
Patients with comorbid coronary artery disease and hypertension: a cross-sectional study with data from the NHANES
Introduction
Coronary artery disease (CAD), characterized by high morbidity, disability, and death, is one of the most common cardiovascular diseases (1,2). According to the American Interactive Summary Health Statistics for Adults [2015–2018], 6.1–6.3% adults suffered from CAD in the USA (3).
Hypertension (HTN) is a common risk factor for CAD because it can promote coronary atherosclerosis and lead to coronary lumen stenosis (4,5). In addition, high systolic blood pressure (SBP) is a risk factor for myocardial fibrosis, myocardial ischemia, and cardiac hypertrophy (6,7). Furthermore, HTN and CAD often coexist, due to shared risk factors and pathophysiological mechanisms, as well as complex interactions (8). The patients with comorbid CAD and HTN have worse outcomes and prognosis than those with single disease. Lubsen et al. explored the 6-year cardiovascular death rate in stable CAD patients with HTN and found that it was 1.68-fold higher than that with normotension (9). Granger et al. revealed that the in-hospital mortality rate of acute coronary syndrome (ACS) patients with HTN was significantly higher than that without HTN (10). Previous studies showed that 50–60% CAD patients had comorbid HTN, and 13% HTN patients had comorbid CAD, implying a high prevalence of comorbid CAD and HTN in the general population (11-13). The high prevalence and worse outcomes of comorbid CAD and HTN could cause a tremendous threat and burden to public health and should be paid more attention by patients, physicians and healthcare provider. To optimize the antihypertensive treatment plan of HTN combined with CAD, and reduce the occurrence and death of cardiovascular events, American Heart Association (AHA)/American College of Cardiology (ACC)/American Society of Hypertension (ASH) and Chinese Society of Cardiology (CSC) issued related scientific statements in succession (8,14). Despite increasingly emerging studies in the field, the prevalence of patients with comorbid CAD and HTN in the entire population still remains unclear.
The National Health and Nutrition Examination Survey (NHANES) is a series of nationwide cross-sectional survey to explore the health and nutritional status of American people. It combines interviews, laboratory tests, and physical examinations of patients, including those with comorbid CAD and HTN (15). In the present cross-sectional study, the prevalence and influence factors of patients with comorbid CAD and HTN were explored and compared by analyzing data obtained from NHANES in two cycles (1999–2000 and 2017–2018). We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2766/rc).
Methods
Study population
The NHANES started half a century ago and has been performed as a series of surveys to assess different population groups in the USA. A multistage, probability sampling design, which samples individuals in strata defined by age, ethnicity and geographic location, has been used to select participants representative of the non-institutionalized USA population. Participants in NHANES have given informed consent for their anonymized data to be used, which was approved by the Institutional Review Board of the Centers for Disease Control. The survey has been conducted in 2-year cycles since 1999–2000 and the most recently completed cycle is 2017–2018. Analysis of anonymous data from an open database was considered as exempt research by the Institutional Review Board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion of participants
All adult participants (≥18 years of age) who were diagnosed with comorbid CAD and HTN in the study periods were included in the present study. HTN was defined according to the 2017 ACC/AHA guideline for the prevention, detection, evaluation, and management of high blood pressure (BP) in adults (16). Briefly, the patients had SBP ≥130 mmHg or diastolic blood pressure (DBP) ≥80 mmHg, a self-report of hypertension diagnosis or were currently taking medication to reduce BP. CAD, also referred to as coronary heart disease or ischemic heart disease, included stable angina, unstable angina, myocardial infarction, and sudden cardiac death (17,18). The participants with self-reported history of HTN and CAD, previously diagnosed by a physician, were included. Those with missing history were excluded. According to the recent dyslipidemia management guidelines published by ACC/AHA and European Society of Cardiology (ESC), the appropriate serum low-density lipoprotein cholesterol (LDL-C) level of CAD patients is defined as <1.8 mmol/L (19,20).
Data collection
Demographic and clinical factors, including age and sex, physical examination [BP, pulse, and body mass index (BMI)], laboratory data (blood lipids, glucose etc.) and questionnaire data (cardiovascular risk factors, comorbidity, and medications) were collected and compared between the participants in the two cycles, 1999–2000 and 2017–2018. Specially, age, sex, smoking, medical history [heart failure, diabetes mellitus (DM), obesity, and chronic kidney disease (CKD)], and medication use [angiotensin converting enzyme inhibitors/angiotensin receptor blocker (ACEI/ARB), β-blocker, calcium channel blocker (CCB), diuretic and statin] were obtained from standardized interviews. SBP, DBP and pulse were measured in the mobile examination center (MEC). Laboratory data including total cholesterol, triglyceride, LDL-C, high-density lipoprotein cholesterol (HDL-C), red blood cell (RBC) count, white blood cell (WBC) count, creatinine, urea nitrogen, uric acid, glucose and glycohemoglobin, were tested with the blood specimen collected in the MEC. BMI was calculated by weight (kg)/height (m)2.
Assessment of influence factors
The changes of influence factors, such as the demographic and clinical factors as well as medication use mentioned above, were compared between the two cycles with the analytic approaches shown in the part of Statistical analysis. HTN control rate and LDL-C control rate were also compared. HTN control rate was defined as the proportion of patients with SBP <130 mmHg and DBP <80 mmHg. LDL-C control rate was defined as the proportion of patients with LDL-C <1.8 mmol/L. Given the prevalence of cardiovascular diseases increase with age, regardless of race and gender, and population ageing present a vital burden for current healthcare (21,22), we performed subgroup analyses between the elder (≥65 years of age) and middle-young (18–65 years of age) populations.
Statistical analysis
The continuous data obtained from the current study, such as age and BP, are presented as mean ± standard deviation (SD) or median (25% percentile, 75% percentile) depending on whether the data were normally distributed. Further, categorical variables, such as the proportion of males and the usage of medications, are presented as percentages. The prevalence of the patients with comorbid CAD and HTN in 1999–2000 and 2017–2018 cycles were age adjusted by the direct method to the USA Census 2000 population. The comparisons of demographic and clinical factors between the two cycles (1999–2000 and 2017–2018) and age subgroups were performed with SAS 9.4 (SAS Institute Inc., NC, USA). In detail, the categorical variables, such as the proportions of males, smoking, medical history and usage of medications, as well as HTN or LDL-C control rate, were compared between the two cycles by chi-squared test. Non-normally distributed data, such as BP, pulse, BMI, serum lipid, glucose and renal function, were compared by Wilcoxon rank test. The statistically significant difference was set at P<0.05 (two-sided).
Results
Selection of eligible participants from NHANES is shown in Figure 1. The prevalence of comorbid CAD and HTN was 5.30% (289/5,448) during the first cycle of data collection [1999–2000] and 6.52% (382/5,856) during the 2017–2018 cycle. After age adjustment by the direct method to the USA Census of 2000 population, the age-adjusted prevalence of patients with comorbid CAD and HTN was significantly higher in 2017–2018 than in 1999–2000 (5.40% vs. 4.22%, P=0.006) (Figure 2).

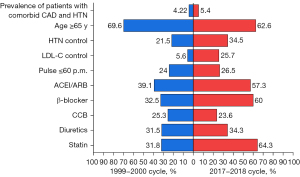
From 1999–2000 to 2017–2018, the age of patients with comorbid CAD and HTN decreased from 71 [63–79] to 69 [61–77] years (P=0.008), and the percentage of those aged ≥65 years of age decreased from 69.6% to 62.6% (P=0.059). On the other hand, the percentage of those aged <65 years of age increased from 30.4% to 37.4%. Compared with the 1999–2000 cycle, the proportions of patients complicated with DM (28.7 vs. 45.6%, P<0.0001), obesity (47.4% vs. 49.6%, P=0.003) and CKD (9.0% vs. 18.6%, P<0.001) all increased in the 2017–2018 cycle among the population with comorbid CAD and HTN (Table 1).
Table 1
| Characteristics | 1999–2000 (n=289) | 2017–2018 (n=382) | P value |
|---|---|---|---|
| Male (%) | 58.1 | 64.1 | 0.130 |
| Age (years) | 71 [63–79] | 69 [61–77] | 0.008 |
| Age (%) | |||
| ≥65 years | 69.6 | 62.6 | 0.059 |
| <65 years | 30.4 | 37.4 | 0.059 |
| SBP (mmHg) | 142 [128–157] | 135 [122–150] | <0.001 |
| DBP (mmHg) | 71 [63–80] | 71 [63–78] | 0.485 |
| HTN control rate (%) | 21.2 | 34.5 | 0.001 |
| Pulse (p.m.) | 68 [62–78] | 68 [60–74] | 0.337 |
| Proportion of pulse ≤60 p.m. (%) | 24.0 | 26.5 | 0.497 |
| BMI (kg/m2) | 28 [25–32] | 30 [26–35] | <0.001 |
| Heart failure (%) | 28.0 | 31.7 | 0.541 |
| Diabetes (%) | 28.7 | 45.6 | <0.001 |
| Obesity (%) | 47.4 | 49.6 | 0.003 |
| CKD (%) | 9.0 | 18.6 | <0.001 |
| Smoking (%) | 23.7 | 27.9 | 0.361 |
Data are presented as percent or median [25% percentile–75% percentile]. SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension; p.m., per minute; BMI, body mass index; CKD, chronic kidney disease.
The median SBP of the included participants in the 2017–2018 cycle was 135 [122–150] mmHg, which was less than the 142 [128–157] mmHg in the 1999–2000 cycle (P=0.001), while the DBP were similar between the two cycles. There was a significant increase in the HTN control rate (34.5% in 2017–2018 cycle vs. 21.2%, P=0.001 in 1999–2000 cycle). The pulse per minute (p.m.) was 68 [62–78] in 1999–2000 cycle and 68 [60–74] in the 2017–2018 cycle (P=0.337), and the proportions of pulse ≤60 p.m. were 24.0% and 26.5% in the 1999–2000 and 2017–2018 cycles (P=0.497), respectively (Table 1).
The values of serum lipids (total cholesterol, triglycerides, and LDL-C) of the included participants in the 2017–2018 cycle, as well as the control rate of LDL-C were significantly lower than in the 1999–2000 cycle (all P<0.05). On the other hand, the values of creatinine, glucose and glycohemoglobin were significantly higher in the 2017–2018 cycle than in the 1999–2000 cycle (both P<0.001) (Table 2).
Table 2
| Laboratory tests | 1999–2000 (n=289) | 2017–2018 (n=382) | P value |
|---|---|---|---|
| Total cholesterol (mmol/L) | 5.1 [4.5–6.0] | 4.3 [3.7–5.0] | <0.001 |
| Triglyceride (mmol/L) | 1.7 [1.3–2.5] | 1.3 [0.9–1.7] | <0.001 |
| LDL-C (mmol/L) | 3.0 [2.6–3.5] | 2.3 [1.8–2.9] | <0.001 |
| LDL-C control rate (%) | 5.6 | 25.7 | <0.001 |
| HDL-C (mmol/L) | 1.1 [1.0–1.4] | 1.2 [1.0–1.5] | 0.01 |
| RBC count (million cells/µL) | 4.6 [4.3–5.0] | 4.6 [4.3–5.0] | 0.797 |
| WBC count (1,000 cells/µL) | 7.1 [6.1–8.6] | 7.1 [5.9–8.6] | 0.714 |
| Creatinine (μmol/L) | 71 [62–88] | 87 [72–110] | <0.001 |
| Urea nitrogen (mmol/L) | 6.1 [4.6–7.5] | 6.1 [5.0–8.2] | 0.302 |
| Uric acid (μmol/L) | 354 [292–434] | 351 [297–410] | 0.349 |
| Glucose (mmol/L) | 5.4 [4.9–6.4] | 5.6 [5.1–6.9] | 0.009 |
| Glycohemoglobin (%) | 5.7 [5.3–6.5] | 6.0 [5.6–6.8] | <0.001 |
Data are presented as percent or median [25% percentile–75% percentile]. LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; RBC, red blood cell; WBC, white blood cell.
Rate of statin usage increased from 31.8% to 64.3%, use of β-blockers increased from 32.5% to 60.0%, and that of ACEIs/ARBs increased from 39.1% to 57.3% among patients with comorbid CAD and HTN (P<0.001) (Table 3).
Table 3
| Medication | 1999–2000 (n=289) | 2017–2018 (n=382) | P value |
|---|---|---|---|
| ACEI/ARB (%) | 39.1 | 57.3 | <0.001 |
| β-blocker (%) | 32.5 | 60.0 | <0.001 |
| CCB (%) | 25.3 | 23.6 | 0.611 |
| Diuretic (%) | 31.5 | 34.3 | 0.444 |
| Statin (%) | 31.8 | 64.3 | <0.001 |
Data are presented as percent. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Subgroup analyses were performed by age (Figure 3, Table 4). Compared with the 1999–2000 cycle, the age-adjusted prevalence of patients with comorbid CAD and HTN among young and middle-aged population (18–65 years of age) increased from 1.81% to 2.73% (P<0.001), and among the elder population (≥65 years of age) from 2.42% to 2.67% (P<0.001). For both cycles, the HTN control rate, SBP and renal function of the middle-young group were superior to those of the elder group, but other aspects of middle-young group, including DBP, pulse, blood lipids and oral mediation rates, were inferior to the latter.
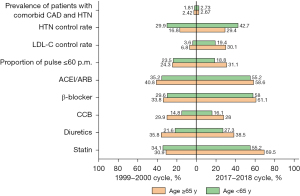
Table 4
| Items | 1999–2000 | 2017–2018 | |||||
|---|---|---|---|---|---|---|---|
| ≥65 | <65 | P value | ≥65 | <65 | P value | ||
| N (%) | 201 (69.6) | 88 (30.4) | – | 239 (62.6) | 143 (37.4) | – | |
| SBP (mmHg) | 145 [132–161] | 134 [122–146] | <0.001 | 137 [127–153] | 129 [118–139] | <0.001 | |
| DBP (mmHg) | 69 [61–76] | 76 [69–83] | 0.001 | 68 [58–77] | 75 [67–81] | <0.001 | |
| HTN control rate (%) | 16.8 | 29.9 | 0.034 | 29.4 | 42.7 | 0.018 | |
| Pulse (p.m.) | 68 [62–76] | 70 [62–84] | 0.185 | 66 [60–72] | 70 [62–82] | <0.001 | |
| Proportion of pulse ≤60 p.m. (%) | 24.3 | 23.5 | 0.895 | 31.1 | 18.8 | 0.012 | |
| Total cholesterol (mmol/L) | 5.0 [4.4–5.8] | 5.5 [4.6–6.1] | 0.065 | 4.2 [3.6–4.9] | 4.4 [3.8–5.3] | 0.127 | |
| Triglycerides (mmol/L) | 1.6 [1.2–2.3] | 2.1 [1.5–3.2] | 0.022 | 1.2 [0.8–1.6] | 1.42 [1.0–1.8] | 0.065 | |
| LDL-C (mmol/L) | 3.0 [2.6–3.5] | 2.9 [2.5–3.7] | 0.968 | 2.2 [1.7–2.6] | 2.6 [2.0–3.3] | 0.009 | |
| LDL-C control rate (%) | 6.8 | 3.6 | 0.558 | 30.1 | 19.4 | 0.117 | |
| HDL-C (mmol/L) | 1.1 [1.0–1.4] | 1.1 [0.9–1.3] | 0.311 | 1.2 [1.0–1.5] | 1.1 [1.0–1.4] | 0.016 | |
| Creatinine (μmol/L) | 79.6 [61.9–97.2] | 70.7 [53–79.6] | 0.001 | 97.3 [76.9–118.5] | 76.9 [66.3–88.8] | <0.001 | |
| Urea nitrogen (mmol/L) | 6.4 [5–8.2] | 5.4 [4.6–6.8] | 0.001 | 6.8 [5.4–9.3] | 5.4 [4.3–6.4] | <0.001 | |
| Glucose (mmol/L) | 5.4 [5.00–6.2] | 5.4 [4.8–6.8] | 0.773 | 5.6 [5.1–6.9] | 5.63 [5.1–7.1] | 0.921 | |
| Glycohemoglobin (%) | 5.7 [5.3–6.3] | 5.6 [5.3–6.8] | 0.758 | 6.1 [5.7–6.8] | 5.9 [5.5–6.8] | 0.099 | |
| ACEI/ARB (%) | 40.8 | 35.2 | 0.372 | 58.6 | 55.2 | 0.524 | |
| β-blocker (%) | 33.8 | 29.6 | 0.474 | 61.1 | 58 | 0.557 | |
| CCB (%) | 29.9 | 14.8 | 0.007 | 28 | 16.1 | 0.008 | |
| Diuretic (%) | 35.8 | 21.6 | 0.017 | 38.5 | 27.3 | 0.025 | |
| Statin (%) | 30.9 | 34.1 | 0.586 | 69.5 | 55.2 | 0.005 | |
Data are presented as percent or median [25% percentile, 75% percentile]. SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension; p.m., per minute; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Discussion
The major finding of our study was that the age-adjusted prevalence of patients with comorbid CAD and HTN increased from 4.22% in the 1999–2000 survey cycle to 5.40% in the 2017–2018 survey cycle. The increased proportion predominantly came from patients aged less than 65 years. The age of patients with comorbid CAD and HTN decreased and the prevalence of complications with DM, obesity and CKD increased together with lower levels of SBP and serum lipids, as well as higher control rates of HTN and LDL-C, due to increased use of statins, ACEIs/ARBs and β-blockers in the 2017–2018 cycle as compared with the 1999–2000 cycle.
Taken together, this study hinted at a significant change in recent disease profile than 20 years ago. We encounter more patients with comorbid CAD and HTN at a younger age and with more complications such as DM, obesity and CKD, but better BP and lipid control due to increased use of effective cardiovascular medications including statins, ACEIs/ARBs and β-blockers.
According to previous research, the age-adjusted prevalence of HTN in the USA was 47.0% in 1999–2000 and slightly decreased to 45.4% in 2017–2018 (23,24). The prevalence of CAD in the USA significantly decreased from 10.3% [2001–2002] to 8.0% [2011–2012], and has stabilized between 6.1% and 6.3% in recent years (25,26). The decreased prevalence of HTN or CAD in high-income countries might be related to improvements in the management of risk factors in addition to medication use. Contrary to the prevalence of CAD or HTN, we found for the first time that the recent prevalence of comorbid CAD and HTN increased than that 20 years ago, which might be due to a decrease in the proportion of patients with only CAD or HTN, because patients with only CAD or HTN are more easily treated than those with comorbid CAD and HTN. Further, subgroup analyses revealed the increased prevalence of comorbid CAD and HTN was predominantly in the middle-young population, due not only to an expanding population of the middle-young but also to a greater increment of prevalence in the middle-young than the elder population. Additionally, DBP, pulse, blood lipids and the oral mediation rates of young-middle patients were inferior to those of their elder counterpart, suggesting they are not giving enough attention and effort to their health management. Therefore, there is clinical benefit to strengthening the screening and treatment of CAD and HTN among the young-middle population.
Dyslipidemia, HTN, DM, obesity and CKD are independent and modifiable risk factors of cardiovascular disease (26,27). The current study revealed that the serum levels of lipids (total cholesterol and LDL-C) and the control rate of LDL-c improved significantly in the 2017–2018 cycle, which could be due to the increased use of statins. Also, the improved HTN control rate may be related to increased use of ACEIs/ARBs and β-blockers, because the use of CCBs or diuretics did not change significantly. However, the proportions of cases complicated with DM, obesity or CKD, as well as the levels of serum glucose, glycohemoglobin and creatinine, increased among American patients with comorbid CAD and HTN in the 2017–2018 cycle than the former period, emphasizing the need for strengthened patient education in the benefits of physical exercise, glucose control and improved renal function.
Notably, ACEIs/ARBs, β-blockers, and statins are the basic and first-line medications of CAD or HTN (8,28) and are widely recommended by the American and European guidelines. However, application is poor in the real world. Spannella et al. found that the prevalence of dyslipidemia was 91.1% among HTN patients, and lipid-lowering drugs were only taken by 23.1% of patients (29). Cheng et al. found at 1–2 years post-ACS, no more than 45% of patients were adherent to statin or β-blocker therapy, with a further decrease over a 10-year follow-up (30). The present study also found that, despite a significantly increased use of these basic medications in the 2017–2018 cycle, there is still much room for improvement of their utilization in future.
The heart rate of patients with CAD is recommended to be no more than 60 beats p.m. (31), but as the heart rate data of the patients in NHANES was not available for the current study, pulse p.m. was used instead. Although there was a considerably increased use of β-blockers in the 2017–2018 cycle than the former, it was evident that nearly two-thirds of the patients failed to achieve the standard pulse p.m., which calls for intensified dosing of β-blockers.
Despite greater use of statins, ACEIs/ARBs, and β-blockers, as well as better control of HTN and serum lipids, it was evident that the proportions of myocardial infarction and heart failure did not significantly decrease among patients with comorbid CAD and HTN in 2017–2018. This could be due to the increasing prevalence of DM and obesity, as well as early-onset CAD and HTN, which counteracted the benefits of increasing use of statins and hypotensive drugs.
Conclusions
Contrary to the decreased prevalence of CAD or HTN, the recent prevalence of patients with comorbid HTN and CAD significantly increased in the USA compared to 20 years ago, predominantly due to an increase among middle-young patients. On the other hand, BP and lipids were better controlled in the 2017–2018 cycle than in the former period, which may be related to increased use of statins, ACEIs/ARBs, and β-blockers, although there is still much room for improvement of their utilization in future. Anyway, it is vital to strengthen the intervention of high cardiovascular risk and the treatment for patients with comorbid CAD and HTN, especially among the young-middle population.
Acknowledgments
Funding: The study was funded by the National Natural Science Foundation of China (No. 81603327), the Major Clinical Study Program of 3-year Action Plan for Promoting Clinical Skills and Clinical Innovation in Municipal Hospitals of Shanghai Shen Kang Hospital Development Center (No. SHDC2020CR1042B) and Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (No. 2058).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2766/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2766/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Analysis of anonymous data from an open database was considered as exempt research by the Institutional Review Board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bauersachs R, Zeymer U, Brière JB, et al. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc Ther 2019;2019:8295054. [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256. [Crossref] [PubMed]
- National Center for Health Statistics. Crude percentages of coronary heart disease for adults aged 18 and over, United States, 2015–2018. National Health Interview Survey. Generated interactively: Oct 23, 2021.
- Task Force on Chinese Guidelines for the Prevention of Cardiovascular Diseases (2017). Chinese guidelines for the prevention of cardiovascular diseases (2017). Zhonghua Xin Xue Guan Bing Za Zhi 2018;46:10-25. [PubMed]
- Weber T, Lang I, Zweiker R, et al. Hypertension and coronary artery disease: epidemiology, physiology, effects of treatment, and recommendations: A joint scientific statement from the Austrian Society of Cardiology and the Austrian Society of Hypertension. Wien Klin Wochenschr 2016;128:467-79. [Crossref] [PubMed]
- Bornstein AB, Rao SS, Marwaha K. Left Ventricular Hypertrophy. (Updated 2021 Aug 11). In: StatPearls [Internet]. Treasure Island: StatPearls Publishing, 2021.
- Kahan T, Bergfeldt L. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart 2005;91:250-6. [Crossref] [PubMed]
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of Hypertension in Patients with Coronary Artery Disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation 2015;131:e435-70. [Crossref] [PubMed]
- Lubsen J, Wagener G, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with symptomatic stable angina and hypertension: the ACTION trial. J hypertens 2005;23:641-8. [Crossref] [PubMed]
- Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345-53. [Crossref] [PubMed]
- Canto JG, Kiefe CI, Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011;306:2120-7. [Crossref] [PubMed]
- Hajar R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017;18:109-14. [Crossref] [PubMed]
- Zang J, Liang J, Zhuang X, et al. Intensive blood pressure treatment in coronary artery disease: implications from the Systolic Blood Pressure Intervention Trial (SPRINT). J Hum Hypertens 2022;36:86-94. [Crossref] [PubMed]
- Chen YD, Sun NLCardiology Branch of Chinese International Exchange and Promotive Association for Medical and Healthcare. The Chinese expert consensus on blood pressure management in patients with comorbid hypertension and coronary artery disease. Zhonghua Yi Xue Za Zhi 2022;102:717-28. [PubMed]
- Center for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. 2021. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed October 21, 2021).
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13-e115. [PubMed]
- Center for Disease Control and Prevention. Coronary Artery Disease (CAD). 2021. Retrieved November 1, 2021. Available online: https://www.cdc.gov/heartdisease/coronary_ad.htm
- Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol 2014;11:276-89. [Crossref] [PubMed]
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082-143. [PubMed]
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. [Crossref] [PubMed]
- Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 2009;25:563-77. vii. [Crossref] [PubMed]
- Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis 2019;6:19. [Crossref] [PubMed]
- Ostchega Y, Fryar CD, Nwankwo T, et al. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017-2018. NCHS Data Brief 2020;1-8. [PubMed]
- Dorans KS, Mills KT, Liu Y, et al. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J Am Heart Assoc 2018;7:008888. [Crossref] [PubMed]
- Yoon SS, Dillon CF, Illoh K, et al. Trends in the Prevalence of Coronary Heart Disease in the U.S.: National Health and Nutrition Examination Survey, 2001-2012. Am J Prev Med 2016;51:437-45. [Crossref] [PubMed]
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. [Crossref] [PubMed]
- Bays HE, Taub PR, Epstein E, et al. Ten things to know about ten cardiovascular disease risk factors. Am J Prev Cardiol 2021;5:100149. [Crossref] [PubMed]
- Olafiranye O, Zizi F, Brimah P, et al. Management of Hypertension among Patients with Coronary Heart Disease. Int J Hypertens 2011;2011:653903. [Crossref] [PubMed]
- Spannella F, Giulietti F, Di Pentima C, et al. Prevalence and Control of Dyslipidemia in Patients Referred for High Blood Pressure: The Disregarded "Double-Trouble" Lipid Profile in Overweight/Obese. Adv Ther 2019;36:1426-37. [Crossref] [PubMed]
- Cheng K, Ingram N, Keenan J, et al. Evidence of poor adherence to secondary prevention after acute coronary syndromes: possible remedies through the application of new technologies. Open Heart 2015;2:e000166. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
(English Language Editor: K. Brown)


