Age at initial diagnosis and prognosis of breast cancer: a nationwide multicenter retrospective study in China
Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer-related death among females. According to Global Cancer Statistics 2020, about 2.3 million new female BC cases were diagnosed, accounting for almost 1 in 4 of all cancer cases among women (1). In 2015, it was estimated that BC accounted for 15% of new cancer cases and 7% of deaths of females due to cancer in China (2). Previous studies have reported the effect of age on clinicopathological characteristics of BC (3). According to female BC statistics from 2017, in the United States, the median age at diagnosis of BC for white and black women was 63 and 59 years, respectively (4). In contrast, the mean age at diagnosis in China was 45–55 years, considerably lower than that of Western women. In addition, studies indicate that there are differences in the clinicopathological characteristics, diagnosis, treatment patterns, and prognosis of BC among patients of different ages (5-8). Therefore, confirming the age distribution is crucial in guiding the treatment and prevention of BC. In most available studies, participants have generally been grouped by matching their age, which was usually adjusted during the data analysis. However, such studies cannot show the full effect of age on the development of BC.
Adjuvant systemic therapy, including chemotherapy, radiotherapy, and endocrine therapy, is a popular BC treatment (9). However, recurrence or metastasis occurs in about 30% of patients with early BC after receiving adjuvant therapy. This means that the proportion of advanced breast cancer (ABC) in China will rise in the next few years, and the burden of BC will be compounded (10). Among the early-stage patients with BC, 30–40% will ultimately develop ABC. Patients with ABC have a 5-year survival rate of approximately 20%, and the overall median survival time is 2 to 3 years (11). In terms of treatment options and efficacy, ABC differs from other BC stages. Reports show that patients with ABC can experience severe financial and psychological stress. However, previous studies have lacked high-quality evidence from hospital-based clinical research. Treatment of ABC still has limitations. China’s population-based cancer registration system continues to improve, providing a basis for cancer prevention and treatment policies. However, population-based cancer registration data lacks information on clinical characteristics, diagnostic methods, treatment options, and prognosis of the disease, making it difficult to provide a reference for ABC treatment guidelines. Recently, hospital-based multicenter clinical epidemiological investigations have commenced (12,13). These studies have focused on clinical characteristics, diagnosis, and treatment characteristics, which could compensate for the disadvantages of population-based cancer registration data. The hospital-based multicenter clinical epidemiological study presented in this article recruited Chinese women newly diagnosed with ABC and retrospectively collected their previous information from medical records. This study aimed to explore the effects of age at initial diagnosis on the clinicopathological characteristics, treatment pattern, and prognosis of BC. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-302/rc).
Methods
Study design
This study was a hospital-based multicenter retrospective epidemiological study. According to the traditional administrative district definition, China was stratified into seven geographical regions: Central China, North China, Northeast China, Northwest China, East China, South China, and Southwest China. Three tertiary hospitals were selected in each of the seven geographic regions. Therefore, a total of 21 hospitals were selected for our study, representing different levels of disease burden and treatment of BC in most areas of China. All the hospitals were required to enroll ABC patients randomly from January 2012 to December 2014. Patients’ general information, clinicopathological features, treatment information, and prognosis were retrospectively collected based on the designed case report form (CRF). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS) (No. 15-115/1042) and individual consent for this retrospective analysis was waived.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) female BC patients newly diagnosed as stage IV or with local recurrence or distant metastasis; (II) admitted to the selected hospitals from 1 January 2012 to 31 December 2014; and (III) complete information of previous history, diagnosis, and treatment history, including surgical pathology results, time, and location of metastasis, detailed therapeutic methods, and protocols after metastasis. Participants who were repeatedly enrolled or with more than 50% missing of their clinicopathological information were excluded. Since we aimed to explore the associations between age at initial diagnosis and risk of recurrence or metastasis, patients with stage IV were also excluded from the study.
Data collection and quality control
To avoid selection bias, the number of cases enrolled in each hospital was determined according to the population density in the region. An enrolment scheme was adopted using alternating prespecified months of inpatient admission from 2012 to 2014. All the hospitals were given random month numbers to enroll patients. Since January and February each year usually include the Chinese traditional spring festival holiday, these two months were excluded. If the number of qualifying cases collected in the selected month was less than the specified number, the cases from the next month were immediately included until enough cases were collected.
The original case information was obtained from the medical record room of each hospital. The following information was retrospectively collected for all enrolled patients: (I) general information, including name, ID number, date of initial diagnosis with BC, inpatient admission date, inpatient discharge date, and diagnosis at discharge; (II) demographic characteristics and BC risk factors, including age at initial diagnosis, nationality, occupation, height, weight, education level and marital status; (III) clinical and imaging diagnostic information when firstly diagnosed with BC; (IV) molecular subtypes; (V) surgical and adjuvant therapy information; and (VI) recurrence and metastasis information including distant metastasis sites, disease-free interval (DFI), endocrine therapy, targeted therapy, and chemotherapy after metastasis. Staging of BC was performed according to the 2003 Union for International Cancer Control tumor-node-metastasis (UICC TNM) staging system, and the histology classification referred to the World Health Organization (WHO) Classification of Tumors of the Breast. Molecular subtypes were identified according to the St. Gallen Guidelines, 2013 (14). (I) Luminal A: estrogen receptor (ER)+, progestogen receptor (PR)+, human epidermal growth factor receptor 2 (HER2)−, and Ki-67 <14%; (II) luminal B: (i) ER+, HER2−, and at least one of Ki-67 >14%, PR−, or low expression, and low recurrence risk based on polygene expression analysis (if applicable); (ii) ER+, HER2+, any Ki-67 and any PR; (III) HER2-enriched: ER−, PR− and HER2+; and (IV) triple-negative: ER−, PR−, and HER2−. DFI was defined as the time from the first diagnosis of BC by surgery or puncture to recurrence or metastasis, whichever was the earliest. Recurrence and distant metastasis were diagnosed by clinical evaluations, including imaging studies or biopsy. Distant metastasis was defined as metastasis of BC that developed beyond the ipsilateral or contralateral breast, chest wall, or regional lymph node, including the ipsilateral axillary, supraclavicular, or internal mammary lymph node. Four main sites of distant metastasis were identified in our study: bone metastasis, liver metastasis, lung metastasis, and brain metastasis.
All the above information was extracted from medical charts to the CRF by local staff after training. The CRF was designed by the experts from the CHCAMS. Epidata was used for the double-entry of data. All databases were checked for consistency and sent to CHCAMS for a logical check. A total of 5% of the CRF were randomly selected for quality control review.
Statistical analysis
Participants were divided into five groups according to their age at diagnosis of BC: <35, 35–44, 45–54, 55–64, and ≥65 years. The chi-square (χ2) test were used to evaluate differences in categorical variables between the different age groups. Kaplan-Meier analysis was used for cumulative disease-free survival rates with a log-rank test to assess the significance among different age groups. Cox proportional hazards regression models were used to determine hazard ratios (HR) and 95% confidence intervals (CI) for the associations between age groups and the risk of recurrence and metastasis. Potential confounders were stage, grade, molecular subtypes, surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, adjuvant radiotherapy, adjuvant endocrine therapy, and family history of BC. In the comparisons, the group aged <35 years was set as the referral group. Subgroup analyses were also conducted according to the metastatic site. All statistical analyses were performed using SPSS software version 23.0 (IBM Corp., Armonk, NY, USA), and a P value <0.05 was considered statistically significant.
Results
Demographic characteristics at initial diagnosis
Data from 1,852 Chinese women with ABC were available for the final analysis (Figure S1). Age at initial diagnosis ranged from 21 to 89 years, with a mean age ± standard deviation (SD) of 46.0±9.9 years. Of the total, 228 (12.3%) patients were aged <35 years at initial diagnosis, 628 (33.9%) were aged 35–44 years, 616 (33.3%) were aged 45–54 years, 318 (17.2%) patients were aged 55–64 years old, and 62 (3.3%) patients were aged ≥65 years old. Among these five age groups, the distribution of body mass index (BMI) (χ2=55.184; P<0.01) and the number of live births (χ2=231.272; P<0.01) were significantly different. The baseline demographic characteristics at initial diagnosis are shown in Table S1.
Clinicopathological characteristics at initial diagnosis
The clinicopathological characteristics at initial diagnosis of patients of different ages are shown in Table 1. A total of 1,433 patients were diagnosed with invasive ductal carcinoma, accounting for 77.4% of the total cases. The distribution of hormone receptor status, HER2 status, the number of lymph node metastasis, and molecular subtypes differed significantly between age groups (all P<0.05). Some 37.4% (119/318) of patients aged 55–64 years were positive for HER2 (χ2=12.510; P<0.05) and 59.6% (136/228) of those aged <35 years were positive for hormone receptor (χ2=24.608; P<0.01). Lymph node metastasis was not present in 41.9% of patients aged ≥65 years. The most common subtype in all patients was luminal B, and the proportion of HER2-enriched subtype (18/62, 29.0%) in patients aged ≥65 was significantly higher than that of the other age groups (χ2=33.613; P<0.01).
Table 1
| Clinicopathological features at initial diagnosis | Age at initial diagnosis | Total | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| <35 years | 35–44 years | 45–54 years | 55–64 years | ≥65 years | ||||
| ER status | 17.952 | <0.01 | ||||||
| + | 124 (54.4%) | 337 (53.7%) | 277 (45.0%) | 165 (51.9%) | 26 (41.9%) | 929 (50.2%) | ||
| − | 70 (30.7%) | 197 (31.4%) | 255 (41.4%) | 121 (38.1%) | 27 (43.5%) | 670 (36.2%) | ||
| Unknown | 34 (14.9%) | 94 (15.0%) | 84 (13.6%) | 32 (10.1%) | 9 (14.5%) | 253 (13.7%) | ||
| PR status | 41.932 | <0.01 | ||||||
| + | 120 (52.6%) | 325 (51.8%) | 254 (41.2%) | 129 (40.6%) | 21 (33.9%) | 849 (45.8%) | ||
| − | 73 (32.0%) | 203 (32.3%) | 279 (45.3%) | 164 (51.6%) | 33 (53.2%) | 752 (40.6%) | ||
| Unknown | 35 (15.4%) | 100 (15.9%) | 83 (13.5%) | 25 (7.9%) | 8 (12.9%) | 251 (13.6%) | ||
| Hormone receptor status | 24.608 | <0.01 | ||||||
| + | 136 (59.6%) | 369 (58.8%) | 308 (50.0%) | 176 (55.3%) | 26 (41.9%) | 1,015 (54.8%) | ||
| − | 58 (25.4%) | 161 (25.6%) | 224 (36.4%) | 109 (34.3%) | 27 (43.5%) | 579 (31.3%) | ||
| Unknown | 34 (14.9%) | 98 (15.6%) | 84 (13.6%) | 33 (10.4%) | 9 (14.5%) | 258 (13.9%) | ||
| HER2 status | 12.510 | <0.05 | ||||||
| + | 77 (33.8%) | 170 (27.1%) | 213 (34.6%) | 119 (37.4%) | 16 (25.8%) | 595 (32.1%) | ||
| − | 107 (46.9%) | 335 (53.3%) | 298 (48.4%) | 145 (45.6%) | 30 (48.4%) | 915 (49.4%) | ||
| Unknown | 44 (19.3%) | 123 (19.6%) | 105 (17.0%) | 54 (17.0%) | 16 (25.8%) | 342 (18.5%) | ||
| Stage at initial diagnosis | 12.845 | 0.12 | ||||||
| I | 23 (10.1%) | 74 (11.8%) | 53 (8.6%) | 35 (11.0%) | 5 (8.1%) | 190 (10.3%) | ||
| II | 103 (45.2%) | 253 (40.3%) | 244 (39.6%) | 115 (36.2%) | 18 (29.0%) | 733 (39.6%) | ||
| III | 102 (44.7%) | 301 (47.9%) | 319 (51.8%) | 168 (52.8%) | 39 (62.9%) | 929 (50.2%) | ||
| Metastatic lymph nodes | 36.780 | <0.01 | ||||||
| 0 | 91 (39.9%) | 205 (32.6%) | 221 (35.9%) | 110 (34.6%) | 26 (41.9%) | 653 (35.3%) | ||
| 1–2 | 44 (19.3%) | 121 (19.3%) | 89 (14.4%) | 43 (13.5%) | 6 (9.7%) | 303 (16.4%) | ||
| 3–8 | 55 (24.1%) | 172 (27.4%) | 152 (24.7%) | 61 (19.2%) | 16 (25.8%) | 456 (24.6%) | ||
| ≥9 | 38 (16.7%) | 130 (20.7%) | 154 (25.0%) | 104 (32.7%) | 14 (22.6%) | 440 (23.8%) | ||
| Pathological type | 13.832 | 0.312 | ||||||
| Carcinoma in situ | 6 (2.6%) | 22 (3.5%) | 16 (2.6%) | 9 (2.8%) | 1 (1.6%) | 54 (2.9%) | ||
| Invasive ductal carcinoma | 180 (78.9%) | 484 (77.1%) | 476 (77.3%) | 238 (74.8%) | 55 (88.7%) | 1,433 (77.4%) | ||
| Others invasive | 22 (9.6%) | 58 (9.2%) | 50 (8.1%) | 40 (12.6%) | 2 (3.2%) | 172 (9.3%) | ||
| Others | 17 (7.5%) | 48 (7.6%) | 57 (9.3%) | 21 (6.6%) | 2 (3.2%) | 145 (7.8%) | ||
| Unknown | 3 (1.3%) | 16 (2.5%) | 17 (2.8%) | 10 (3.1%) | 2 (3.2%) | 48 (2.6%) | ||
| Grade | 7.284 | 0.51 | ||||||
| G1 | 4 (1.8%) | 6 (1.0%) | 10 (1.6%) | 10 (3.1%) | 0 (0.0%) | 30 (1.6%) | ||
| G2 | 80 (35.1%) | 205 (32.6%) | 191 (31.0%) | 113 (35.5%) | 22 (35.5%) | 611 (33.0%) | ||
| G3 | 45 (19.7%) | 108 (17.2%) | 121 (19.6%) | 65 (20.4%) | 14 (22.6%) | 353 (19.1%) | ||
| Unknown | 99 (43.4%) | 309 (49.2%) | 294 (47.7%) | 130 (40.9%) | 26 (41.9%) | 858 (46.3%) | ||
| Molecular subtypes by post-operative pathology | 33.613 | <0.01 | ||||||
| Luminal A | 30 (13.2%) | 84 (13.4%) | 60 (9.7%) | 25 (7.9%) | 7 (11.3%) | 206 (11.1%) | ||
| Luminal B | 80 (35.1%) | 172 (27.4%) | 179 (29.1%) | 100 (31.4%) | 10 (16.1%) | 541 (29.2%) | ||
| HER2-enriched | 32 (14.0%) | 88 (14.0%) | 101 (16.4%) | 47 (14.8%) | 18 (29.0%) | 286 (15.4%) | ||
| Triple negative | 34 (14.9%) | 76 (12.1%) | 113 (18.3%) | 66 (20.8%) | 9 (14.5%) | 298 (16.1%) | ||
| Unknown | 52 (22.8%) | 208 (33.1%) | 163 (26.5%) | 80 (25.2%) | 18 (29.0%) | 521 (28.1%) | ||
ER/PR positive was defined as >1% of tumor cell nuclei staining positively with any intensity. Hormone receptor status positive was defined as ER positive or PR positive. HER2 positive was defined as HER2 membrane staining scored 3+ by immunohistochemistry or amplification by fluorescence in situ hybridization. BC, breast cancer; ER, estrogen receptor; PR, progestogen receptor; HER2, human epidermal growth factor receptor 2.
Treatment before developing recurrence and metastasis
There were significant differences in the types of treatment among patients of different ages. Patients aged ≥65 years had lower percentages of receiving surgery (87.1%), adjuvant chemotherapy (61.3%), and endocrine therapy (30.6%) than those of the other age groups (all P<0.05; Table 2).
Table 2
| Treatment | Age at initial diagnosis | Total | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| <35 years | 35–44 years | 45–54 years | 55–64 years | ≥65 years | ||||
| Surgery | 27.354 | <0.01 | ||||||
| No | 7 (3.1%) | 15 (2.4%) | 19 (3.1%) | 17 (5.3%) | 8 (12.9%) | 66 (3.6%) | ||
| Conservative surgery | 186 (81.6%) | 525 (83.6%) | 533 (86.5%) | 266 (83.6%) | 49 (79.0%) | 1,559 (84.2%) | ||
| Total mastectomy | 31 (13.6%) | 85 (13.5%) | 62 (10.1%) | 31 (9.7%) | 5 (8.1%) | 214 (11.6%) | ||
| Neoadjuvant chemotherapy | 1.799 | 0.77 | ||||||
| No | 170 (74.6%) | 465 (74.0%) | 442 (71.8%) | 233 (73.3%) | 48 (77.4%) | 1,358 (73.3%) | ||
| Yes | 53 (23.2%) | 154 (24.5%) | 160 (26.0%) | 76 (23.9%) | 12 (19.4%) | 455 (24.6%) | ||
| Adjuvant chemotherapy | 58.554 | <0.01 | ||||||
| No | 15 (6.6%) | 65 (10.4%) | 60 (9.7%) | 53 (16.7%) | 24 (38.7%) | 217 (11.7%) | ||
| Yes | 208 (91.2%) | 551 (87.7%) | 539 (87.5%) | 260 (81.8%) | 38 (61.3%) | 1,596 (86.2%) | ||
| Adjuvant radiotherapy | 9.469 | 0.05 | ||||||
| No | 107 (46.9%) | 320 (51.0%) | 318 (51.6%) | 188 (59.1%) | 34 (54.8%) | 967 (52.2%) | ||
| Yes | 115 (50.4%) | 289 (46.0%) | 273 (44.3%) | 124 (39.0%) | 22 (35.5%) | 823 (44.4%) | ||
| Adjuvant endocrine therapy | 44.369 | <0.01 | ||||||
| No | 88 (38.6%) | 294 (46.8%) | 354 (57.5%) | 186 (58.5%) | 40 (64.5%) | 962 (51.9%) | ||
| Yes | 134 (58.8%) | 320 (51.0%) | 243 (39.4%) | 120 (37.7%) | 19 (30.6%) | 836 (45.1%) | ||
Age of initial diagnosis and recurrence/distant metastasis
The relationship between age at initial diagnosis and recurrence and metastasis status of ABC is shown in Table 3. Age at initial diagnosis was neither associated with the recurrence and metastasis status nor the number of metastases (all P>0.05). Compared with the other age groups, patients aged <35 years were more likely to have bone metastasis (χ2=10.215; P<0.05).
Table 3
| Recurrence and metastasis | Age at initial diagnosis | Total | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| <35 years | 35–44 years | 45–54 years | 55–64 years | ≥65 years | ||||
| Recurrent-metastasis status | 11.055 | 0.2 | ||||||
| Local recurrence | 29 (12.7%) | 118 (18.8%) | 109 (17.7%) | 51 (16.0%) | 11 (17.7%) | 318 (17.2%) | ||
| Distant metastasis | 165 (72.4%) | 399 (63.5%) | 399 (64.8%) | 205 (64.5%) | 43 (69.4%) | 1,211 (65.4%) | ||
| Both | 31 (13.6%) | 89 (14.2%) | 85 (13.8%) | 52 (16.4%) | 3 (4.8%) | 260 (14.0%) | ||
| Unknown | 3 (1.3%) | 22 (3.5%) | 23 (3.7%) | 10 (3.1%) | 5 (8.1%) | 63 (3.4%) | ||
| Number of metastatic sites | 12.982 | 0.11 | ||||||
| None | 3 (1.3%) | 5 (0.8%) | 11 (1.8%) | 7 (2.2%) | 1 (1.6%) | 27 (1.5%) | ||
| 1–2 | 174 (76.3%) | 490 (78.0%) | 504 (81.8%) | 248 (78.0%) | 54 (87.1%) | 1,470 (79.4%) | ||
| ≥3 | 51 (22.4%) | 131 (20.9%) | 100 (16.2%) | 63 (19.8%) | 6 (9.7%) | 351 (19%) | ||
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 1 (1.6%) | 2 (0.1%) | ||
| Metastatic sites | ||||||||
| Bone | 104 (45.6%) | 251 (40.0%) | 215 (34.9%) | 124 (39.0%) | 19 (30.6%) | 713 (38.5%) | 10.215 | <0.05 |
| Liver | 50 (21.9%) | 128 (20.4%) | 143 (23.2%) | 65 (20.4%) | 7 (11.3%) | 393 (21.2%) | 5.381 | 0.25 |
| Lung | 73 (32%) | 170 (27.1%) | 170 (27.6%) | 109 (34.3%) | 19 (30.6%) | 541 (29.2%) | 6.923 | 0.14 |
| Brain | 7 (3.1%) | 23 (3.7%) | 16 (2.6%) | 8 (2.5%) | 1 (1.6%) | 55 (3.0%) | 1.973 | 0.74 |
In Cox regression analyses, crude and fully adjusted HR and 95% CI for the associations between age groups and recurrence or distant metastasis status are shown in Table 4. Compared with patients aged <35 years, the risk of recurrence or distant metastasis in those aged 55–64 years was significantly higher (HRage 55–64 =1.24, 95% CI: 1.04–1.47), and the risk of bone metastasis and lung metastasis in those aged 35–44 years were lower (HRbone metastasis =0.74, 95% CI: 0.59–0.93; HRlung metastasis =0.70, 95% CI: 0.53–0.93). After adjusting for stage, grade, molecular subtype, surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, adjuvant radiotherapy, adjuvant endocrine therapy, and family history of BC, patients aged 55–64 years had an increased risk of recurrence or distant metastasis by 1%, but HR did not have statistical significance (HRage 55–64 =1.01, 95% CI: 0.77–1.32). The protective effect of aging for patients aged 35–44 years on bone metastasis and lung metastasis were still significant (HRbone metastasis =0.69, 95% CI: 0.48–0.98; HRlung metastasis =0.48, 95% CI: 0.31–0.74).
Table 4
| Recurrence/metastasis condition | Age at initial diagnosis, HRs (95% CI) | ||||
|---|---|---|---|---|---|
| <35 years | 35–44 years | 45–54 years | 55–64 years | ≥65 years | |
| Recurrence and metastasis | |||||
| Univariate analysis | Reference | 0.87 (0.74–1.01) | 1.07 (0.91–1.25) | 1.24 (1.04–1.47) | 1.28 (0.95–1.72) |
| Multivariate analysis† | Reference | 0.84 (0.66–1.06) | 0.87 (0.68–1.10) | 1.01 (0.77–1.32) | 0.76 (0.49–1.19) |
| Bone metastasis | |||||
| Univariate analysis | Reference | 0.74 (0.59–0.93) | 0.82 (0.64–1.04) | 1.05 (0.80–1.37) | 0.83 (0.49–1.40) |
| Multivariate analysis† | Reference | 0.69 (0.48–0.98) | 0.72 (0.50–1.04) | 0.87 (0.57–1.31) | 0.36 (0.15–0.86) |
| Liver metastasis | |||||
| Univariate analysis | Reference | 0.87 (0.61–1.22) | 1.18 (0.84–1.65) | 1.21 (0.82–1.79) | 0.67 (0.29–1.58) |
| Multivariate analysis† | Reference | 0.88 (0.53–1.44) | 1.16 (0.71–1.89) | 1.05 (0.60–1.84) | 0.55 (0.19–1.65) |
| Lung metastasis | |||||
| Univariate analysis | Reference | 0.70 (0.53–0.93) | 0.91 (0.69–1.21) | 1.32 (0.98–1.79) | 1.33 (0.79–2.24) |
| Multivariate analysis† | Reference | 0.48 (0.31–0.74) | 0.62 (0.41–0.94) | 1.00 (0.63–1.56) | 0.50 (0.21–1.21) |
| Brain metastasis | |||||
| Univariate analysis | Reference | 1.10 (0.44–2.71) | 0.95 (0.37–2.45) | 1.18 (0.41–3.41) | 0.83 (0.10–6.91) |
| Multivariate analysis† | Reference | 2.48 (0.42–14.52) | 1.01 (0.17–6.06) | 2.42 (0.38–15.55) | 1.97 (0.13–29.14) |
†, adjusted for TNM stage, grade, molecular subtypes, surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, adjuvant radiotherapy, adjuvant endocrine therapy, and family history of BC. HR, hazard ratio; CI, confidence interval; BC, breast cancer.
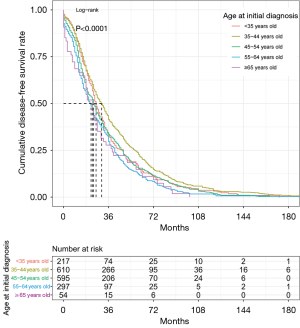
Cumulative recurrence/metastasis rate
Figure 1 compares the recurrence/metastasis rates of different age groups. The median DFI was 26.0 months. All patients’ cumulative recurrence/metastasis rates at 36 months of DFI were the most significant. Individuals aged 35–44 years had a significantly better prognosis, followed by participants aged <35, 45–54, 55–64, and ≥65 years (P<0.01).
Discussion
In recent years, the rising incidence of ABC has become a major public health concern. A multicenter nationwide study in China showed that more than 20% of patients with BC were diagnosed at stage III or IV (12). A study showed that about 65% of patients with BC were diagnosed with ABC in China (10). Whether patients with ABC could benefit from surgical extirpation of the primary lesion is still uncertain. However, most data on BC in China have been collected from surgeons; therefore, the disease characteristics of ABC may have been underestimated. In this study, we found many differences in clinical characteristics and treatment selection among patients of different ages.
The incidence of BC has been rising annually among younger women, and the peak age of BC death in China was lower than that for women from other countries (15). The median age at diagnosis of BC in China is 45–55 years compared with 55–65 years in Western countries, with patients aged under 35 years accounting for about 10–15% of women with BC (10). In the present study, the median age at initial diagnosis (39–53 years) was not consistent with the results of a previous study about BC in China, indicating that the age at initial diagnosis of ABC patients is earlier than that of patients with other types of BC. The possible reasons could be: (I) according to the Chinese National Breast Cancer Screening Guidelines, women younger than 45 were defined as a general risk group, and regular screening is not recommended for this population; (II) younger females with BC are in their prime years of childbearing, and their levels of progesterone and estrogen are higher, which easily promotes BC progression; (III) in younger women, early diagnosis of BC is more difficult because younger women generally have denser breast tissue than older women that is likely responsible for the decreased accuracy of film mammography; and (IV) younger populations usually have poorer health awareness compared to middle-aged and elderly individuals, which may cause the higher proportion of missed and delayed diagnoses of BC among young females.
In this study, we found that age at initial diagnosis was significantly related to hormone receptor status and HER2 status but not to tumor stage, grade, or pathological type. Clinical research shows that compared with older individuals, younger women tend to present more frequently with BC that has poor prognostic features such as a higher T stage, positive lymph nodes, and a high proliferating fraction (16,17). In our study, the proportion of advanced disease (stages III) was higher among older patients than in younger ones. Although Louwman et al. (18) reported the same result, our finding was contrary to previous studies. For example, Wang’s (19) study shows that young patients were more likely to be PR positive and HER2 positive, and the proportion of PR+ and HER2+ in the young group was higher than that in the old group. Our study showed that the proportion of hormone receptor positivity declined with age. In contrast, the proportion of HER2+ in older women (55–64 years) was highest (37.4%), closely followed by the 45–54 years group (34.6%) and the <35 years group (33.8%), which is inconsistent with the current findings. The observed differences correlated with the inclusion criteria, sample sizes, and grouping criteria of age. The participants in our study were all patients with ABC, whereas the majority of studies have not specified the tumor stage of patients. The inconsistency between the results of our study and existing studies could reflect the difference between ABC and overall BC. In addition, we divided the participants into five age groups, so it was possible to better observe the age distribution of molecular features to some extent.
Further, in the treatment decision-making of BC, it is necessary to fully consider the patient’s tolerance to treatment-related adverse effects. Older women usually had multiple diseases, and the molecular characteristics in BC tumors of younger and older women were different. Therefore, treatment decisions for older women were complex. We found that, with increasing age, the proportion of patients treated with surgery decreased, because in older patients, the organ systems that decline during aging lead to their inadequate tolerance to complications and treatment-related adverse reactions. In treating BC in older patients, individualized surgical strategies should be formulated according to the patient’s characteristics. Overall, optimizing chemotherapeutic regimens could reduce the risk of recurrence in BC patients, especially those under the age of 50. This conclusion was also confirmed in our study. However, according to Chinese guidelines for the treatment of ABC (20), the age of patients should not be the only reason for receiving undertreatment (older patients) or over-treatment (younger patients). When making treatment decisions, clinical doctors should consider factors such as hormone receptor and HER2 status, prior therapies (efficacy, toxicity, tolerance, etc.), DFI, tumor burden (the number of metastatic sites and the specific site of metastases), age, general status, menstrual status, and comorbidities. At the same time, appropriate adjustments should be made to the treatment strategies according to the severity of clinical symptoms, the need to control the disease and/or symptoms quickly, and the patient’s social, economic, and psychological factors.
Furthermore, the relationship between the age of the patient and prognosis remains a controversial aspect of the natural history of BC, which is of considerable practical importance to clinicians (21). Available evidence suggests that young age (22), particularly younger than 35 years, is a significant risk factor for local recurrence in women with early-stage BC after breast-conserving therapy (23,24). Young patients may have some characteristics that promote local recurrence. A review of the epidemiology of BC by Anders et al. (25) that compared younger and older BC patients found that the percentage of HER2-positive and triple-negative molecular types in young patients, which were usually associated with worse clinical outcomes, was much higher, leading to their poor tumor differentiation, high malignancy, rapid growth, and high risk of recurrence and metastasis. However, there were few studies and sample sizes available for this review’s 10-year local recurrence rate, which may have led to false-negative results (25). A previous study recruited 3,064 patients who underwent breast conservation surgery between 1988 and 2001 at the Mayo Clinic to evaluate the risk factors of local recurrence, and the results indicated that young age (<40 years) increased the risk of local recurrence after breast-conserving treatment (26). A study in China showed that both younger (aged <35 years) and older (aged ≥65 years) BC patients had poor prognoses (27). In our study, we analyzed the disease-free survival of different age groups. Our results indicated that being 55–65 years old correlated with a higher risk of recurrence and metastasis among patients with BC, even though it was not an independent prognostic factor, which is inconsistent with current studies. Generally, young BC patients usually have better physique and can tolerate traumatic treatments, such as surgery and chemotherapy. Therefore, clinicians tend to develop more aggressive treatment regimens for young women to prevent local recurrence. Adjuvant radiotherapy and/or chemotherapy treatment could be useful to lower the risk of local recurrence or metastasis. Contrary to expectations, older patients (aged ≥65 years) in our study had a higher stage at initial diagnosis, but univariate analysis results showed that most patients 55 years or older did not receive adjuvant chemotherapy or adjuvant radiotherapy. This finding means that undertreatment might also contribute to worse prognoses among older patients, implying that low doses of chemotherapy or radiotherapy should be used with caution in older patients with BC. Further, complicated diseases of older patients may represent an important contributing factor for undertreatment of older patients.
Finally, a certain degree of missing complicated disease data existed in our study. Despite this, we compared the proportion of the main three complicated diseases, including hypertension, diabetes, and coronary heart disease, in patients with complicated disease information. We found that older patients significantly had these diseases (P<0.01). Chemotherapeutic regimens commonly used after operation for BC include cyclophosphamide methotrexate fluoroucil (CMF) and anthracyclines-based regimens (28). For first-line chemotherapeutic regimens for ABC, doxorubicin, an anthracycline drug, is recommended in chemonaïve cases. Concerning concomitant anthracycline therapy, cardiotoxicity with anthracyclines is generally known to be dose-dependent (29). Older patients usually have poor tolerance to the treatment, and the use of anthracyclines could bring a higher risk of cardiotoxicity to them; therefore, older patients are recommended to receive attenuated doses of chemotherapy, which may result in a lower survival rate in the older group compared to the younger group. After adjusting for stage at initial diagnosis, grade, molecular subtype, family history of BC, and different types of therapy, the risk of recurrence and metastasis among older patients was not significantly higher than younger patients, corroborating the hypothesis mentioned above.
There are some advantages of the present study. Our multicenter retrospective hospital-based clinical epidemiology study was the first to explore the characteristics of ABC for women in China. All the data was collected from 21 tertiary hospitals in seven geographical regions, representing different cancer burden levels, diagnoses, and treatments. Convenience sampling was adopted, and the sample size was allocated according to the month to reduce the bias caused by the time of initial diagnosis. However, the project was limited in several ways. First, this study was a retrospective cross-sectional survey and the included patients’ information was based on medical records, leading to recall bias. Second, this study did not examine the consistency of molecular typing between relapsed and metastatic tumor and primary tumor. Finally, because the medical records of each hospital are different, there are certain data missing. Further population-based research should be undertaken to explore the diagnosis and treatment information of ABC.
Conclusions
Age at initial diagnosis is related to the clinicopathological characteristics and treatment pattern of patients with BC. Although the risk of site-specific metastasis varies by age, age is not an independent factor influencing the risk of total recurrence and metastasis. In accordance with current clinical practice guidelines for BC, however, precise treatment should be chosen personally for patients of different ages.
Acknowledgments
Thanks to the participants and health workers of the present study for their contributions. Thanks to Maria Jose Gonzales and Mohamed S. Bangura from Dalian Medical University for providing assistance with polishing the manuscript.
Funding: This work was supported by the Investigator-initiated program of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. CH-BC-045).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-302/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-302/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-302/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-302/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences (No. 15-115/1042) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Yang HJ, Yu XF, He XM, et al. Age interactions in breast cancer: an analysis of a 10-year multicentre study in China. J Int Med Res 2012;40:1130-40. [Crossref] [PubMed]
- DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439-48. [Crossref] [PubMed]
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/Ethnicity and Age Distribution of Breast Cancer Diagnosis in the United States. JAMA Surg 2018;153:594-5. [Crossref] [PubMed]
- Mehdi I, Monem AA, Al Bahrani B, et al. Breast cancer molecular subtypes in oman: correlation with age, histology, and stage distribution–- analysis of 542 cases. Gulf J Oncolog 2014;1:38-48. [PubMed]
- Anderson WF, Pfeiffer RM, Dores GM, et al. Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1899-905. [Crossref] [PubMed]
- Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 2011;29:3885-91. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018;379:111-21. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Chinese Anti-Cancer Association, Committee of Breast Cancer Society. Chinese expert consensus on the clinical diagnosis and treatment of advanced breast carcinoma(2018). Zhonghua Zhong Liu Za Zhi 2018;40:703-13. [PubMed]
- Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011;11:364. [Crossref] [PubMed]
- Jia MM, Lin X, Liu P, et al. A Multi-Center Study of Automated Breast Ultrasound System for the Diagnosis of Breast Cancer in China. Annals of Global Health 2017;83:104-5. [Crossref]
- Harbeck N, Thomssen C, Gnant M, St. . Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 2013;8:102-9. [Crossref] [PubMed]
- Yan Y, Chen Y, Jia H, et al. Patterns of Life Lost to Cancers with High Risk of Death in China. Int J Environ Res Public Health 2019;16:2175. [Crossref] [PubMed]
- Kim JK, Kwak BS, Lee JS, et al. Do very young Korean breast cancer patients have worse outcomes? Ann Surg Oncol 2007;14:3385-91. [Crossref] [PubMed]
- Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol 2010;21:1974-81. [Crossref] [PubMed]
- Louwman WJ, Vulto JC, Verhoeven RH, et al. Clinical epidemiology of breast cancer in the elderly. Eur J Cancer 2007;43:2242-52. [Crossref] [PubMed]
- Wang H. What problems are Chinese young abreast cancer patients facing? 2019. Available online: http://cancer.39.net/a/190922/7485555.html
- Breast Cancer Expert Committee of National Cancer Quality Control Center. Cancer Drug Clinical Research Committee of China Anti-Cancer Association. Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2020 Edition). Zhonghua Zhong Liu Za Zhi 2020;42:781-97.
- Martínez MT, Oltra SS, Peña-Chilet M, et al. Breast Cancer in Very Young Patients in a Spanish Cohort: Age as an Independent Bad Prognostic Indicator. Breast Cancer (Auckl) 2019;13:1178223419828766. [Crossref] [PubMed]
- He XM, Zou DH. The association of young age with local recurrence in women with early-stage breast cancer after breast-conserving therapy: a meta-analysis. Sci Rep 2017;7:11058. [Crossref] [PubMed]
- Figueiredo JC, Ennis M, Knight JA, et al. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res Treat 2007;105:69-80. [Crossref] [PubMed]
- Liu N, Li P, Wang J, et al. Psychometric properties of the Breast Cancer Awareness Measurement among Chinese women: a cross-sectional study. BMJ Open 2020;10:e035911. [Crossref] [PubMed]
- Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237-49. [Crossref] [PubMed]
- Miles RC, Gullerud RE, Lohse CM, et al. Local recurrence after breast-conserving surgery: multivariable analysis of risk factors and the impact of young age. Ann Surg Oncol 2012;19:1153-9. [Crossref] [PubMed]
- Li H, Zuo TT, Zeng HM, et al. Clinical features and prognostic analysis of female breast cancer in different diagnosed ages. Zhonghua Zhong Liu Za Zhi 2021;43:126-31. [PubMed]
- Zhang J, Liu Y. HER2 over-expression and response to different chemotherapy regimens in breast cancer. J Zhejiang Univ Sci B 2008;9:5-9. [Crossref] [PubMed]
- Sato A, Yoshihisa A, Miyata-Tatsumi M, et al. Valvular heart disease as a possible predictor of trastuzumab-induced cardiotoxicity in patients with breast cancer. Mol Clin Oncol 2019;10:37-42. [PubMed]
(English Language Editors: C. Mullens and J. Jones)