and then extracted after 10 seconds to obtain the lavage solution. This procedure was repeated 3 times. A drop of alveolar lavage fluid was placed in a blood counting chamber, and the total number of white blood cells (WBCs) was detected under a microscope. The collected alveolar lavage fluid was centrifuged for 5 minutes at 4 ℃ at 1,000 r/min, and the supernatant was sucked out and stored at −80 ℃ for later use. The precipitate was suspended in PBS, fixed by smearing, stained with Wright's stain, and the number of WBCs, neutrophils (Ns), lymphocyte (LYM), and mononuclear macrophage lineage cells (MMLCs) were read under a microscope.
Pathological assessment of lung tissue
Lung tissue was fixed in 10% neutral formalin solution overnight, embedded in paraffin, and then segmented into 4-mm-thick sections. The paraffin was removed and stained with hematoxylin eosin (HE) to observe lung injury and infiltration of inflammatory cells. Van Gieson (VG) staining was performed to observe airway remodeling and pulmonary vascular remodeling indicators. Airway remodeling indicators included outer diameter, wall thickness, tube wall area, and total trachea area of bronchioles with diameter ≤100 µm (the shortest diameter/longest diameter >0.7), which were measured in 5 random fields under 200× microscope. The ratio of the wall thickness to the outer diameter of the trachea (MT%) and the ratio of the tube wall area to the total area of the trachea (MA%) were then calculated. The stained lung tissue sections were observed under a microscope (100–200×) to find the complete cross-section of the intrapulmonary bronchus. Image assessment software was employed to appraise the lumen internal circumference (Pi) and tube wall area (WA). The measured values were normalized by Pi and the thickness of the bronchial tube wall was expressed by WAPi. Three bronchi were selected for each section, and airway measurement was carried out independently by 2 researchers, with the average value of the 2 researchers as the measurement value. To obtain pulmonary vascular remodeling indicators, image analysis was performed on muscular arteries (MA) with a diameter larger than 50 µm and less than 100 µm under 400× microscope. The ratio of pulmonary arterial wall thickness to vascular external diameter (WT%) and ratio of vascular area to total vascular area (WA%) were measured.
Determination of inflammatory factor levels
The content of inflammatory factors tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and (IL-6) in serum, BALF, and lung tissue was observed through enzyme-linked immunosorbent assay (ELISA) kit as per the instructions.
In vitro experiment of human bronchial epithelial cells
Preparation of extracts from cigarette smoke
Two unfiltered cigarettes were pumped continuously with a negative pressure driving device, with combustion completed within 10 minutes. The smoke was inhaled through an outlet with negative pressure suction, dissolved in 50 mL Dulbecco’s Modified Eagle Medium (DMEM) to form a suspension, and then shaken. The pH value of the suspension was adjusted to 7.4 with 1 mol/L sodium hydroxide (NaOH), and the suspension was then filtered through a 0.22 µm microporous membrane to make 100% cigarette smoke extract (CSE).
Establishment of human bronchial epithelial cell model in vitro
Human bronchial epithelial cell line (16-HBE) was cultivated in DMEM comprising 10% fetal bovine serum (FBS) and incubated with 5% CO2 at 37 ℃ and saturated humidity. The cells were inoculated into 96-well plates (1×104 cells/well) and cultured for 24 hours. Next, 4% CSE and 0.1 µg/mL lipopolysaccharide were added to the cell culture medium to induce an in vitro COPD-16-HBE cell model. After 24 hours, cell survival rate was counted under a microscope, and the supernatant and cells were collected for detection of inflammatory factors.
Detection of inflammatory factors in cell supernatant
In the COPD-16-HBE cell model, DEX (10−6 mol/L) was added, followed by 10−5 mol/L betulin, and 10−4 mol/L betulin culture for 24 hours. The cell supernatant was collected, and the supernatants of normal 16-HBE cells and COPD-16-HBE cells were also collected. The expression levels of inflammatory factors were observed through ELISA.
Betulin mechanism of action experiment
Cell transfection
The 16-HBE cells were inoculated into 6-well plates (5×104 cells/well) and cultured with DMEM comprising 10% FBS in an incubator with 5% CO2 at 37 ℃. When the cells grew to 50–70% confluence, short hairpin RNAs (shRNAs) were transfected into cells at a final concentration of 80 nmol/L with TransLipid Transfection Reagent in accordance with the protocols. Before transfection, cells were cultivated in 2 mL serum-free DMEM for 2 hours. At 6 hours after transfection, the medium was substituted with conventional DMEM comprising 10% FBS. The experimental settings included normal (vector group), P2X7 overexpression [circular RNA (circ)P2X7 group], negative shRNA (shRNA-control group), interference vector 1 (P2X7-shRNA-1 group), and interference vector 2 (P2X7-shRNA-2 group). Six hours after transfection, quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed to assess the effectiveness of cell transfection. After sufficient homogenization at low temperature, the extraction of total RNA was obtained with Trizol reagent. DNA was synthesized with 5 µL of the extracted total RNA in a 20 µL reverse transcription system. Primers upstream and downstream of the target gene (P2X7) were added with 5 µL complementary DNA (cDNA) as the template, and PCR amplification was performed in a 50 µL system.
Determination of key proteins
After 16-HBE cells were treated with P2X7 overexpression/stable knockdown and 4% CSE, 10−6 mol/L DEX, 10−5 mol/L betulin, or 10−4 mol/L betulin culture for 24 hours, the relative messenger RNA (mRNA) expression levels of key proteins in cells were detected through RT-qPCR. The levels of key proteins in cells were analyzed through Western blot, and the protein content was ascertained through bicinchoninic acid assay (BCA). The protein specimens underwent sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred to a polyvinylidene difluoride (PVDF) membrane, blocked with 5% skim milk powder at ambient temperature for 2 hours, and incubated with primary antibodies P2X7, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively. The membrane was washed with tris-buffered saline with Tween (TBST) and subsequently reacted with secondary antibodies coupled with horseradish peroxidase. The membrane was rinsed with TBST and then developed with enhanced chemiluminescence reagent. The optical density of the main band was determined through grayscale imaging software (UVP, UK).
Statistical analysis
SPSS 22.00 was used for data statistics and GraphPad Prism 8 software was used for mapping. Measurement data are expressed as , and t-test was used for comparison between groups. P<0.05 indicated that the difference was statistically significant.
Results
In vivo animal experiment results
Pulmonary ventilation function
Compared with the control group, MVV, PEF, FEV, and CL in the model group were significantly decreased, while RE and RI were substantially augmented (all P<0.05). In comparison to the model group, pulmonary ventilation function of the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups was improved; MVV, PEF, FEV, and CL were significantly increased; and RE and RI were substantially diminished (all P<0.05). There was no significant difference in pulmonary ventilation function between any 2 of the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups (all P>0.05). Detailed information is shown in Table 1 and Figure 1.
Table 1
| Group | MVV (mL) | RE (kPa) | RI (kPa) | CL (mL/cm) | PEF (mL/s) | FEV 0.3/FCV |
|---|---|---|---|---|---|---|
| Control | 131.89±11.74 | 31.85±6.17 | 22.81±2.72 | 0.29±0.07 | 30.40±5.15 | 83.02±6.67 |
| Model | 100.59±11.77* | 46.74±7.61* | 40.32±2.95* | 0.03±0.01* | 21.13±3.21* | 67.76±3.38* |
| DEX | 124.49±10.17*# | 38.39±4.96*# | 33.35±4.21*# | 0.17±0.03*# | 26.34±2.69# | 74.10±6.69*# |
| Betulin (20 mg/kg) | 122.93±9.21*# | 40.17±3.82*# | 37.95±3.78*# | 0.10±0.03*# | 26.98±2.87# | 72.93±6.35*# |
| Betulin (40 mg/kg) | 128.51±7.76*# | 38.18±4.52*# | 35.06±3.72*# | 0.14±0.05*# | 25.43±4.65# | 77.86±8.22*# |
The data was expressed as (). *P<0.05 when compared to control; #P<0.05 when compared to model. MVV, maximal voluntary ventilation; DEX, dexamethasone; RE, expiratory resistance; RI, inspiratory resistance; CL, lung compliance; PEF, peak expiratory flow; FEV, forced expiratory volume; FCV, forced vital capacity.

Effects of betulin on inflammation in COPD mice
Compared to the control group, WBCs, Ns, LYM, and MMCLs in the model group increased significantly (all P<0.05). In comparison to the model group, WBCs, Ns and MMLC in the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups decreased (all P<0.05). Furthermore, there was no significant difference in WBCs content between the DEX and betulin (20 mg/kg) groups (P>0.05). The WBCs content of the betulin (40 mg/kg) group was higher than that of the DEX and betulin (20 mg/kg) groups, and the difference was significant (both P<0.05). Detailed information is shown in Table 2 and Figure 2.
Table 2
| Group | WBCs ×108 | N ×108 | LYM ×108 | MMLC ×108 |
|---|---|---|---|---|
| Control | 1.69±0.32 | 0.15±0.023 | 1.19±0.26 | 0.29±0.058 |
| Model | 2.75±0.20* | 0.45±0.071* | 1.75±0.44* | 0.37±0.016* |
| DEX | 2.19±0.48*# | 0.19±0.027*# | 1.58±0.20*# | 0.33±0.021# |
| Betulin (20 mg/kg) | 2.26±0.31*# | 0.22±0.026*# | 1.65±0.27*# | 0.32±0.010# |
| Betulin (40 mg/kg) | 2.55±0.18*#ab | 0.18±0.015*# | 1.54±0.23*# | 0.31±0.021# |
The data was expressed as (). *P<0.05 when compared to control; #P<0.05 when compared to model; aP<0.05 when compared to DEX; bP<0.05 when compared to Betulin (20 mg/kg). DEX, dexamethasone; WBCs, white blood cells; LYM, lymphocyte; MMLC, mononuclear macrophage cell.

The expression levels of inflammatory factors related to BALF, serum, and lung tissue in each group were determined. The findings demonstrated that in comparison to the control group, the levels of TNF-α, IL-6, and IL-1β in BALF, serum, and lung tissue in the model group were substantially enhanced (all P<0.05). In comparison to the model group, the levels of TNF-α, IL-1β, and IL-6 in the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups were substantially diminished (all P<0.05). Detailed information is shown in Table 3 and Figure 3.
Table 3
| Group | TNF-ɑ (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| BALF | |||
| Control | 78.23±19.34 | 12.54±2.20 | 42.48±18.75 |
| Model | 693.34±68.42* | 60.92±5.06* | 274.67±15.17* |
| DEX | 349.67±62.35*# | 38.38±6.65*# | 143.01±27.37*# |
| Betulin (20 mg/kg) | 331.25±89.07*# | 43.95±8.31*#a | 160.29±19.45*#a |
| Betulin (40 mg/kg) | 385.06±42.64*# | 40.02±4.80*# | 154.84±10.54*#a |
| Serum | |||
| Control | 85.28±43.30 | 12.25±2.67 | 41.08±13.60 |
| Model | 693.75±62.21* | 33.02±2.38* | 275.33±15.94* |
| DEX | 285.85±25.23*# | 24.37±2.38*# | 136.90±18.47*# |
| Betulin (20 mg/kg) | 325.54±65.29*# | 26.62±1.88*# | 144.02±17.32*# |
| Betulin (40 mg/kg) | 310.47±54.02*# | 23.11±3.53*# | 127.81±17.24*# |
| Lung tissue | |||
| Control | 194.99±48.79 | 14.43±2.96 | 33.14±7.05 |
| Model | 929.15±24.44* | 51.98±4.18* | 278.84±4.36* |
| DEX | 387.22±52.17*# | 36.37±2.74*# | 163.75±36.68*# |
| Betulin (20 mg/kg) | 379.85±59.84*# | 34.89±1.37*# | 163.63±41.38*# |
| Betulin (40 mg/kg) | 410.04±57.53*#a | 32.55±8.41*# | 164.45±18.99*# |
The data was expressed as (). *P<0.05 when compared to control; #P<0.05 when compared to model; a, P<0.05 when compare to DEX. DEX, dexamethasone; TNF-ɑ, tumor necrosis factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; BALF, bronchoalveolar lavage fluid; DEX, dexamethasone.

Pulmonary pathology in COPD mice
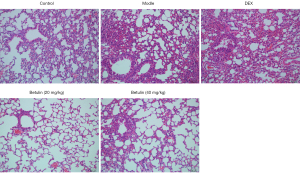
HE staining was employed for determining the lung pathological damage of mice in each group, as shown in Figure 4. Moderate inflammatory cell infiltration was observed in alveolar walls in the model group in comparison to the control group. The main type of inflammatory cells were mononuclear macrophages. Mild goblet cell metaplasia occurred in the bronchial epithelium of the lung, and a notable number of inflammatory cells were observed around the trachea. The bronchial epithelium in the lung was moderately deformed and necrotic, with mucus or cell obstruction in the lumen and pigmentation in the tube wall. The pathological score was 5.97±1.06, indicating that the model was effectively replicated. In comparison to the model group, the lesion degree of the DEX group was slightly reduced, and a notable number of inflammatory cells infiltrated the alveolar cells and the alveolar wall. The alveolar wall of the betulin (40 mg/kg) group was slightly infiltrated with inflammatory cells of the same type as above. There was slight goblet cell metaplasia in the bronchial epithelium of the lung, and a very small amount of inflammatory cell infiltration was detected around the trachea. A few bronchial epithelial cells in the lung were mildly degenerate and necrotic, with a small amount of mucus or cell obstruction in the lumen, and a very small amount of pigmentation in the wall of the tube. The pathological score was 3.5±0.22. The pathological score of the betulin (20 mg/kg) group was 3.5±0.22, which was further improved compared with the betulin (40 mg/kg) group. These results indicated that betulin could improve COPD lung injury to some extent.
Airway and vascular remodeling were analyzed in each group. The findings showed that MT%, MA%, WAPi, WT%, and WA% in the model group were substantially decreased in comparison to the control group (all P<0.05). In comparison to the model group, MT%, MA%, WAPi, and WT% in the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups were significantly diminished (all P<0.05). There were no significant differences in airway and vascular remodeling among the DEX, betulin (20 mg/kg), and betulin (40 mg/kg) groups (all P>0.05). Detailed information is shown in Table 4 and Figure 5.
Table 4
| Group | MT% | MA% | WAPi | WT% | WA% |
|---|---|---|---|---|---|
| Control | 5.79±0.99 | 14.85±3.21 | 62.97±7.10 | 17.24±2.87 | 40.63±4.19 |
| Model | 8.41±1.26* | 21.66±3.38* | 116.22±14.42* | 22.85±2.29* | 45.14±4.47* |
| DEX | 7.07±1.47*# | 15.54±2.76# | 99.45±22.27*# | 19.09±2.76# | 42.55±3.60 |
| Betulin (20 mg/kg) | 7.11±0.43*# | 15.67±2.94# | 94.92±15.49*# | 19.84±1.71# | 42.09±3.01 |
| Betulin (40 mg/kg) | 6.81±0.56*# | 16.33±2.78*# | 98.34±9.32*# | 20.26±2.63# | 42.32±2.85 |
The data was expressed as (). *P<0.05 when compared to control; #P<0.05 when compared to model. DEX, dexamethasone; MT%, ratio of the wall thickness to the outer diameter of the trachea; MA%, ratio of the tube wall area to the total area of the trachea; WAPi, thickness of the bronchial tube wall area; WT%, ratio of pulmonary arterial wall thickness to wall thickness; WA%, ratio of vascular area to total vascular area.

Results of betulin on COPD in vitro
Effects of betulin concentration on proliferation of 16-HBE cells
The influence of various concentrations of betulin on the proliferation of 16-HBE cells was analyzed. The results showed that DEX and betulin demonstrated no impact on the proliferation of 16-HBE cells (P>0.05). The survival rate of 16-HBE cells diminished substantially after CSE stimulation, and CSE inhibited the proliferation of 16-HBE cells (P<0.05). After DEX and betulin treatment, the inhibiting influence of CSE on 16-HBE cell proliferation was reversed, and the survival rate of 16-HBE cells was remarkably increased (P<0.05). Detailed information is shown in Table 5.
Table 5
| Group | 16-HBE cell survival rate (%) | Group | 16-HBE cell survival rate (%) |
|---|---|---|---|
| Controls | 100 | 5% CSE | 47.52±2.09 |
| DEX | 108.54±7.46 | 5% CSE + DEX | 66.89±1.01a |
| Betulin (10−5 mol/L) | 110.29±8.35 | 5% CSE + betulin (10-5 mol/L) | 70.73±2.01ab |
| Betulin (10−4 mol/L) | 109.72±7.74 | 5% CSE + betulin (10-4 mol/L) | 65.00±1.71a |
The data was expressed as (). aP<0.05 when compared to 5% CSE; bP<0.05 when compared to 5% CSE + DEX. DEX, dexamethasone; HBE, human bronchial epidermal; CSE, cigarette smoke extract; DEX, dexamethasone.
Expression levels of inflammatory factors in supernatant of 16-HBE cells
The expression levels of inflammatory factors in the supernatant of 16-HBE cells were determined. The findings demonstrated that TNF-α, IL-1β, and IL-6 levels in the 5% CSE group were substantially augmented compared to the control group (all P<0.05). In comparison to the 5% CSE group, levels of TNF-α, IL-1β, and IL-6 in the 5% CSE + DEX, 5% CSE + betulin (10−5 mol/L), and 5% CSE + betulin (10−4 mol/L) groups were remarkably diminished (all P<0.05). Furthermore, the levels of IL-1β and IL-6 in 5% the CSE + betulin (10−5 mol/L) group were substantially lower than those in the 5% CSE + DEX group (P<0.05). Detailed information is shown in Table 6 and Figure 6.
Table 6
| Group | TNF-ɑ (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Control | 534.52±63.29 | 590.33±45.18 | 472.88±40.09 |
| 5% CSE | 899.65±89.94* | 1107.49±114.42* | 1344.465±126.39* |
| 5% CSE + DEX | 544.04±61.32c | 661.24±49.08*c | 882.83±79.16*c |
| 5% CSE + Betulin (10−5 mol/L) | 554.73±51.26c | 581.74±49.19cd | 685.12±58.51*cd |
| 5% CSE + Betulin (10−4 mol/L) | 729.11±63.78*c | 823.36±75.52*cd | 1113.34±59.05*cd |
The data was expressed as (). *P<0.05 when compared to control; cP<0.05 when compared to 5% CSE; dP<0.05 when compared to 5% CSE + DEX. DEX, dexamethasone; TNF-ɑ, tumor necrosis factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; CSE, cigarette smoke extract.

Molecular mechanisms of betulin palliative therapy for COPD based on gated ion channel P2X7 receptor targets
Expression of P2X7 protein in airway tissues of COPD model mice
The expression of P2X7 protein in airway tissues of COPD mice was ascertained through Western blot. The findings illustrated that the expression of P2X7 protein in airway tissues of COPD mice was substantially increased, and the expression of P2X7 protein in the betulin group was reduced. Details are shown in Figure 7.

RT-qPCR analysis on key proteins in cells with P2X7 overexpression and stable knockdown
After transfection, the relative expression level of P2X7 in the vector group was 1.56±0.24, and that of the circP2X7 group was 4.07±0.38. This indicated that compared to the blank control vector, transfection of P2X7 overexpression vector significantly augmented the expression level of P2X7 in 16-HBE cells (P<0.05). The relative expression level of P2X7 in the shRNA-control group was 1.63±0.31, and that of the P2X7-shRNA-1 and P2X7-shRNA-2 groups were 0.18±0.009 and 0.25±0.11, respectively. This indicated that in comparison to the blank control vector, the expression level of P2X7 in 16-HBE cells was significantly decreased after transfection and knockdown of the vector (P<0.05). This study successfully constructed 16-HBE which overexpressed and knocked down P2X7.
After 16-HBE cells with P2X7 gene silencing and overexpression were treated with betulin and DEX, total proteins were obtained, and mRNA relative expression levels of key proteins were observed. The findings revealed that the mRNA expression of ERK, JNK, rho-associated protein kinase (ROCK), NF-κB, and p38 protein in the 5% CSE group was enhanced in comparison to that in the control group. After DEX and betulin intervention, mRNA relative expression levels of ERK, JNK, ROCK, NF-κB, and p38 protein were significantly decreased (all P<0.05). In 16-HBE cells transfected with P2X7-shRNA-1, relative mRNA expression levels of ERK, JNK, ROCK, NF-κB, and p38 in the 5% CSE group were substantially increased in comparison to the control group (all P<0.05). After DEX and betulin intervention, the relative mRNA expression levels of key proteins did not change significantly (all P>0.05). Details are shown in Table 7 and Figure 8.
Table 7
| Group | ERK | JNK | ROCK | NF-κB | p38 |
|---|---|---|---|---|---|
| CircP2X7 | |||||
| Control | 1.18±0.19 | 1.18±0.19 | 1.22±0.37 | 1.61±0.14 | 0.94±0.09 |
| 5% CSE | 4.45±0.83* | 4.45±0.83* | 4.69±0.56* | 6.19±0.72* | 2.46±0.28* |
| 5% CSE + DEX | 2.18±0.31*c | 2.18±0.31*c | 2.25±0.24*c | 1.76±0.15*c | 1.10±0.18*c |
| 5% CSE + betulin (10−5 mol/L) | 2.15±0.32*c | 3.15±0.32*c | 1.61±0.12*c | 2.68±0.38*c | 1.63±0.21*c |
| 5% CSE + betulin (10−4 mol/L) | 2.21±0.19*c | 2.21±0.19*c | 1.42±0.13*c | 2.54±0.26*c | 1.77±0.14*c |
| P2X7-shRNA-1 | |||||
| Control | 1.58±0.12 | 1.60±0.14 | 0.88±0.07 | 2.74±0.56 | 1.54±0.29 |
| 5% CSE | 1.89±0.10* | 1.93±0.13* | 3.54±0.31* | 3.38±0.22* | 1.90±0.10* |
| 5% CSE + DEX | 1.93±0.19* | 1.87±0.16* | 3.48±0.22* | 3.36±0.29* | 1.90±0.23* |
| 5% CSE + betulin (10−5 mol/L) | 1.91±0.13* | 1.88±0.14* | 3.41±0.25* | 3.34±0.27* | 1.86±0.22* |
| 5% CSE + betulin (10−4 mol/L) | 1.93±0.15* | 1.92±0.12* | 3.46±0.36* | 3.44±0.26* | 1.92±0.20* |
The data was expressed as (). *P<0.05 when compared to control; cP<0.05 when compared to 5% CSE. DEX, dexamethasone; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; ROCK, rho-associated protein kinase; NF-κB, nuclear factor-κB.

Western blot analysis on pathway-related proteins in cells with P2X7 gene silencing and overexpression
Key proteins were explored by Western blot. The outcomes showed that the protein expression of ERK, JNK, ROCK, NF-κB, and p38 in the model group was enhanced in comparison to that in the control group. After DEX and betulin intervention, the protein expression of ERK, JNK, ROCK, NF-κB, and p38 was decreased. In 16-HBE cells transfected with P2X7-shRNA-1, the expression of ERK, JNK, ROCK, NF-κB, and p38 protein was substantially enhanced in the model group in comparison to the control group. After DEX and betulin intervention, the expression levels of key proteins were not significantly changed. Details are shown in Figure 9.

Discussion
Betulin, also known as betula camphor, is a pentacyclic triterpenoid compound. It was discovered by scientist Johann Tobias Lowitz in 1788 and is abundant in white birch, jujube seed, and other plants. The content of betulin is highest in the bark of white birch, which is up to 30%. Study has shown that betulin has antitumor and liver protection functions, is involved in antioxidant activity, and plays an immune regulation role (9). In addition, betulin shows significant anti-inflammatory activity due to its affinity for glucocorticoid receptors. There are multiple inflammatory cell infiltrates in the airway and lung parenchyma of COPD patients, and the types of inflammatory cell infiltrates are relevant to the severity of the disease. According to a recent study, Chunhua et al. (10) found that betulin reversed pathological injury of lung tissue in COPD mice. In addition, betulin is capable of hindering the overproduction of proinflammatory cytokines TNF-a, IL-6, and IL-1β. Yue et al. (8) also found that betulin could inhibit the increased alkaline phosphatase (AKP) and albumin (ALB) levels in the lung tissue of mice induced by fine particle matter (PM2.5), reverse the inhibition of superoxide dismutase activity in BALF by PM2.5, and improve the levels of TNF-α and IL-6 in BALF. The above studies indicate that betulin has an important anti-inflammatory impact on COPD. Herein, a COPD rat model was established and betulin was administered for a period of time. After treatment, the pulmonary ventilation function of the mice was significantly improved, and the levels of inflammatory factors (TNF-α, IL-1β, and IL-6) in BALF, serum, and lung tissues were significantly decreased. The pathological changes of lung tissue were further observed, and it was found that betulin could reverse the pathological degree of lung tissue, and the remodeling indicators of lung airway and pulmonary vessels were significantly changed. These results indicated that betulin could substantially ameliorate the inflammation and pathological injury of COPD mice and promote the remodeling of pulmonary blood vessels and pulmonary airway, which was similar to the effect of DEX. In addition, betulin demonstrated no impact on the proliferation of 16-HBE cells in vitro, and the level of inflammatory factors in the supernatant of betulin-treated 16-HBE cells was significantly reduced, indicating that betulin could hinder the release of inflammatory factors through 16-HBE cells.
P2X7 is an adenosine triphosphate (ATP)-gated ion channel that performs an essential task in regulating inflammation (11-13). Studies have shown that P2X7 receptor is significantly upregulated in diverse inflammatory pathological states and can affect the expression and release of inflammatory mediators, participate in inflammatory responses and immune responses, and induce cell damage and even apoptosis (14,15). P2X7 receptor can mediate sodium ion (Na+) and calcium ion (Ca2+) influx and potassium ion K+ outflow, and activate phospholipase A2, phospholipase D, MAPK, and NF-κB, causing the generation and release of IL-6, IL-1β, and TNF-a, take part in the pathogenesis of inflammatory diseases, and activate calcium-dependent phosphatase/nuclear factor of activated T cells (NFAT), resulting in the synthesis of proinflammatory factors including cycde-2 (COX-2) and inducible-nitric oxide synthase (iNOS) (16,17). However, current research on P2X7 is mainly focused on diabetes, cardiovascular disease, and cancer. Its role in respiratory disease is rarely reported. A recent study showed that P2X7 reduces inflammatory responses in vivo by attenuation of selective antagonists in vitro and P2X7 knockout in mice, suggesting that P2X7 is a potential therapeutic target for inflammatory diseases (18). Therefore, P2X7 receptor-mediated inflammatory response may be a potential target for COPD treatment, improving drug tolerance, and a key fundamental approach for the development of new targeted therapeutic drugs. These results showed that betulin could significantly relieve smoke-induced COPD and reduce serum inflammatory factors. However, it has not been clear whether betulin can regulate airway inflammation and thus play a therapeutic role in COPD via mediating P2X7 receptor. In this study, 16-HBE which overexpressed and knocked down P2X7 was constructed and treated with 5% CSE and DEX (10-6 mol/L), betulin (10-5 mol/L), and betulin (10-4 mol/L). In 16-HBE cells transfected with circP2X7, mRNA expression of ERK, JNK, ROCK, NF-κB, and p38 proteins was increased in the 5% CSE group compared to the control group. After DEX and betulin intervention, the relative mRNA expression of ERK, JNK, ROCK, NF-κB, and p38 protein decreased significantly. In 16-HBE cells transfected with P2X7-shRNA-1, mRNA relative expression levels of ERK, JNK, ROCK, NF-κB, and p38 protein in the 5% CSE group were significantly increased compared to the control group. Similar results were obtained by measuring the expression of key proteins. These results suggested that betulin exerted its therapeutic effect by inhibiting the P2X7 signaling pathway.
In conclusion, betulin could improve lung pathological injury, improve pulmonary ventilation function, and diminish the level of inflammatory factors in COPD mice, mainly by playing a therapeutic role through the P2X7 signaling pathway.
Acknowledgments
Funding: The study was supported by the 2021 Henan Province Key R&D and Promotion Special Project (Science and Technology) (No. 212102310192) and the Key Project of Medical Science and Technology of Henan Province in 2021 and 2022 (No. SBGJ202102171).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2629/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2629/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2629/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017;389:1931-40. [Crossref] [PubMed]
- Wheaton AG, Liu Y, Croft JB, et al. Chronic Obstructive Pulmonary Disease and Smoking Status - United States, 2017. MMWR Morb Mortal Wkly Rep 2019;68:533-8. [Crossref] [PubMed]
- Ritchie AI, Baker JR, Parekh TM, et al. Am J Respir Crit Care Med 2021;204:14-22. Update in Chronic Obstructive Pulmonary Disease 2020. [Crossref] [PubMed]
- GOLD. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of COPD (2018 report) [EB/OL]. [2020-07-08]. Available online: http://www.goldcopd.org.Accessed June 2018.
- Rush B, Hertz P, Bond A, et al. Use of Palliative Care in Patients With End-Stage COPD and Receiving Home Oxygen: National Trends and Barriers to Care in the United States. Chest 2017;151:41-6. [Crossref] [PubMed]
- Bloom CI, Slaich B, Morales DR, et al. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J 2018;51:1701879. [Crossref] [PubMed]
- Tuli HS, Sak K, Gupta DS, et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants (Basel) 2021;10:2663. [Crossref] [PubMed]
- Yue Q, Deng X, Li Y, et al. Effects of Betulinic Acid Derivative on Lung Inflammation in a Model of Chronic Obstructive Pulmonary Disease Induced by Particulate Matter 2.5. Med Sci Monit 2021;27:e928954. [Crossref] [PubMed]
- Kıran İ, Çiftçi GA, Eklioğlu ÖA, et al. Bacterial Biotransformation and Anticancer Activities of Betulin against A549, HepG2 and 5RP7 Cancer Cell Lines. Anticancer Agents Med Chem 2021;21:1581-93. [Crossref] [PubMed]
- Chunhua M, Long H, Zhu W, et al. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed Pharmacother 2017;85:679-86. [Crossref] [PubMed]
- Oskolkova OV, Godschachner V, Bochkov VN. Off-Target Anti-Inflammatory Activity of the P2X7 Receptor Antagonist AZ11645373. Inflammation 2017;40:530-6. [Crossref] [PubMed]
- Sekar P, Huang DY, Hsieh SL, et al. AMPK-dependent and independent actions of P2X7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun Signal 2018;16:83. [Crossref] [PubMed]
- Oliveira-Giacomelli Á, Petiz LL, Andrejew R, et al. Role of P2X7 Receptors in Immune Responses During Neurodegeneration. Front Cell Neurosci 2021;15:662935. [Crossref] [PubMed]
- Liang S, Xu C, Li G, et al. P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine. Neurochem Int 2010;57:705-12. [Crossref] [PubMed]
- Qin X, Huang C, Wu K, et al. Anti-coronavirus disease 2019 (COVID-19) targets and mechanisms of puerarin. J Cell Mol Med 2021;25:677-85. [Crossref] [PubMed]
- Hopp SC. Targeting microglia L-type voltage-dependent calcium channels for the treatment of central nervous system disorders. J Neurosci Res 2021;99:141-62. [Crossref] [PubMed]
- Jiang LH, Caseley EA, Muench SP, et al. Structural basis for the functional properties of the P2X7 receptor for extracellular ATP. Purinergic Signal 2021;17:331-44. [Crossref] [PubMed]
- Cao SH, Yuan SP, Hou Q. Advance in the research on P2X7 and inflammatory respiratory diseases. Yao Xue Xue Bao 2013;48:1183-8. [PubMed]
(English Language Editor: A. Muijlwijk)