Efficacy and safety of fecal microbiota transplantation for the induction of remission in active ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon with periodic oscillations of relapse and remission (1). Conventional medical treatments including sulfasalazine, 5-aminosalicylate, 6-mercaptopurine, azathioprine, steroids, immune modulating agents such as cyclosporine, and biologicals agents such as anti-tumor necrosis factor drugs can provide remissions but cannot much alter disease course and are associated with risks such as the non-adherence, side effects (hepatitis, pancreatitis, lupus, hematological alterations, hepatotoxicity, etc.), and dysplasia (2).
Fecal microbiota transplantation (FMT) is the transfer of fecal microbes from healthy individuals to patients (3). FMT modifies microbial composition and creates a taxonomic equilibrium between gut viruses, bacteria, and fungi to restore microbial homeostasis (4). Advantages of FMT include easy acquisition, use of samples from multiple donors in a single transplant, convenience in storage, and more than one modes of administration. FMT is found to be highly efficacious in treating recurrent clostridium difficile infection (CDI) with cure rates of 87–90% (5,6). FMT is also a potential therapy for inflammatory bowel disease and irritable bowel syndrome. It has shown potential in treating hepatic encephalopathy, autism, and metabolic syndrome. Other potential areas in which FMT has been shown beneficial effects include modulating the response to chemotherapy, overcoming multidrug resistance, and treating conditions involving the gut-brain axis (4,7). For many other indications, the data on FMT are limited, as randomized controlled trials (RCTs) are scarce and typically constrained by small populations, and often have conflicting results (7).
The first case of successful treatment of UC with FMT was reported in 1989 by a physician who treated himself with FMT via an enema after 11 years of illness. The remission was inflammation free and was achieved without the use of additional drugs (8). Since then, many reports and RCTs have been published on this subject. Despite the publication of several reports on the efficacy of FMT, the effectiveness of FMT in UC treatment remains debatable. A previous meta-analysis found FMT a promising treatment for active UC (9). A network meta-analysis also showed that FMT was comparable to infliximab, and vedolizumab in terms of their absolute effects or relative ranks. In that study, no statistical differences were found between the efficacy of biological agents, tofacitinib, and FMT (10). However, some recent investigations have reported conflicting outcomes (11-13). A study found FMT comparable to 5-aminosalicylate in achieving clinical remission (11) whereas another study reported similar rates of clinical remission in FMT and placebo treated patients (12) and a study found UC exclusion diet to be more beneficial than FMT in achieving remission (13). Safety profiles were similar in comparative groups of these studies. Thus, the scenario urged for a systematic review and to perform meta-analyses of statistical indices for refining the existing evidence. The present study sought to evaluate the efficacy and safety of FMT in patients with active UC by performing a meta-analysis of the RCTs available in the literature to obtain more precise information on this subject. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3236/rc).
Methods
Search strategies
We searched the Cochrane, Embase, PubMed, and Web of Science databases from their inception to December 2021. The literature search was performed using the key terms with following strategy: ((FMT or fecal microbial transplant or bacteriotherapy) OR ((faecal or fecal or feces or faeces or stool) AND (transplant* or microbiota or transfusion or implant* or instillation or donor* or enema or reconstitution or infusion* or transfer*))) AND (UC or ulcerative colitis). Two authors screened each record and retrieved article independently and then unified their outputs. Any discrepancies were resolved through discussion with the senior authors. The literature search was restricted to original research articles published in English language.
Inclusion and exclusion criteria
To be eligible for inclusion in the present study, the articles had to meet the following inclusion criteria: (I) report on a RCT study comprising patients with active UC; (II) report on the efficacy (clinical remission and/or endoscopic remission), and safety outcomes of the FMT treatment; and (III) compare FMT to a placebo or a suitable comparator. Patients receiving FMT through any delivery route, including oral, nasogastric, colonoscopic, or enema administration, were eligible for inclusion. The PICOS: Patients, UC; Intervention, FMT; Control, Placebo or suitable comparator; Outcomes, remission rate/SAE rate; Study design, RCT. Studies on animals or cell lines, reviews, case reports, case series, retrospective studies, cohort studies, and non-RCTs were excluded from the study, as were study reports with incomplete or insufficient data. In relation to duplicate publications, the latest published article was included in the study.
Data extraction
The data were searched and extracted by 2 authors independently. Any discrepancies were resolved through discussion with the senior authors. The information extracted from the articles included the authors’ names, publication dates, countries, number of patients, severity of UC, FMT dosages, delivery routes, control interventions, follow-up time, clinical remission, endoscopic remission, and serious adverse events (SAEs). All the extracted data were then synthesized and tabulated in datasheets according to the analysis requirements.
Quality assessment
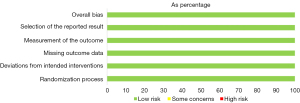
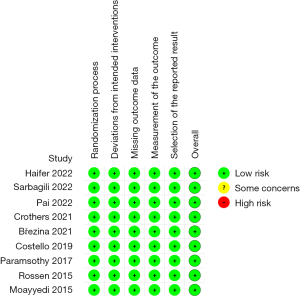
The quality assessment of the included studies was performed with the version 2 of the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2.0) using an Excel Tool for the implementation of RoB 2. The RoB 2.0 assesses the risk of bias in a randomized trial under five domains: (I) randomization; (II) deviations from intended interventions; (III) missing outcome data; (IV) outcome measurement; and (V) selection of the reported results. A study with all low-risk domains is considered to have low risk of bias whereas a study with high risk of bias in at least one domain or some concerns in more than one domain is considered to have high risk of bias.
Statistical analysis
We tested the presence of statistical heterogeneity in the reported outcomes with the Chi2 test and estimated it as a percentage with the I2 index. The risk ratios (RRs) were calculated to estimate the differences in the remission rates and the risk of SAEs between the FMT-treated and control patients. Review Manager software (version 5.4.1; Cochrane Collaboration; Copenhagen, Denmark) was used for the meta-analyses of RRs of remission or SAEs between treated and control groups. We used random-effects model (REM) for a meta-analysis when the I2 value was >50%, and otherwise a fixed-effects model (FEM) was used. To investigate the sources of heterogeneity, subgroup analyses were performed with respect to the mode of administration, the number of donors, antibiotic pretreatment, and total FMT dosage. Subgroup analyses were also performed to evaluate the heterogeneity arising from control treatments (placebo vs autologous FMT). Sensitivity analyses were performed to evaluate the robustness of the outcomes. All the statistical tests were 2-tailed, and outcome data with P values <0.05 were considered statistically significant.
Publication bias assessment was performed with Begg’s test and Egger’s test using Stata software (version 16; Stata Corporation, College Station, Texas, USA) after the visual examination of funnel plot corresponding to the meta-analysis of clinical remission rates.
Results
Search results and study characteristics
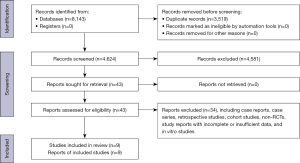
The literature search yielded a total of 8,143 citations, of which 3,519 were duplications. After deleting the duplicates, 4,624 studies were reviewed to determine their eligibility based on their titles and abstracts. After the 2 reviewers had independently screened the titles and abstracts, 43 studies on UC patients who received FMT remained. Of these studies, 9 were RCTs evaluating the efficacy of FMT in patients with active UC, and these articles were included in the meta-analysis (11-19) (Figure 1). A total of 425 patients were enrolled in these studies; 213 FMT-treated and 212 control patients. These studies were conducted in the United States, Australia, Canada, the Czech Republic, Israel, and the Netherlands. Table 1 summarizes the research focused demographic, clinical, analytical, and FMT features of these studies.
Table 1
| Study | Country | Sample size (FMT/ control) | Severity | Type of patients | Type of donor |
Type of faces | Delivery route | Total dosage | Pre-antibiotics | Control | Time of evaluation | Clinical remission (FMT/control) | Endoscopic remission (FMT/control) | Serious adverse events (FMT/control) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haifer 2022 | Australia | 15/20 | Mild to moderate (Mayo score of 4–10 and endoscopic Mayo subscore ≥ 1) | Adult | Single donor | Frozen | FMT capsules | 102.9 g | Yes | Placebo | 8 weeks | 11/5 | 7/3 | 2/1 |
| Sarbagili 2022 | Israel | 19/15 | Active UC (SCCAI 5–11 and endoscopic Mayo score of 2–3) | Adult | Single donor | Frozen | Colonoscopy and retention enema | 133.3 g | No | UCED | 8 weeks | 4/6 | 3/4 | 0/0 |
| Pai 2021 | Canada | 12/12 | Active UC | Pediatric | Multiple donors | Frozen | Retention enema | 600 g | No | Placebo | 30 weeks | 5/4 | NR | 5/1 |
| Crothers 2021 | USA | 6/6 | Mild to moderate (Mayo score of 4–10; endoscopic Mayo subscore ≥1, a rectal bleeding subscore ≥1, and a stool frequency subscore ≥1) | Adult | Multiple donors | Frozen | Colonoscopy and FMT capsules | ~62 g | Yes | Placebo | 12 weeks | 2/0 | NR | 1/1 |
| Březina 2021 | Czech Republic | 21/22 | Mild to moderate active left-sided UC (Mayo score of 4–10, and endoscopy subscore ≥2) | Adult | Single donor | Frozen | Retention enema | 500 g | No | 5-ASA enemas | 12 weeks | 12/8 | 3/3 | 4/1 |
| Costello 2019 | Australia | 38/35 | Mild to moderate (Mayo score of 3–10 and endoscopic subscore ≥2) | Adult | Multiple donors (3-4) | Frozen | Colonoscopy and retention enema | 100 g | No | Autologous FMT | 8 weeks | 18/6 | 4/0 | 3/2 |
| Paramsothy 2017 | Australia | 41/40 | Mild to moderate (Mayo score of 4–10) | Adult | Multiple donors (3-7) | Frozen | Colonoscopy and retention enema | 1537.5 g | No | Placebo | 8 weeks | 18/8 | 5/3 | 2/1 |
| Rossen 2015 | Netherland | 23/25 | Mild to moderate (SCCAI 4–11, and endoscopic subscore ≥1) | Adult | Single donor | Fresh | Naso-duodenal infusions | ~240 g | No | Autologous FMT | 12 weeks | 7/8 | 2/2 | 2/2 |
| Moayyedi 2015 | Canada | 38/37 | Mild to moderate (Mayo score of 3–10, and endoscopic subscore ≥1) | Adult | Single donor | Fresh or frozen | Retention enema | 300 g | No | Placebo | 7 weeks | 9/2 | 9/2 | 3/2 |
RCTs, randomized controlled trials; FMT, fecal microbiota transplantation; SCCAI, simple clinical colitis activity index; NR, no record; UCED, UC Exclusion Diet.
The majority of the included studies used clinical response or remission as the primary endpoint, but some studies used a combination of endoscopic improvement or remission as the primary endpoint. The definitions of clinical remission, endoscopic remission, and SAEs were not uniform across these trials; however, all the 9 studies reported outcome data for clinical remission, and SAEs, and 7 studies reported endoscopic remission data.
Quality assessment
All the studies were classified as high-quality studies according to the RoB 2.0. Figures 2,3 show the quality of each included study.
Efficacy of FMT in UC patients
Clinical remission
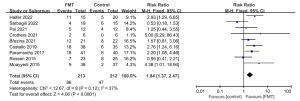
All the included RCTs reported outcome data on clinical remission after FMT. When the data were pooled, 86 of the 213 patients (40.4%) in the FMT group and 47 of the 212 patients (22.2%) in the control group achieved clinical remission [RR: 1.84; 95% confidence interval (CI): 1.37, 2.47; P<0.0001]. This meta-analysis showed low statistical heterogeneity between the studies (Chi2=12.67, P=0.12, I2=37%; Figure 4). Sensitivity analyses found these results stable. There was no indication of publication bias upon examining the symmetry of funnel plot corresponding to this meta-analysis. Publication bias tests also endorsed this observation [Begg’s test: Adjusted Kendall’s score =0±9.59; P=1.000 (Figure S1) and Egger’s test: bias coefficient: 0.366 (−3.025, 3.757); P=0.806].
Figures S2-S5 show the subgroup analyses based on the mode of administration, the number of donors, antibiotic pretreatment, and total FMT dosage. When the route of administration of FMT was studied, in comparison to the controls the pooling of data from 6 studies showed that administering the FMT via the lower gastrointestinal tract had a beneficial effect (RR: 1.72; 95% CI: 1.06, 2.78; Chi2=8.62; P=0.13; I2=42%; REM), while the pooling of data from 3 studies showed that administering the FMT via the upper gastrointestinal tract had no benefit (RR: 1.86; 95% CI: 0.73, 4.77; Chi2=4.07; P=0.13; I2=51%; REM). Compared to the control groups in 4 RCTs, a significant beneficial effect was observed when FMT with multiple donors was used (RR: 2.17; 95% CI: 1.36, 3.46; Chi2=1.78; P=0.62; I2=0%, REM), but no such effect was observed when FMT with a single donor was used in 5 RCTs (RR: 1.51; 95% CI: 0.80, 2.85; Chi2=9.50; P=0.05; I2=58%; REM). The pooling of data from 4 studies showed that FMT with a total dosage of ≥300 grams had a significant beneficial effect (RR: 1.85; 95% CI: 1.22, 2.83; Chi2=2.46; P=0.48; I2=0%; REM), but the pooling of data from 5 studies showed that FMT with a total dosage of <300 grams had no benefit (RR: 1.60; 95% CI: 0.77, 3.32; Chi2=10.12; P=0.04; I2=60%; REM). In comparison to the control group, FMT exhibited a significantly better effect in both the with and without antibiotic pretreatment subgroups. In subgroup analysis with regards to control treatment, there was no significant difference between the placebo and autologous FMT in clinical remission rate (Figure S6).
Endoscopic remission
Endoscopic remission was achieved by more patients receiving FMT than by those receiving control therapies. The pooled rate of endoscopic remission was 16.9% (33 of 195 patients) in the FMT group and 8.76% (17 of 194 patients) in the control group (RR: 1.94; 95% CI: 1.14, 3.31; P=0.01; Chi2=6.92; P=0.33; I2=13%; Figure 5).
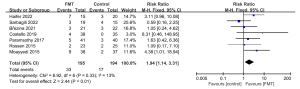
The subgroup analyses examining the mode of administration, the number of donors, antibiotic pretreatment, total FMT dosage, and control treatment found no significant differences between the subgroups (see Figures S7-S11).
Safety of FMT in UC
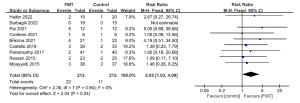
Adverse events requiring treatment, hospitalization, surgery, or death during the FMT process were designated as SAEs. The FMT group had a pooled SAE rate of 10.3% (22 of 213 patients), while the control group had a rate of 5.19% (11 of 212 patients) (RR: 2.05; 95% CI: 1.03, 4.09; P=0.04; Chi2=2.36; P=0.94; I2=0%; Figure 6). However, in the sensitivity analysis, the difference in the SAEs between the FMT and control groups was not significant.
Discussion
This meta-analysis of RCTs found that FMT has significantly higher clinical and endoscopic remission rates than control treatments. We identified 425 patients with active UC, of whom 213 received FMT. Overall, 40.4% of the UC patients and 22.2% of the control patients achieved clinical remission. Our meta-analysis outcomes showed the efficacy of FMT in inducing remission in mild to moderate active UC patients in the short term only. Very few RCTs have examined the outcomes of maintenance treatment with FMT. A RCT published in 2019 showed that maintenance FMT treatment in UC patients who were in in clinical remission may help sustain clinical, endoscopic, and histological remission (20).
The FMT group had a higher number of SAEs than the control group; however, in the sensitivity analysis, the difference in the SAEs between the FMT and control groups was not significant. Thus, further research is required to determine whether or not FMT causes more SAEs than placebo or other regimens. When the data of all of the 9 RCTs were pooled, the SAEs were not rare (10.3%); thus, SAEs need to be carefully monitored throughout FMT treatment. However, some of the reported SAEs may not have been linked to FMT. For example, the misdiagnosis of Crohn’s disease as UC and the identification of cervical cancer during therapy were included as SAEs in some studies (18,19), but they were not found to be significantly associated with FMT. Additionally, the most common SAEs were CDI (11,12,16,17,19) and exacerbations of UC (11-17,19). In a systematic review of 50 studies on FMT comprising 1,089 patients, the total incidence rate of SAEs was 9.2% (21) which is similar to the figure we found. High-quality RCTs are needed to verify the incidence of SAEs among FMT patients (21).
Despite the fact that there was no substantial statistical heterogeneity in the meta-analyses, there were discrepancies among the included studies in terms of donor selection, total dosage, delivery route, and whether or not antibiotic pretreatment was employed. The dosage of FMT varied from trial to trial, and there was no uniform standard. In most of the cases, the feces specimens were frozen, and fresh stool specimens were used in only 1 study (19). Frozen feces may have advantages over fresh feces in relation to the preparation, storage, monitoring, and delivery of FMT (22). Quantitative polymerase chain reaction analyses have shown that frozen and lyophilized FMT products can be stored for up to 7 months without any change in the microbial composition or therapeutic strength (23). A recent animal study showed that frozen FMT had a better therapeutic effect than fresh FMT (24). In the present study, feces from a single donor were used in some trials (11,13,14,18,19), while mixed specimens from multiple donors were used in others (12,15-17). Several studies used upper gastrointestinal administration (14,15,18), including nasogastric tubes and capsules, while others used lower gastrointestinal administration (11-13,16,17,19), such as enteroscopies or enemas. These discrepancies could have affected our study’s findings. Data were insufficient to perform meta-regression, however, to understand these findings, subgroup analyses were performed. In relation to clinical remission, FMT with a high dosage, mixed stool specimens from multiple donors, and the lower gastrointestinal tract as the route of administration were found to be significantly better than low-dose FMT, stool specimen from single donor, and upper gastrointestinal tract as route of administration, respectively. However, for endoscopic remission, no significant differences were observed among the subgroups.
Our meta-analysis showed that multi-donor FMTs may offer an advantage for clinical remission. Some studies using single-donor FMTs have found preliminary evidence that some donor FMTs are significantly more effective than others, and another study showed that some donor FMTs had an increased risk of adverse events, which suggests that donor selection affects FMT outcomes (13,19). The composition of donor gut flora may affect FMT efficacy in UC patients (4). In inflammatory bowel disease patients, usually the firmicutes are decreased and proteobacteria taxa are increased (25). In an RCT, the presence of Fusobacterium spp. was associated with a lack of remission (17). Higher donor richness was linked to a successful transplant in a study employing 16S ribosomal deoxyribose nucleic acid pyrosequencing to evaluate fecal microbiota (26). The extent to which donor’s microbial taxa engraft and reverse the dysbiosis associated with a specific disease phenotype also affects subject’s response to FMT (4). Previous evidence on its benefit in disease remission has shown that precisely processed FMT can minimize donor-dependent constraints and provide a well-defined flora blend. Such preparations will take FMT more similar to prebiotics that can enable whole fecal transplantations to be avoided (27). Further studies are required to investigate the factors affecting the efficacy of FMT in different disease states.
Conclusions
This meta-analysis of RCTs showed that FMT had significant advantages in terms of clinical and endoscopic remission in patients with mild to moderate active UC. The FMT group had a significantly higher incidence of SAEs than the control group; however, more data is needed to confirm this difference. Additionally, further RCTs and long-term observational registries are needed to reach a consensus on donor selection, total dosage, FMT delivery route, and antibiotic pretreatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3236/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3236/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756-70. [Crossref] [PubMed]
- Navaneethan U, Shen B. Pros and cons of medical management of ulcerative colitis. Clin Colon Rectal Surg 2010;23:227-38. [Crossref] [PubMed]
- Gupta A, Khanna S. Fecal Microbiota Transplantation. JAMA 2017;318:102. [Crossref] [PubMed]
- Ng SC, Kamm MA, Yeoh YK, et al. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2020;69:83-91. [Crossref] [PubMed]
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407-15. [Crossref] [PubMed]
- Kelly CR, Kahn S, Kashyap P, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015;149:223-37. [Crossref] [PubMed]
- Waller KMJ, Leong RW, Paramsothy S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J Gastroenterol Hepatol 2022;37:246-55. [Crossref] [PubMed]
- Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989;1:164. [Crossref] [PubMed]
- Liu X, Li Y, Wu K, et al. Fecal Microbiota Transplantation as Therapy for Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2021;2021:6612970. [Crossref] [PubMed]
- Zhou HY, Guo B, Lufumpa E, et al. Comparative of the Effectiveness and Safety of Biological Agents, Tofacitinib, and Fecal Microbiota Transplantation in Ulcerative Colitis: Systematic Review and Network Meta-Analysis. Immunol Invest 2021;50:323-37. [Crossref] [PubMed]
- Březina J, Bajer L, Wohl P, et al. Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis-Results of a Randomized Controlled Trial. J Clin Med 2021;10:2753. [Crossref] [PubMed]
- Pai N, Popov J, Hill L, et al. Results of the First Pilot Randomized Controlled Trial of Fecal Microbiota Transplant In Pediatric Ulcerative Colitis: Lessons, Limitations, and Future Prospects. Gastroenterology 2021;161:388-393.e3. [Crossref] [PubMed]
- Sarbagili Shabat C, Scaldaferri F, Zittan E, et al. Use of fecal transplantation with a novel diet for mild to moderate active ulcerative colitis: The CRAFT UC randomized controlled trial. J Crohns Colitis 2022;16:369-78. [Crossref] [PubMed]
- Haifer C, Paramsothy S, Kaakoush NO, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol 2022;7:141-51. [Crossref] [PubMed]
- Crothers JW, Chu ND, Nguyen LTT, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol 2021;21:281. [Crossref] [PubMed]
- Costello SP, Hughes PA, Waters O, et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019;321:156-64. [Crossref] [PubMed]
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218-28. [Crossref] [PubMed]
- Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015;149:110-118.e4. [Crossref] [PubMed]
- Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015;149:102-109.e6. [Crossref] [PubMed]
- Sood A, Mahajan R, Singh A, et al. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J Crohns Colitis 2019;13:1311-7. [Crossref] [PubMed]
- Wang S, Xu M, Wang W, et al. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One 2016;11:e0161174. [Crossref] [PubMed]
- Lee CH, Steiner T, Petrof EO, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2016;315:142-9. [Crossref] [PubMed]
- Jiang ZD, Alexander A, Ke S, et al. Stability and efficacy of frozen and lyophilized fecal microbiota transplant (FMT) product in a mouse model of Clostridium difficile infection (CDI). Anaerobe 2017;48:110-4. [Crossref] [PubMed]
- Zhu F, Ke Y, Luo Y, et al. Effects of Different Treatment of Fecal Microbiota Transplantation Techniques on Treatment of Ulcerative Colitis in Rats. Front Microbiol 2021;12:683234. [Crossref] [PubMed]
- Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr 2022;76:489-501. [Crossref] [PubMed]
- Vermeire S, Joossens M, Verbeke K, et al. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis 2016;10:387-94. [Crossref] [PubMed]
- Wilson BC, Vatanen T, Cutfield WS, et al. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol 2019;9:2. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)