
Small intestinal bacterial overgrowth is associated with clinical relapse in patients with quiescent Crohn’s disease: a retrospective cohort study
Introduction
Crohn’s disease (CD), a kind of inflammatory bowel disease (IBD), is a persistent and irreversible inflammatory disorder with an unknown cause that has a usual state marked by remission and relapse (1). Unfortunately, the illness flare-ups occur randomly and can be difficult to predict (2). Frequent relapse of CD will seriously affect the physical and mental health of patients, resulting in loss of confidence in treatment and poor quality of life. Although a large number of studies over the years have examined clinical, environmental, microbial, genetic, endoscopic, serologic and fecal markers, there are no recognized reliable indicators to predicting prognosis (2). Increasing evidence suggests that gut microbial dysbiosis is linked to the onset and course of CD (3-7). Recently, a study by Braun et al. analyzing the 16S ribosomal RNA (rRNA) gene sequence in fecal samples reported that in individuals with CD, changes in the abundance of Christensenellaceae, S24.7, and Gemellaceae can predict future flare-ups (6).
Small intestinal bacterial overgrowth (SIBO) is characterized by an excess quantity of small intestinal bacteria, resulting in gastrointestinal (GI) disorders such as diarrhoea, bloating, stomach pain, and weight reduction (8), which is a manifestation of gut microbial dysbiosis. SIBO is common in people who suffer from CD. According to a recent meta-analysis of 11 studies, the incidence of SIBO in patients with CD was 25.4%, which was ten-fold higher than that in non-IBD controls (9). This is likely because patients with CD might have fistulas, strictures, damaged ileocecal valves, and reduced intestinal motility (10,11). Based on previous literature, SIBO is linked to elevated serum endotoxin and production of inflammatory cytokines/mediators stimulated by bacterial products (12). Besides, microscopic and macroscopic inflammatory changes are also commonly observed in individuals with SIBO (13). Thus, we hypothesize that in the case of SIBO, bacterial “toxins” or products of bacterial disrupting the intestinal mucosal barrier and inducing an immune response may contribute to intestinal inflammation and injury, which may result in the relapse of CD.
However, until now, the prognostic value of SIBO for CD has not been evaluated. The information may provide support for the integrated treatment of patients with CD, targeting inflammation and SIBO, and thus further improving patients’ lives. Therefore, we aimed to investigate whether SIBO detected by a lactulose breath test (LBT) could predict clinical relapse in hospitalised individuals with quiescent CD. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3335/rc).
Methods
Study design and patients
This was a retrospective observational cohort study of adult patients (≥18 years) with quiescent CD who were managed at General Hospital of Eastern Theater Command between 2016 and 2020. Using the electronic medical records to identify all quiescent CD patients who underwent LBT to diagnose SIBO between January 2016 and June 2020. All patients were in clinical remission for at least 1 month. The exclusion criteria included age <18 years, pregnancy, concomitant serious illness, and duration of clinical remission <1 month. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of General Hospital of Eastern Theater Command (No. 2022DZGZR-097) and individual consent for this retrospective analysis was waived.
The Crohn’s Disease Activity Index (CDAI) was utilized to determine CD activity, and the disease was considered to be quiescent if the patient was in clinical remission (CDAI <150 points) (14). We followed all enrolled patients for 18 months or until the primary endpoint of clinical relapse by searching the outpatient and inpatient electronic medical record system for clinical data on patient re-visits or contacting patients by phone. The primary endpoint was clinical relapse within 18 months, described as aggravating symptoms with a CDAI score >150, therapeutic intensification, CD-related surgery, or progression of the illness behavior as per the Montreal classification. The size of the sample was determined by how many cases were eligible during the study period.
Data
We searched the electronic medical records to obtain key attributes at baseline, including age, sex, body mass index (BMI), state of disease (active or in remission), smoking status, disease location and behavior (according to the Montreal classification), symptoms, surgical history, current and previous medication, and possible associated chronic diseases. To evaluate inflammation, baseline laboratory indicators including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), platelets, hemoglobin, albumin, interleukin-6 (IL-6), and fecal calprotectin (FC) levels were measured.
During the subsequent 18-month follow-up, we identified whether patients experienced clinical relapse by retrospectively assessing clinical parameters from patients’ medical records: CDAI scores, changes in CD-related medications, CD-related surgery, and progression of disease behavior according to Montreal classification.
Lactulose Hydrogen-Methane Breath Test
The hallmark for diagnosing SIBO is small bowel aspirate and culture. A concentration ≥103 colony forming units per millilitre (cfu/mL) is now regarded as a diagnostic indicator for SIBO (8,11). However, small intestinal aspiration and culture are invasive, time-consuming, and costly, which limits their widespread application. In clinical practice cultures have been largely replaced by breath tests. Breath testing is a valuable tool, using the amount of hydrogen (H2) and/or methane (CH4) gases in the breath produced by bacterial fermentation of unabsorbed carbohydrates to diagnose SIBO; it is non-invasive, simple, and cheap. Our study used the LBT for the diagnosis of SIBO with a sensitivity and specificity of 31–68% and 44–100% respectively (15). The day before the LBT, patients were directed to consume a diet that was low in fibre and carbohydrates and to fast for 8–12 h before the test. For 2 h before or during the test, smoking and physical activity were prohibited. On the day of the test, the oral cavity was kept clean. After a baseline breath sample, a 10 g dose of lactulose was administered. Samples were collected in bags (NAMEF, Beijing, China) every 30 minutes for 150 minutes and analyzed immediately using chromatography (QuinTron Instrument Company, Milwaukee, WI, USA) for both H2 and CH4 levels.
Based on the manufacturer’s recommendations, SIBO diagnosis could be established if any one of the following conditions was present within 90 minutes of oral lactulose: (I) elevated breath hydrogen level ≥20 ppm; (II) elevated breath methane level ≥12 ppm; and (III) an increase in combined breath hydrogen and methane level ≥15 ppm.
Statistical analysis
Continuous statistics with a normal distribution were summarized using the mean ± standard deviation (SD), and differences between groups were compared using independent sample t-tests. Continuous statistics with a non-normal distribution were summarized using medians [interquartile range (IQR)], and differences between groups were compared using Mann-Whitney U tests. Categorical variables were summarized using numbers (percentages), and differences between groups were compared using the χ2 (chi-squared) test or Fisher’s exact test.
Kaplan-Meier curves of the time-to-relapse and log-rank tests were performed to estimate the prognostic value of SIBO. Patients who did not relapse or did not report for follow-up before 18 months were censored at 18 months. The Cox proportional hazards model was utilized to identify factors associated with clinical relapse. All variables with P<0.05 in the log-rank test were integrated into a multivariate Cox regression model, which included SIBO, penetrating disease behavior, CRP, hemoglobin, ESR, IL-6 and albumin. The associations between outcomes and variables were represented as a hazard ratio (HR) and 95% confidence interval (CI). Using SPSS (version 21, IBM Corporation, Armonk, NY, USA), statistical significance was set at two-sided P<0.05.
Results
Study population
Seventy-three patients with CD who met our inclusion criteria (Figure 1) were identified. The basic attributes of all patients are presented in Table 1. Overall, 34 patients (46.6%) tested positive for SIBO. A total of 57.5% were male, and the average age was 26.0 years (IQR, 20.0–41.0 y) at the time of the breath test. The median disease period was 36.4 months (IQR, 16.3–65.6 m). Only two patients were smokers. Fifty-three patients (72.6%) reported no GI symptoms, 17 patients (23.3%) had mild abdominal pain, and four patients (5.5%) had bloating. Most of the patients (83.6%) did not adjust maintenance treatment within 3 months of inclusion. Table 2 displays the comparison of characteristics among individuals with and without SIBO. The groups appeared well balanced at baseline. No statistically significant differences were observed in the demographics, disease phenotypes, previous and current treatments, and laboratory parameters among patients with and without SIBO.
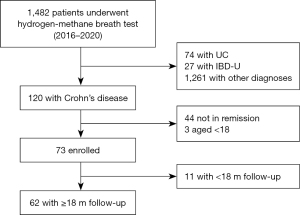
Table 1
| Variables | Patients |
|---|---|
| Males | 42 (57.5) |
| Age at diagnosis (years) | 23.0 (17.5–34.5) |
| Age at breath test (years) | 26.0 (20.0–41.0) |
| Disease duration (months) | 36.4 (16.3–65.6) |
| Median length of hospitalization (days) | 4 [2–6] |
| SIBO+ | 34 (46.6) |
| CDAI | 72.5±38.1 |
| Montreal location | |
| L1 (ileal) | 23 (31.5) |
| L2 (colonic) | 8 (11.0) |
| L3 (ileocolonic) | 41 (56.2) |
| Isolated L4 (upper GI tract) | 1 (1.4) |
| Small bowel lesions | 65 (89.0) |
| Montreal behavior at diagnosis | |
| B1 (non-stricturing, non-penetrating) | 40 (54.8) |
| B2 (stricturing) | 29 (39.7) |
| B3 (penetrating) | 4 (5.5) |
| P (perianal disease) | 32 (43.8) |
| Previous bowel resection | 12 (16.4) |
| Extraintestinal manifestations | 10 (13.7) |
| Smokers | 2 (2.7) |
| Previous medication | |
| 5-ASA | 72 (98.6) |
| Steroids | 13 (17.8) |
| Immunomodulator | 19 (26.0) |
| Anti-TNF | 7 (9.6) |
| EEN | 61 (83.6) |
| Current medication | |
| 5-ASA | 54 (74.0) |
| Steroids | 3 (4.1) |
| Immunomodulator | 11 (15.1) |
| Anti-TNF | 5 (6.8) |
| EEN | 54 (74.0) |
Data are n (%), mean ± SD, or median (IQR). n, number; SD, standard deviation; IQR, interquartile range; SIBO, small intestinal bacterial overgrowth; CDAI, Crohn’s Disease Activity Index; GI, gastrointestinal; 5-ASA, 5-aminosalicylic acid; TNF, tumor necrosis factor; EEN, exclusive enteral nutrition.
Table 2
| Variables | SIBO+ (n=34) | SIBO− (n=39) | P |
|---|---|---|---|
| Males | 17 (50.0) | 25 (64.1) | 0.224 |
| Age at diagnosis (years) | 20.0 (15.5–38.5) | 23.0 (21.0–33.0) | 0.711 |
| Disease duration (months) | 44.4 (10.3–85.6) | 38.6 (18.7–70.0) | 0.916 |
| Montreal location | 0.455 | ||
| L1 (ileal) | 13 (38.2) | 10 (25.6) | 0.248 |
| L2 (colonic) | 4 (11.8) | 4 (10.3) | 1.000 |
| L3 (ileocolonic) | 17 (50.0) | 24 (61.5) | 0.322 |
| Isolated L4 (upper GI tract) | 0 (0.0) | 1 (2.6) | 1.000 |
| Small bowel lesions | 31 (91.2) | 34 (87.2) | 0.865 |
| Montreal behavior at diagnosis | |||
| B1 (non-stricturing, non-penetrating) | 15 (44.1) | 25 (64.1) | 0.087 |
| B2 (stricturing) | 16 (47.1) | 13 (33.3) | 0.232 |
| B3 (penetrating) | 3 (8.8) | 1 (2.6) | 0.511 |
| P (perianal disease) | 15 (44.1) | 17 (43.6) | 0.964 |
| Previous bowel resection | 8 (23.5) | 4 (10.3) | 0.127 |
| Current medication | |||
| 5-ASA | 23 (67.6) | 31 (79.5) | 0.250 |
| Steroids | 0 (0.0) | 3 (7.7) | 0.289 |
| Immunomodulator | 5 (14.7) | 6 (15.4) | 0.936 |
| Anti-TNF | 3 (8.8) | 2 (5.1) | 0.874 |
| EEN | 23 (67.6) | 31 (79.5) | 0.250 |
| WBC (×109/L) | 6.4 (4.4–7.5) | 4.7 (3.3–5.0) | 0.207 |
| Hemoglobin (g/L) | 130 (112–142) | 128 (112–142) | 0.082 |
| Platelet count (×109/L) | 288 (166–384) | 197 (163–254) | 0.116 |
| Albumin (g/L) | 44.8 (41.9–46.7) | 42.3 (35.3–44.8) | 0.974 |
| C-reactive protein (mg/L) | 2.2 (0.9–5.0) | 1.9 (0.5–7.3) | 0.946 |
| ESR (mm/h) | 13 [6–29] | 7 [5–21] | 0.189 |
| Interleukin-6 (ng/L) | 8.24 (5.44–11.01) | 6.08 (4.78–10.14) | 0.476 |
| Fecal calprotectin (μg/g), n=56 | 489.1 (397.0–682.9) | 205.1 (41.1–852.4) | 0.416 |
Data are n (%) or median (IQR). n, number; IQR, interquartile range; CD, Crohn’s disease; SIBO, small intestinal bacterial overgrowth; 5-ASA, 5-aminosalicylic acid; TNF, tumor necrosis factor; EEN, exclusive enteral nutrition; WBC, white blood cell count; ESR, erythrocyte sedimentation rate.
SIBO to predict clinical relapse
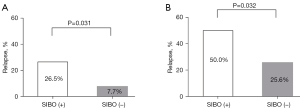
After a median follow-up of 16.4 months, 27 (37.0%) of the 73 patients experienced disease relapse, with a median time-to-relapse of 13.9 months. The overall cumulative number of clinical relapses (rates) was five (6.8%), 12 (16.4%), 20 (27.4%), and 27 (37.0%) at 3, 6, 12, and 18 months follow-up, respectively (Figure 2A). Among these, 10 individuals with clinical relapse were identified in the SIBO-positive group, and 17 patients were found to be in the SIBO-negative group (Figure 2B). The percentage of patients with CD who relapsed during follow-up was consistently higher among patients with SIBO as compared to those without SIBO, and this was statistically significant at the 6-month (26.5% vs. 7.7%, P=0.031) (Figure 3A) and 18-month (50.0% vs. 25.6%, P=0.032) follow-ups (Figure 3B).
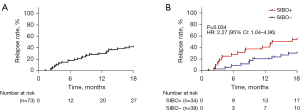
The standard attributes of individuals with and without clinical relapse at the 18-month follow-up are shown in Table 3. Patients who experienced clinical relapse exhibited an increased frequency of SIBO (63.0% vs. 37.0%, P=0.032), penetrating disease behavior (14.8% vs. 0%, P=0.031), and perianal disease (59.3% vs. 34.8%, P=0.042) compared to that of the remission group. Furthermore, the CRP, ESR, FC, and IL-6 levels were elevated in patients who relapsed (P=0.0004, P=0.001, P=0.01, and P=0.0004, respectively), while hemoglobin was reduced (P=0.048). To analyze the risk of clinical relapse in relation to SIBO, the time-to-relapse curves were constructed for the 18 months of the study and stratified according to SIBO (Figure 2B). According to the log-rank test, there were significant variations in relapse rates among individuals with and without SIBO (P=0.034).
Table 3
| Variables | Relapse (n=27) | Remission (n=46) | P |
|---|---|---|---|
| Males | 17 (63.0) | 25 (54.3) | 0.472 |
| Age at diagnosis (years) | 27.0 (17.0–39.0) | 22.0 (18.0–29.5) | 0.567 |
| Disease duration (months) | 83.03 (5.93–86.43) | 38.0 (20.9–54.5) | 0.458 |
| SIBO+ | 17 (63.0) | 17 (37.0) | 0.032 |
| Montreal location | |||
| L1 (ileal) | 6 (22.2) | 17 (37.0) | 0.191 |
| L2 (colonic) | 3 (11.1) | 5 (10.9) | 1.000 |
| L3 (ileocolonic) | 18 (66.7) | 23 (50.0) | 0.166 |
| Isolated L4 (upper GI tract) | 0 (0.0) | 1 (2.2) | 1.000 |
| Small bowel lesions | 25 (92.6) | 40 (87.0) | 0.722 |
| Montreal behavior at diagnosis | |||
| B1 (non-stricturing, non-penetrating) | 12 (44.4) | 28 (60.9) | 0.173 |
| B2 (stricturing) | 11 (40.7) | 18 (39.1) | 0.892 |
| B3 (penetrating) | 4 (14.8) | 0 (0.0) | 0.031 |
| P (perianal disease) | 16 (59.3) | 16 (34.8) | 0.042 |
| Previous bowel resection | 4 (14.8) | 8 (17.4) | 1.000 |
| Smoker | 0 (0.0) | 2 (4.3) | 0.527 |
| Current medication | |||
| 5-ASA | 19 (70.4) | 35 (76.1) | 0.591 |
| Steroids | 1 (3.7) | 2 (4.3) | 1.000 |
| Immunomodulator | 5 (18.5) | 6 (13.0) | 0.770 |
| Anti-TNF | 1 (3.7) | 4 (8.7) | 0.737 |
| EEN | 22 (81.5) | 32 (69.6) | 0.263 |
| WBC (×109/L) | 5.9±2.5 | 4.8±1.6 | 0.948 |
| Hemoglobin (g/L) | 124±16 | 125±19 | 0.048 |
| Platelet count (×109/L) | 287±101 | 216±75 | 0.187 |
| Albumin (g/L) | 43.3±6.8 | 41.6±5.1 | 0.060 |
| C-reactive protein (mg/L) | 3.4 (1.8–7.0) | 1.4 (0.5–3.0) | 0.0004 |
| ESR (mm/h) | 13 [6–24] | 7 [6–23] | 0.001 |
| Interleukin-6 (ng/L) | 9.83 (6.88–18.32) | 5.43 (4.79–7.81) | 0.0004 |
| Fecal calprotectin (μg/g), n=56 | 696.6 (449.9–847.0) | 72.3 (35.1–461.1) | 0.010 |
| CDAI | 81.86±34.90 | 64.05±42.08 | 0.301 |
Data are n (%), mean ± SD, or median (IQR). CD, Crohn’s disease; SD, standard deviation; IQR, interquartile range; SIBO, small intestinal bacterial overgrowth; 5-ASA, 5-aminosalicylic acid; TNF, tumor necrosis factor; EEN, exclusive enteral nutrition; WBC, white blood cell count; ESR, erythrocyte sedimentation rate; CDAI, Crohn’s Disease Activity Index.
Finally, we carried out univariate and multivariate analyses using the Cox proportional hazard model to assess the predictive capacities of different potential risk elements for clinical relapse in individuals with CD. The univariate and multivariate analyses outcomes are shown in Table 4. The univariate Cox proportional hazards analysis showed that SIBO (HR 2.27, 95% CI: 1.04–4.96; P=0.04) and penetrating disease behavior (HR 4.15, 95% CI: 1.43–12.10; P=0.009) were linked to a higher risk of clinical relapse. There were also associations between clinical relapse and CRP (P=0.002), hemoglobin (P=0.02), ESR (P=0.011), IL-6 (P=0.004), and albumin (P=0.038) levels. We found no associations were found with age, sex, smoking status, disease location, previous intestinal resection, current treatment, and previous treatment. The multivariate Cox proportional hazards analysis demonstrated that SIBO (HR, 2.79; 95% CI, 1.20–6.51; P=0.017) and penetrating disease behavior (HR, 3.66; 95% CI, 1.06–12.63; P=0.040) were the only independent risk factors for clinical relapse.
Table 4
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| SIBO(+) | 2.27 (1.04–4.96) | 0.040 | 2.79 (1.20–6.51) | 0.017 | |
| Penetrating disease behavior (Montreal B3) | 4.15 (1.43–12.10) | 0.009 | 3.66 (1.06–12.63) | 0.040 | |
| Hemoglobin (g/L) | 0.98 (0.96–1.00) | 0.020 | |||
| C-reactive protein (mg/L) | 1.04 (1.01–1.06) | 0.002 | |||
| ESR (mm/h) | 1.03 (1.01–1.05) | 0.011 | |||
| Interleukin-6 (ng/L) | 1.03 (1.01–1.05) | 0.004 | |||
| Albumin (g/L) | 0.94 (0.8–1.00) | 0.038 | |||
HR, hazard ratio; SIBO, small intestinal bacterial overgrowth; ESR, erythrocyte sedimentation rate.
Discussion
Alterations in the GI microbiome occur in patients with CD. Numerous studies have suggested a link between SIBO and CD (10,16-23), but the significance of SIBO in diagnosing quiescent CD has not been extensively researched. In this study, we explored whether SIBO is associated with subsequent clinical relapse of CD. To our knowledge, the present study is the first to find that SIBO is independently related to a higher risk of clinical relapse in individuals with quiescent CD.
In this study, 46.6% of patients tested positive for SIBO, which is similar to previous research findings (15–62%) (17,18,20,21,23) and suggests that SIBO is common in patients with CD. According to the findings of the latest meta-analysis, the mean occurrence of SIBO in patients with CD was 25.4%, which is lower than that reported in this study (9). This discrepancy may be due to differences in the hydrogen-methane breath test methodology, diagnostic criteria, and population characteristics.
Over the 18-month follow-up period of this study, we demonstrated that SIBO has a good capacity to anticipate clinical relapse in quiescent CD patients. First, we found that the relapse group had significantly increased chances of having SIBO compared to the non-relapse group. Furthermore, the time-to-relapse curves showed that patients with SIBO had a 2.27-fold elevated risk of clinical relapse compared to those without SIBO. Finally, the multivariate Cox regression model analysis outcome demonstrated that SIBO was an independent predictive factor for clinical relapse in CD. In addition to SIBO, penetrating disease behavior was also independently linked to clinical relapse in our analysis. Penetrating type disease is frequently referred to as an adverse prognostic factor in CD, especially when predicting first surgery and postoperative recurrence (1,24). In this study, patients with CD and penetrating behavior had a 3.66 (95% CI, 1.06–12.63, P=0.04) chance of clinical relapse.
Some published studies have reported a link between higher baseline FC and the risk of CD relapse (1,25,26). In our study, we found that FC at baseline was dramatically increased in the relapse group compared to the non-relapse group (P=0.01); however, statistical significance was not observed in the Cox proportional hazard regression model. It must be highlighted that the current study did not intend to identify such a link, and nearly a third of the patients did not have baseline FC measurements. Also, young age at diagnosis, disease location, and smoking were not found to be risk factors in the cases that were analyzed, even though other studies reported them as such (2,27-29).
Although the mechanism of SIBO leading to CD relapse is still unclear, one possible factor may be intestinal inflammation. Studies have suggested that microscopic and macroscopic inflammatory changes are common in individuals with SIBO, although this was not investigated in the current study. Toskes et al. (30) reported that chronic inflammatory cells have been found in the lamina propria of rats with SIBO. In addition, evidence from humans shows that intraepithelial lymphocyte counts and local lamina propria immunoglobulin A plasma cells are increased in SIBO (31-33). Macroscopic changes, such as mucosal oedema, disturbed vascular pattern, erosion, and, rarely ulceration, could also be present in individuals with SIBO. A previous study found small intestinal mucosal injury in people with chronic intestinal dysmotility (CID) using capsule endoscopy and hypothesized that this may be due to bacterial overgrowth (34). Another study found that LBT-positive patients had severe nonsteroidal anti-inflammatory drug (NSAID)-induced small intestinal damage (35). Based on the above evidence, we speculate that bacterial “toxins” or products of bacterial metabolism may disrupt the intestinal mucosal barrier and induce an immune response and that this may contribute to intestinal inflammation and injury, which may lead to CD relapse. However, in the CD group, endoscopic mucosal healing was not evaluated to rule out intestinal activity. Thus, further clinical and basic studies are required to clarify the underlying mechanisms.
We also assessed the association between SIBO and the clinical data of people with CD. Numerous investigations have observed that surgical procedures, as well as strictures or fistulae, are risk factors for the presence of SIBO in people with CD (10,16,17,20,22). We found that patients who were SIBO-positive showed higher rates of stricturing phenotypes and intestinal resection than SIBO-negative patients, although the difference was not statistically significant.
However, whether SIBO is the basis or result of CD remains unclear. On the one hand, patients with CD are prone to developing SIBO compared to healthy people because of fistulas, strictures, destruction of the ileocecal valve, and reduced intestinal motility. However, SIBO may induce intestinal inflammation through bacterial “toxins” and bacterial products, which may complicate the patient’s prognosis and clinical course. Although one previous study speculated that there is a potential two-way pathological connection between CD and SIBO (20), this is the first observation that SIBO is independently linked to clinical relapse of CD.
There were several limitations in this study that should be noted. Firstly, this was a retrospective study and the sample size was small. Furthermore, LBT is not the hallmark for SIBO diagnosis; however, it is a relatively quick, simple, and non-invasive surrogate test with acceptable accuracy. Finally, we did not perform endoscopy to determine the connection between SIBO and intestinal inflammation in this CD population, which is an aspect that deserves further evaluation in future studies.
In conclusion, the present study showed that SIBO appears to be an independent indicator of clinical relapse in people with quiescent CD. The detection of SIBO may be a valuable option for the prognostic assessment of patients in clinical remission. However, more trials with larger sample sizes are needed in the future to better understand the prognostic value of SIBO and the effect of proactive treatment of SIBO on the prognosis of CD.
Acknowledgments
We thank Editage (www.editage.cn) for English language editing.
Funding: The work was supported by the National Natural Science Foundation of China (No. 81873559).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3335/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3335/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3335/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of General Hospital of Eastern Theater Command (No. 2022DZGZR-097) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis 2017;11:3-25. [Crossref] [PubMed]
- Liverani E, Scaioli E, Digby RJ, et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol 2016;22:1017-33. [Crossref] [PubMed]
- Aldars-García L, Chaparro M, Gisbert JP. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms 2021;9:977. [Crossref] [PubMed]
- Kabeerdoss J, Sankaran V, Pugazhendhi S, et al. Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India. BMC Gastroenterol 2013;13:20. [Crossref] [PubMed]
- Pavel FM, Vesa CM, Gheorghe G, et al. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics (Basel) 2021;11:1090. [Crossref] [PubMed]
- Braun T, Di Segni A, BenShoshan M, et al. Individualized Dynamics in the Gut Microbiota Precede Crohn's Disease Flares. Am J Gastroenterol 2019;114:1142-51. [Crossref] [PubMed]
- Bamba S, Inatomi O, Nishida A, et al. Relationship between the gut microbiota and bile acid composition in the ileal mucosa of Crohn's disease. Intest Res 2021; Epub ahead of print. [Crossref] [PubMed]
- Pimentel M, Saad RJ, Long MD, et al. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am J Gastroenterol 2020;115:165-78. [Crossref] [PubMed]
- Shah A, Morrison M, Burger D, et al. Systematic review with meta-analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment Pharmacol Ther 2019;49:624-35. [Crossref] [PubMed]
- Ghoshal UC, Yadav A, Fatima B, et al. Small intestinal bacterial overgrowth in patients with inflammatory bowel disease: A case-control study. Indian J Gastroenterol 2022;41:96-103. [Crossref] [PubMed]
- Rezaie A, Pimentel M, Rao SS. How to Test and Treat Small Intestinal Bacterial Overgrowth: an Evidence-Based Approach. Curr Gastroenterol Rep 2016;18:8. [Crossref] [PubMed]
- Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol 2006;4:11-20. [Crossref] [PubMed]
- Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol 2010;16:2978-90. [Crossref] [PubMed]
- Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439-44. [Crossref] [PubMed]
- Khoshini R, Dai SC, Lezcano S, et al. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008;53:1443-54. [Crossref] [PubMed]
- Rana SV, Sharma S, Malik A, et al. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig Dis Sci 2013;58:2594-8. [Crossref] [PubMed]
- Sánchez-Montes C, Ortiz V, Bastida G, et al. Small intestinal bacterial overgrowth in inactive Crohn's disease: influence of thiopurine and biological treatment. World J Gastroenterol 2014;20:13999-4003. [Crossref] [PubMed]
- Greco A, Caviglia GP, Brignolo P, et al. Glucose breath test and Crohn's disease: Diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol 2015;50:1376-81. [Crossref] [PubMed]
- Lee JM, Lee KM, Chung YY, et al. Clinical significance of the glucose breath test in patients with inflammatory bowel disease. J Gastroenterol Hepatol 2015;30:990-4. [Crossref] [PubMed]
- Ricci JER. Small-Intestinal Bacterial Overgrowth is Associated With Concurrent Intestinal Inflammation But Not With Systemic Inflammation in Crohn's Disease Patients. J Clin Gastroenterol 2018;52:530-6. [Crossref] [PubMed]
- Cohen-Mekelburg S, Tafesh Z, Coburn E, et al. Testing and Treating Small Intestinal Bacterial Overgrowth Reduces Symptoms in Patients with Inflammatory Bowel Disease. Dig Dis Sci 2018;63:2439-44. [Crossref] [PubMed]
- Bertges ER, Chebli JMF. Prevalence and factors associated with small intestinal bacterial overgrowth in patients with crohn's disease: a retrospective study at a referral center. Arq Gastroenterol 2020;57:283-8. [Crossref] [PubMed]
- Gu P, Patel D, Lakhoo K, et al. Breath Test Gas Patterns in Inflammatory Bowel Disease with Concomitant Irritable Bowel Syndrome-Like Symptoms: A Controlled Large-Scale Database Linkage Analysis. Dig Dis Sci 2020;65:2388-96. [Crossref] [PubMed]
- Gionchetti P, Dignass A, Danese S, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis 2017;11:135-49. [Crossref] [PubMed]
- Jung ES, Lee SP, Kae SH, et al. Diagnostic Accuracy of Fecal Calprotectin for the Detection of Small Bowel Crohn's Disease through Capsule Endoscopy: An Updated Meta-Analysis and Systematic Review. Gut Liver 2021;15:732-41. [Crossref] [PubMed]
- Liu F, Lee SA, Riordan SM, et al. Global Studies of Using Fecal Biomarkers in Predicting Relapse in Inflammatory Bowel Disease. Front Med (Lausanne) 2020;7:580803. [Crossref] [PubMed]
- Torres J, Caprioli F, Katsanos KH, et al. Predicting Outcomes to Optimize Disease Management in Inflammatory Bowel Diseases. J Crohns Colitis 2016;10:1385-94. [Crossref] [PubMed]
- Pozios I, Seeliger H, Lauscher JC, et al. Risk factors for upper and lower type prolonged postoperative ileus following surgery for Crohn's disease. Int J Colorectal Dis 2021;36:2165-75. [Crossref] [PubMed]
- Fan C, You J, Zhao H, et al. Analysis of Risk Factors and Nursing Intervention Measures Affecting Nutritional Status of Children with Crohn's Disease. Evid Based Complement Alternat Med 2021;2021:5416487. [Crossref] [PubMed]
- Toskes PP, Giannella RA, Jervis HR, et al. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology 1975;68:1193-203. [Crossref] [PubMed]
- Bushyhead D, Quigley EM. Small Intestinal Bacterial Overgrowth. Gastroenterol Clin North Am 2021;50:463-74. [Crossref] [PubMed]
- Chojnacki C, Mikulska P, Knopik-Dąbrowicz A, et al. The role of intraepithelial lymphocytes in pathogenesis of small intestinal bacterial overgrowth syndrome. Pol Merkur Lekarski 2021;49:23-7. [PubMed]
- Chen B, Zhu S, Du L, et al. Reduced interstitial cells of Cajal and increased intraepithelial lymphocytes are associated with development of small intestinal bacterial overgrowth in post-infectious IBS mouse model. Scand J Gastroenterol 2017;52:1065-71. [Crossref] [PubMed]
- Hoog CM, Lindberg G, Sjoqvist U. Findings in patients with chronic intestinal dysmotility investigated by capsule endoscopy. BMC Gastroenterol 2007;7:29. [Crossref] [PubMed]
- Muraki M, Fujiwara Y, Machida H, et al. Role of small intestinal bacterial overgrowth in severe small intestinal damage in chronic non-steroidal anti-inflammatory drug users. Scand J Gastroenterol 2014;49:267-73. [Crossref] [PubMed]


