Lower mean corpuscular hemoglobin concentration is associated with poorer outcomes in intensive care unit admitted patients with acute myocardial infarction
Introduction
Hematological parameters, easily obtained with low cost using the modern hematological analyzers, are widely used in risk stratification, diagnosis, and prognosis estimation for patients with cardiovascular diseases (1-3). For example, higher red blood cell distribution width (RDW) (4), neutrophil to lymphocyte ratio (5), mean corpuscular volume (MCV) (6), platelet count (7) and white blood cell (WBC) (8) count are associated with poorer outcomes in patients with acute myocardial infarction (AMI), while decreased hemoglobin is associated with higher mortality (9). In addition, serum potassium level can also impact the outcomes of AMI patients (10,11). Since these routine laboratory tests are usually mutually correlated (e.g., WBC and RDW (12), RDW and hemoglobin (12), platelet count and potassium (13), platelet count and RDW (14), neutrophil count and RDW (15), the confounding effects of another tests cannot thus be ignored when the prognostic value of an interesting test is evaluated. However, no previous study has evaluated the prognostic values of these laboratory tests simultaneously to the best of our knowledge.
In this study, we studied the prognostic values of hematological parameters and serum ion levels (including potassium, sodium and chloride) in adult AMI patients admitted to intensive care unit (ICU).
Materials and methods
Data source
Similar to our previous work (16), this study is based on a publicly accessible critical care database named Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC II) (17). This database consisted of more than 30,000 patients admitted to ICU of Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) from 2001 to 2008 (18). Clinical variables, including demographics characteristics, laboratory tests, microbiological findings and outcomes were all recorded in this database. Accessing process for MIMIC II was well documented by Dr. Zhang (19).
The establishment of this database is approved by the Institutional Review Boards (IRB) of the Massachusetts Institute of Technology (MIT, Cambridge, MA, USA) and BIDMC. All patients in this database are de-identified to protect their privacy. Therefore, patients consent was waived in this study.
Data extraction
Structure query language (SQL) with pgAdmin (version 1.12.3), an open source development and administration platform for PostgreSQL, was used to extract data from MIMIC II. We restricted the search to adult patients (more than 15 years old) with AMI using International Classification of Diseases (ICD)-9 code between 410.00 and 410.92. The order or priority of ICD-9 code was not limited. Hematological parameters on admission were extracted, including WBC and its subpopulations, red blood cell (RBC) and its indices [MCV, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), RDW, hematocrit and hemoglobin] and platelet count. In addition, serum sodium, potassium, chloride levels and simplified acute physiology score I (SAPS I) were also extracted.
For patients admitted to ICU for more than one time, only data of initial admission were used. If patients received a laboratory test more than one time during their hospitalization, only the initial test results were included.
Statistical analyses
Mann-Whitney U test was used to compare continuous variables because all of them were not normally distributed. Chi-square test was used to compare categorical variables. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value of laboratory tests for hospital mortality. Forward conditional logistic regression model was used to analyze the association between laboratory tests and hospital mortality. The long term prognostic values of laboratory tests were evaluated using Cox hazard regression model and Kaplan-Meier curve analysis. All analyses were performed using SPSS 18.0, and a P value less than 0.05 was considered statistically significant.
Results
Characteristics of subjects
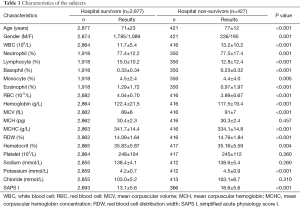
As shown in Table 1, 3,304 subjects were included in this study. A total of 427 subjects died in hospital (hospital non-survivors) and their clinical characteristics were compared with hospital survivors. Generally, hospital non-survivors were older and had significantly higher WBC count, neutrophil percentage, MCV, RDW, potassium and SAPS I (P<0.01 for all), while their RBC, hemoglobin, MCHC, hematocrit, percentages of lymphocyte, monocyte, basophil and eosinophil were significantly lower (P<0.01 for all). Therefore, these laboratory tests were included into further analyses.
Full table
Predictive value of hematological parameters and potassium for hospital mortality
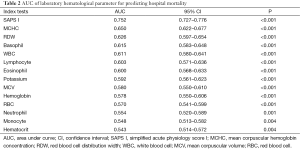
Table 2 lists the areas under ROC curves (AUC) of hematological parameters and potassium. SAPS I, a well-known prognostic factor for critical ill patients, had the highest AUC, followed by MCHC, RDW, basophil, WBC, lymphocyte percentage, eosinophil percentage, potassium, MCV, hemoglobin, RBC, neutrophil percentage, monocyte percentage, and hematocrit.
Full table
Associations between hospital mortality and both hematological parameters and potassium
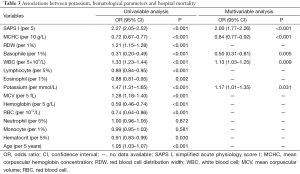
We then analyzed the associations between hospital mortality and both hematological parameters and potassium using logistic regression model. As shown in Table 3, in univariable analysis, all laboratory tests except neutrophil and monocyte percentages were associated with hospital mortality. However, in multivariable analysis, only SAPS I, MCHC, basophil, WBC and potassium were independently associated with hospital mortality.
Full table
Long-term prognostic values of hematological parameters and potassium
The long-term prognostic values of hematological parameters and potassium were analyzed using Cox regression model and the results were listed in Table 4. In univariable analysis, all laboratory tests except monocyte were significantly associated with long-term mortality. However, in multivariable analysis, only SAPS I, MCHC, RDW, WBC, lymphocyte percentage, potassium and neutrophil percentage were independently associated with long term mortality.
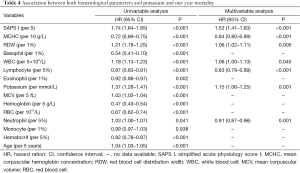
Full table
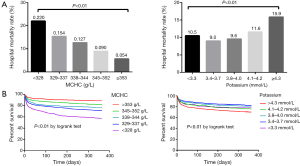
The subjects were then divided into five groups based on the MCHC and potassium, and the hospital mortality in all groups was shown in Figure 1A. Obviously, hospital mortality was significantly lower with the increase of MCHC. For potassium, hospital mortality was higher with the increase of potassium (i.e., potassium values >3.4 mmol/L). However, the subjects with potassium <3.3 mmol/L had higher hospital mortality than those with potassium between 3.4 and 4.0 mmol/L, indicating that there may be a U-shape (or J-shape) relationship between hospital mortality and potassium. Figure 1B is Kaplan-Meier curves depicting the prognostic values of MCHC and potassium. Subjects with higher MCHC had significantly lower mortality risk (P<0.01 by log-rank test). When potassium was >3.4 mmol/L, higher mortality risk was observed, and subjects with potassium <3.3 mmol/L also had higher mortality risk.
Discussion
In this study, we investigated the prognostic values of hematological parameters and serum ion levels in adult AMI patients admitted to ICU. Some findings in our study were consistent with previous investigations. Specifically, increased RDW (4) and WBC (8) were found to be associated with poorer outcomes after AMI. However, our study has two strengths: firstly, to the best of our knowledge, this is the first study that investigated the prognostic values of these laboratory tests simultaneously, so that the confounding effects of these laboratory tests on AMI outcomes could be minimized. Therefore, the results of our study may be more reliable than previous investigations. Second, for the first time, we found that decreased MCHC was independently associated with poorer prognosis, either indicated by hospital mortality or 1-year mortality of AMI.
We compared the clinical characteristics of hospital survivors and non-survivors, and found that some of laboratory tests were associated with hospital outcomes, such as RDW and potassium. These findings are partially consistent with previous reports (4-11). However, we noted that percentages of basophil and eosinophil were decreased in hospital non-survivors, suggesting that eosinophil and basophil percentages are potential prognostic factors for AMI and thus unmasking a potential (new) of these blood cells in the complications of acute coronary syndrome. Indeed, using ROC curve analysis, we found that the AUC of basophil and eosinophil for predicting hospital mortality were approximately 0.60, which are comparable to those of WBC and RDW, two well-recognized prognostic factors in AMI. To our knowledge, this is the first study reported that eosinophil and basophil are prognostic factors for AMI. Jiang et al. (20) found that eosinophil percentage was inversely correlated with cardiac troponin I level in AMI. Although that study suggested that eosinophil percentage was a potential prognostic factor, the prognostic value of eosinophil percentage was not further investigated. We believe that decreased eosinophil percentage in AMI patients may be attributed to the following reasons. Firstly, AMI is an inflammatory related disease, which may increase neutrophil percentage, and therefore, eosinophil percentage is decreased (21,22). Second, during the development of AMI, cortisol level is increased (23), which can decrease the peripheral eosinophil percentage (24,25), as also noticed in additional studies investigating the acute behavior of eosinophils in stress conditions (26). Although we failed to find the independent prognostic value of eosinophil for AMI, this may be due to the small sample size of our study, and we believe that further studies with large sample sizes may be needed to investigate the prognostic value of eosinophil percentage in patients with this condition.
The most novel and important finding of our study is that increased MCHC is independently associated with decreased hospital and 1-year mortality. The mechanisms under increased MCHC and better outcomes of AMI patients are largely unknown. MCHC is defined as the average hemoglobin level in a RBC. We hypothesized that inflammation and iron status may partially explain the association between increased MCHC and poorer outcomes in AMI patients. It is widely-accepted that inflammatory response plays a crucial role in the occurrence and development of AMI (27,28). Increased inflammatory markers, such as CRP, predict poor prognosis of AMI (29,30), and anti-inflammatory agents such as corticosteroid can decrease mortality in AMI patients (31). Inflammatory response may lead to iron deficiency (32,33), which decrease MCHC (34). Therefore, similar to RDW (35), MCHC can be regarded as an inflammatory marker and thus can affect the prognosis after AMI. Our hypothesis was supported by a recently published work, which shown that MCHC was negatively correlated with RDW in AMI patients (36). Actually, higher serum iron was proved to be associated with better outcomes in AMI patients (37).
Our study has some limitations. Firstly, this is a retrospective analysis; therefore, subject selection bias cannot be ignored. Second, since some of the prognostic factors, such as cardiac troponin, in some patients were not available in MIMIC II, we could not investigate whether the prognostic value of MCHC was independent of these prognostic factors. Finally, it should be noted that the subjects enrolled in this study were admitted to ICU, thus conclusions of this study cannot be extended to the AMI patients admitted to other departments, such as cardiology department or emergency department.
In conclusion, we found that hematological parameters and serum potassium can provide prognostic information in AMI patients. MCHC was an independent prognostic factor for both short and long term outcomes of AMI.
Acknowledgements
Funding: This study was funded by the National Natural Science Foundation of China (No. 81302541) and Key Program of Nanjing Military Command of PLA (No. 15DX007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethic review board,and informed consent was waived in this study.
References
- Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost 2015;21:139-43. [Crossref] [PubMed]
- Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J 2013;40:17-29. [PubMed]
- Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 2015;7:E402-11. [PubMed]
- Sun XP, Chen WM, Sun ZJ, et al. Impact of red blood cell distribution width on long-term mortality in patients with ST-elevation myocardial infarction. Cardiology 2014;128:343-8. [Crossref] [PubMed]
- Gazi E, Bayram B, Gazi S, et al. Prognostic Value of the Neutrophil-Lymphocyte Ratio in Patients With ST-Elevated Acute Myocardial Infarction. Clin Appl Thromb Hemost 2015;21:155-9. [Crossref] [PubMed]
- Franczuk P, Kaczorowski M, Kucharska K, et al. Could an analysis of mean corpuscular volume help to improve a risk stratification in non-anemic patients with acute myocardial infarction? Cardiol J 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Goliasch G, Forster S, El-Hamid F, et al. Platelet count predicts cardiovascular mortality in very elderly patients with myocardial infarction. Eur J Clin Invest 2013;43:332-40. [Crossref] [PubMed]
- Grzybowski M, Welch RD, Parsons L, et al. The association between white blood cell count and acute myocardial infarction in-hospital mortality: findings from the National Registry of Myocardial Infarction. Acad Emerg Med 2004;11:1049-60. [Crossref] [PubMed]
- Anker SD, Voors A, Okonko D, et al. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: data from the OPTIMAAL trial. Eur Heart J 2009;30:1331-9. [Crossref] [PubMed]
- Ma W, Liang Y, Zhu J, et al. Serum Potassium Levels and Short-Term Outcomes in Patients With ST-Segment Elevation Myocardial Infarction. Angiology 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Choi JS, Kim YA, Kim HY, et al. Relation of serum potassium level to long-term outcomes in patients with acute myocardial infarction. Am J Cardiol 2014;113:1285-90. [Crossref] [PubMed]
- Guimarães PO, Sun JL, Kragholm K, et al. Association of standard clinical and laboratory variables with red blood cell distribution width. Am Heart J 2016;174:22-8. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Guidi GC. Adjustment of serum potassium for age and platelet count. A simple step forward towards personalized medicine. Clin Chem Lab Med 2015;53:e325-7. [Crossref] [PubMed]
- Lappegård J, Ellingsen TS, Vik A, et al. Red cell distribution width and carotid atherosclerosis progression. The Tromsø Study. Thromb Haemost 2015;113:649-54. [Crossref] [PubMed]
- Vaya A, Hernández JL, Zorio E, et al. Association between red blood cell distribution width and the risk of future cardiovascular events. Clin Hemorheol Microcirc 2012;50:221-5. [PubMed]
- Hu ZD, Wei TT, Tang QQ, et al. Prognostic value of red blood cell distribution width in acute pancreatitis patients admitted to intensive care units: an analysis of a publicly accessible clinical database MIMIC II. Clin Chem Lab Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Saeed M, Villarroel M, Reisner AT, et al. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Crit Care Med 2011;39:952-60. [Crossref] [PubMed]
- Zhang Z, Chen K, Ni H, et al. Predictive value of lactate in unselected critically ill patients: an analysis using fractional polynomials. J Thorac Dis 2014;6:995-1003. [PubMed]
- Zhang Z. Accessing critical care big data: a step by step approach. J Thorac Dis 2015;7:238-42. [PubMed]
- Jiang P, Wang DZ, Ren YL, et al. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron Artery Dis 2015;26:101-6. [Crossref] [PubMed]
- Carbone F, Nencioni A, Mach F, et al. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 2013;110:501-14. [Crossref] [PubMed]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002;53:31-47. [Crossref] [PubMed]
- Bain RJ, Poeppinghaus VJ, Jones GM, et al. Cortisol level predicts myocardial infarction in patients with ischaemic chest pain. Int J Cardiol 1989;25:69-72. [Crossref] [PubMed]
- Finn AV. Eosinophils: an overlooked player in acute myocardial infarction? Coron Artery Dis 2015;26:99-100. [Crossref] [PubMed]
- Sabag N, Castrillón MA, Tchernitchin A. Cortisol-induced migration of eosinophil leukocytes to lymphoid organs. Experientia 1978;34:666-7. [Crossref] [PubMed]
- Lippi G, Banfi G, Montagnana M, et al. Acute variation of leucocytes counts following a half-marathon run. Int J Lab Hematol 2010;32:117-21. [Crossref] [PubMed]
- Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am 2007;91:553-72. ix. [Crossref] [PubMed]
- Fang L, Moore XL, Dart AM, et al. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol 2015;12:305-12. [PubMed]
- Raposeiras-Roubín S, Barreiro Pardal C, Rodiño Janeiro B, et al. High-sensitivity C-reactive protein is a predictor of in-hospital cardiac events in acute myocardial infarction independently of GRACE risk score. Angiology 2012;63:30-4. [Crossref] [PubMed]
- Correia LC, Lima JC, Rocha MS, et al. Does high-sensitivity C-reactive protein add prognostic value to the TIMI-Risk Score in individuals with non-ST elevation acute coronary syndromes? Clin Chim Acta 2007;375:124-8. [Crossref] [PubMed]
- Giugliano GR, Giugliano RP, Gibson CM, et al. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol 2003;91:1055-9. [Crossref] [PubMed]
- Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol 2009;46:387-93. [Crossref] [PubMed]
- McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev 2009;67:100-4. [Crossref] [PubMed]
- Archer NM, Brugnara C. Diagnosis of iron-deficient states. Crit Rev Clin Lab Sci 2015;52:256-72. [Crossref] [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [PubMed]
- Vayá A, Rivera L, de la Espriella R, et al. Red blood cell distribution width and erythrocyte deformability in patients with acute myocardial infarction. Clin Hemorheol Microcirc 2015;59:107-14. [PubMed]
- Huang CH, Chang CC, Kuo CL, et al. Serum iron concentration, but not hemoglobin, correlates with TIMI risk score and 6-month left ventricular performance after primary angioplasty for acute myocardial infarction. PLoS One 2014;9:e104495. [Crossref] [PubMed]