A bibliometric analysis of the application of artificial intelligence to advance individualized diagnosis and treatment of critical illness
Introduction
Due to the deepened understanding of critical illness and advancements in treatment, the mortality rate of critically ill patients in the emergency department (ED) and intensive care unit (ICU) has gradually been decreasing (1). However, the pathophysiology of these patients is extremely complex, and it is sometimes difficult to make a clear differential diagnosis. Further, critically ill patients, such as those with sepsis, acute respiratory distress syndrome (ARDS), and infectious diseases, comprise a heterogenous population (2). Therefore, rapid diagnosis and personalized treatment based on dynamic and complex clinical situations are needed to improve the strategy making and prognosis of these patients (3).
Artificial intelligence (AI) refers to the ability of a computer or machine to perform tasks generally conducted by a human being (4). It was originally applied to image recognition, and later applied to critical care medicine to assist with difficult decision making (5). As the latest technologic advance in medicine, AI based on big-data has been conducted in most medical fields, especially in ICU, where is brimming with high volume of complex information (6).
It has been reported that AI can make an accurate diagnosis by using a pathology graphic and text analysis system and identify the subtypes of sepsis and ARDS from the Medical Information Mart for Intensive Care (MIMIC) database (7). What’s more, a lot of hard work has been made to develop AI extensions both to the consolidated standards of reporting trials (CONSORT) and standards for reporting of diagnostic accuracy studies (STARD) guidelines (8,9). Thus, the CONSORT-AI and STARD-AI can provide more evidence-based materials to the practice guidelines. It should also be noted that the most utilized methods are unsupervised learning techniques which work like a “black box”. Thus, the underlying process of getting the outcomes or results is not clear to the user (10-12). There were, however, studies about AI application to advance individualized medical decisions in ICU are fragmented over past decade. Herein, we aimed to conduct a bibliometric analysis and describes the details of published documents qualitatively and quantitatively in this field.
Registered trials have played an important role in changing daily clinical practice, especially during the coronavirus disease 2019 (COVID-19) pandemic (13). With the development of new drugs and therapies, individualized diagnosis and personalized treatment are urgently needed. However, information about the application of AI to critically ill patients is limited, and the application and development directions of AI for the next decade have not yet been clarified.
Thus, we conducted a bibliometric analysis to provide a comprehensive review of the AI field in critical care medicine and identify the currently solved and unsolved issues. Through analysis of the registered clinical studies, we also aimed to reveal the hotspots and future directions in this area.
Methods
Data sources and search strategy
Articles published from January 2011 to December 2021 were screened by two authors of the research team in the Web of Science (WoS) core collection database for bibliometric analysis. Additionally, a cross-sectional analysis of relevant studies that had been registered at ClinicalTrials.gov in the same period was carried out.
Search strategy was based on “PICO” framework. P—population/problem, which referred to the critical illness. I—intervention, in our study it comprised of various AI methods and machine-learning technologies. C—comparison, which indicated the difference between AI assistance and manual handling. O—outcome, which outlined the results of individualized treatment and diagnosis (14,15). Search strategy was adopted from previous work and experts’ opinions (15,16). We refined the query to include keywords related to critical illness (“critical care/treatment”, “intensive care”, “intensive care unit”, “ICU”, “emergency medicine/treatment/service/care/department”, and “EICU”), AI technologies (“artificial intelligence”, “AI”, “algorithmic prognostication”, “computational intelligence”, “machine learning”), and individualized treatment and diagnosis (“individual”, “personalized”) in both Medical Subject Headings (MESH) and titles. The data for bibliometric analysis were extracted and downloaded in text format from the database, including details of the publication, authors, and titles.
There are two standard weight attributes for an item which are defined as the links attribute and the total link strength attribute. They represent the number of links of an item with other items and the total strength of the links of an item with other items, respectively. For example, in the case of co-authorship links between researchers, the links attribute indicates the number of co-authorship links of a given researcher with other researchers. The total link strength attribute indicates the total strength of the co-authorship links of a given researcher with other researchers. Both weight attributes were calculated by VOSviewer with full counting. Further, the full data of the trials, including study type, participant age, gender, status, and study outcomes, were recorded from ClinicalTrials.gov.
Inclusion and exclusion criteria
The articles for bibliometric analysis were restricted to original articles with English written. The exclusion criteria were as follows: (I) documents were written in non-English language and (II) documents were classified into non-original article type according to the classification service.
Statistical analysis
The intrinsic functions of the WoS core collection database were applied to describe the basic characteristics of the retrieved publications. The online analysis platform of literature metrology (http://bibliometric.com/) successfully validated for bibliometric analysis and was used to determine the international cooperative relations in this study (17,18).
VOSviewer (version 1.6.17; Leiden University, The Netherlands) was mainly used to construct and visualize co-occurrence networks of co-authorship, co-occurrence, citation, keywords, and themes extracted from literature. CiteSpace (Version 5.8 R3; Chen Meichao, Drexel University, Philadelphia, PA, USA) was used to reveal the evolution and turning point of the research field. Other analysis tools such as Bibliographic Item Co-Occurrence Matrix Builder version 2.0 (BICOMB; Lei Cui, China Medical University, Shenyang, China) and Graphical Clustering Toolkit version 1.0 (gCLUTO; University of Minnesota, Minneapolis, MN, USA), were also applied to provide matrix of keywords clustering results. The categorical variables of the studies registered at ClinicalTrial.gov were described by frequencies and percentages.
Keywords occurred more than five times were defined as high-frequency keywords in VOSviewer and keywords burst defined as the occurrence of the keywords varied greatly over one year by CiteSpace (19). All clusters were calculated by VOSviewer, CiteSpace and gCLUTO with its own clustering algorithm.
Results
Publications output
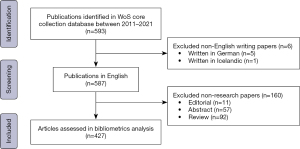
A total of 593 eligible publications were selected, of which 432 (72.8%) were original articles, 93 (15.7%) were reviews, 64 (10.8%) were conference papers, and 5 were other types of articles. It should be noted that almost half of the articles (34.6%) were published in 2021. Ultimately, 427 English written research articles were included in the bibliometric analysis (Figure 1).
Growth trend of publications
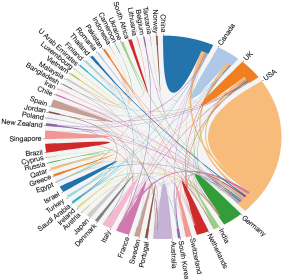
A rapid increase in the number of articles published in this field from 2011 to 2021 were detected, for which there was a wide global geographic distribution (Figure 2). The number of articles published in 2021 (161 articles) was about 35 times higher than that in 2011. Further, 54 countries contributed to publications on the application of AI in the ED and ICU. The United States of America (USA) published the most articles (n=201), followed closely by China (n=57).

Bibliometric analysis of the keywords
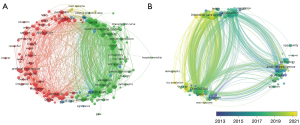
The keywords were identified by VOSviewer and CiteSpace. Both tools extracted all keywords from the included articles based on the citation index service of WoS core collection database. Finally, a total of 2,504 keywords were identified from the included articles. Among them, 93 keywords were high frequency and included in the analysis. The co-occurrence of high-frequency keywords were divided into six clusters with a total link strength of 1,835 (Figure 3A). “Machine learning” was the most frequent keyword (147 occurrences, 165 links, and 395 total link strengths), and was linked to 88 other high-frequency keywords (Figure 3B). “Artificial intelligence” (51 occurrences, 52 links, and 136 total link strengths), “risk” (43 occurrences, 61 links, and 146 total link strengths), “models” (42 occurrences, 63 links, and 144 total link strengths), and “mortality” (37 occurrences, 59 links, and 149 total link strengths) were the other four top high frequency keywords. Other high frequency keywords that ranked in the top ten included “classification”, “prediction”, “care”, “deep learning”, and “COVID-19”.

The top 15 keywords with the strongest burst values are summarized in Figure 3C. From 2011 to 2021, “big-data” had the strongest burst strength (strength: 6.74), followed by “machine learning algorithm” (strength: 5.51), “patient care” (strength: 4.51), “real time” (strength: 3.95), and “deep learning” (strength: 3.79). In 2017, the most frequently observed keyword bursts included “precision medicine” (strength: 3.66), “heart rate” (strength: 3.66), and “treatment decision” (strength: 3.05), to the exclusion of “big-data” and “machine learning algorithm”. The two keywords that maintained high burst values from 2019 to 2021 were “deep learning” (strength: 3.79) and “machine learning” (strength: 2.89).
Co-citation clustering and time evolution analysis
The whole silhouette [defined as the homogeneity of a cluster, values ranges (0–1), ≥0.7 represent the clustering is efficient and convincing] of the co-cited references clustering was 0.93, and the Q score was 0.88 [defined as modularity, values ranges (0–1), ≥0.3 represent the clustering is significant] by analysis tool CiteSpace. Articles on AI application research in the ED and ICU were divided into 81 clusters, and the clusters of the largest connected components (as labeled by the log likelihood ratios) are shown in Figure 4A. The top five largest clusters were “COVID-19” (#0, size: 54, silhouette: 0.94), “artificial intelligence” (#1, size: 44, silhouette: 0.90), “optimization” (#2, size: 38, silhouette: 0.94), “triage” (#3, size: 16, silhouette: 0.96), and “risk prediction” (#35, size: 3, silhouette: 0.99). Additionally, the time evolution analysis indicated that the top five largest clusters were highly cited after 2013 (Figure 4B).

Research themes and topic trends
In the application of AI, four theme clusters were found among the ED and ICU studies (Figure 5A). The red cluster mainly represents the fundamental and new research areas in the application of AI. The green cluster shows the prediction model establishment. The blue cluster represents the performance and future prospect of AI in COVID-19 treatment. The yellow cluster indicates the outcomes of patients with critical illness. Figure 5B shows the trends of these topics, and the dot color, which ranges from purple to yellow, illustrates different research times. Recently, studies have mainly focused on the relation of machine learning models and clinical outcomes.
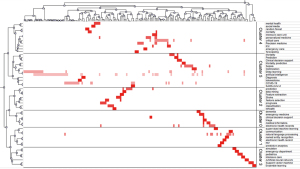
According to the matrix generated by BICOMB and bi-clustering by gCLUTO, the keywords that appeared more than three times were divided into six clusters (Figure 6). The different clusters of the keywords represent the application of AI in different disease study fields. For example, Cluster 0 represents the application of AI to dementia, Cluster 1 represents the application of AI technology to human immunodeficiency virus research, Cluster 3 and Cluster 4 summarize the algorithmic design in the pediatric and mental health fields, respectively. In addition, Cluster 5 focuses on the clinical application of AI to sepsis, COVID-19, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Analysis of co-authorship
According to the domestic and international literature search, 56 countries published relevant papers. Figure 7 displays the cooperative relationship among the countries or regions. Notably, with 205 articles, the USA had the highest frequency of interactions with other countries (20 links, and 94 total link strength). The main active collaborators with the USA were China, England, and Canada. Of the 1,018 institutions, 34 institutions that had published five or more papers were included in the analysis. Harvard Medical School had the most institutional partnerships with 17 organizations (25 total link strength), and their main partner was Massachusetts General Hospital (4 link strength), followed by Massachusetts Institute of Technology (18 total link strength), and Stanford University (16 total link strength). Additionally, with six articles and 28 citations, Zhejiang University had no links to other organizations.
Analysis of the registered trials
From 1st January, 2011 to 31st December, 2021, 130 trials were identified that examined the application of AI in the ED and ICU. Among them, 59 (45.4%) were observational study and 57 (43.8%) are applied to prospective data. Additionally, 25 trials (19.2%) had recruited children as participants. In terms of the status of the trials, only 36 (27.7%) had been completed. The results of most (125 trials, 96.2%) were not able to be retrieved during our study period. Further details are provided in Table 1.
Table 1
| Characteristics | n (%) |
|---|---|
| Study type | |
| Interventional | 71 (54.6) |
| Observational | 59 (45.4) |
| Time perspective | |
| Prospective | 57 (43.8) |
| Retrospective | 17 (13.1) |
| Others | 56 (43.1) |
| Participant age (years) | |
| <18 | 25 (19.2) |
| 18–64 | 114 (87.7) |
| ≥65 | 116 (89.2) |
| Trial status | |
| Not recruiting | 20 (15.4) |
| Recruiting | 47 (36.2) |
| Completed | 36 (27.7) |
| Suspended | 1 (0.8) |
| Terminated/withdrawn | 7 (5.4) |
| Unknown | 6 (4.6) |
| Results | |
| With results | 5 (3.8) |
| Without result | 125 (96.2) |
| Funding source | |
| Industry | 15 (11.5) |
| Other | 115 (88.5) |
| Locations | |
| America | 85 (65.4) |
| Asia | 21 (16.2) |
| Europe | 52 (40.0) |
| Middle East | 5 (3.8) |
The registered trials had three predominant basic objectives. First, a great number of trials sought to evaluate or create deep learning and machine learning models for predicting the clinical or cognitive status of patients admitted to the ED or ICU. Notably, the algorithms used in these studies sought to assist practitioners in patient care decision making. Second, other trials sought to establish a medical database that included standardized, structured clinical diagnosis, and treatment information, and provide a multi-modal data technology system for future AI technology. Third, other trials sought to assess AI-assisted operations or robotic surgery in the improvement of public health.
Discussion
In this study, we conducted a bibliometric analysis of the application of AI in critical illness and undertook a comprehensive review of the relevant trials registered at ClinicalTrials.gov. Though 427 articles from WoS core collection database and 130 clinical trials from ClinicalTrials.gov were limited quantities according to our study, the results based on authoritative databases illustrated the achievement of AI application in individualized diagnosis and treatment were gradually increased. Therefore, it has great importance and applicability in the clinical practice. One attractive result from our analysis was that the number of published articles has continued to increase annually, and in the last decade, the global geographic distribution of the articles has been wide. The relevant articles focused on the following four aspects: (I) AI application; (II) prediction model establishment; (III) COVID-19 treatment; and (IV) outcome assessment. Among the trials that had been registered, only 3.8% (5 trials) of them had reached exact conclusions which favored the application of AI.
With the development of modern science, more external life support equipment has been applied in the treatment of critically ill patients, such as mechanical ventilation (MV), extracorporeal membrane oxygenation (ECMO), and continuous renal replacement therapy (CRRT). Thus, the success rate of resuscitation in the ED or ICU is gradually improving and a great volume of data can be collected during the hospital stay (20,21). “Big Data”, “Machine Learning” and “AI” have already formed an “iron triangle” (22,23), and our bibliometric analysis revealed that most of these terms were keywords in the included studies.
Critical illness management always requires rapid diagnosis and timely treatment. However, sometimes decision making is very difficult due to the similarity of the clinical symptoms. Thus, AI assistance has been applied based on “Big Data” analyses to establish prediction models for disease diagnosis and outcome evolution (24,25). For example, multimodal models based on dynamically associated biological markers and basic characteristics of the hosts have already been established for risk and mortality prediction in ICU patients with COVID-19 (24,26). Meanwhile, natural language processing (NLP) plays another important role in extracting valuable information, which can provide high-quality data for AI and benefit for treatment strategy making, dosage of medications adjusting and parameters changing in MV (27). Furthermore, medical imaging, offers potential prospects for diagnosing, monitoring and surveying complications (28). It is also reported that the combination of AI and specimen morphological recognition has a promising future in differential diagnosis of interstitial exudative inflammation of lung in ICU (29).
Due to the heterogeneity of the diseases and population, individualized treatment is recommended in the ICU. It has been demonstrated that AI assistance can clearly distinguish phenotype and retype in sepsis, ARDS and other symptom complexes (30,31). Thus, the combination of a reliable prediction model and personalized medicine may improve the outcome. However, both internal and external validation is required to establish a credible prediction model. Digital twin, a virtual/digital replica of physical entities aimed to access cost-effective simulations, was always combined with AI analysis tools, which can quickly evaluate many possible ICU treatment alternatives. It is still in its infancy and data collection and team establishment are quite difficult. Unify data and model standards, share data and models, innovate on service and establish forums would make research and development of digital twins more coherent in critical care medicine (32,33). Meanwhile, it is also important to strengthen the cooperative relationship between different countries and institutions to make up for the small sample size of a single institution.
The ethical issue of AI is another unavoidable matter and it has already become one of the mostly controversial challenge of emergency and critical care medicine. AI and algorithms are established from the electronic systems and database and algorithms written may naturally contain errors which may lead to unforeseen consequences. Thus, current regulatory oversight is quite necessary and “double check” is recommended (34,35).
Clinical trials play an important role in the diagnosis and treatment of critically ill patients. The safety and effectiveness of new drugs and therapies have been tested in these trials, and some of them might be suggested in practice guidelines and recommendations as changes to the treatment strategy (36,37). ClinicalTrials.gov is a public registry platform that increases the transparency of research projects in progress or those that have been completed (38). As many as 130 trials have been registered on this platform, and 43.8% of them are applied to prospective data which will coincide with the development in critical care medicine. It can be expected that they may have more impact and can provide more practical instruction. Notably, only 27.7% of the registered trials have been completed and only 3.8% of the clinical trials have presented favorable results. Thus, the feasibility of these trials may still be problematic, and a high-quality control process and good coordination among multidisciplinary teams are needed.
The present study provided a comprehensive review of the application of AI in the ED and ICU, but still has some limitations. First, the WoS core collection database and ClinicalTrials.gov are updated dynamically, and thus, the most recent data might be missing from this study. Second, we only included English written articles and trials in the final analysis. However, the findings of articles and trials written in other languages may not concur with our results. Thus, bibliometric analysis and review of the registered trials should also be dynamic and updated in the future.
Conclusions
The application of AI to critical illness for individualized diagnosis and treatment is a research field with great potential. Cooperation between agencies engaged in AI research needs to be strengthened. A growing number of trials focusing on decision-making assistance and prediction model establishment have been registered at ClinicalTrial.gov, and a quality-control process should be implemented to ensure research completion.
Acknowledgments
We would like to thank L. Huleatt and J. Jones for language editing of our manuscript.
Funding: This work was supported by Shanghai Shenkang Hospital Development Center (No. SHDC12018106), the National Key Research and Development Program of China (No. 2017YFC0909002), and the National Natural Science Foundation of China (No. 81974251).
Footnote
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-913/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-913/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zimmerman JJ, Harmon LA, Smithburger PL, et al. Choosing Wisely For Critical Care: The Next Five. Crit Care Med 2021;49:472-81. [Crossref] [PubMed]
- Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019;321:2003-17. [Crossref] [PubMed]
- Nguyen D, Ngo B, vanSonnenberg E. AI in the Intensive Care Unit: Up-to-Date Review. J Intensive Care Med 2021;36:1115-23. [Crossref] [PubMed]
- Mathur P, Burns ML. Artificial Intelligence in Critical Care. Int Anesthesiol Clin 2019;57:89-102. [Crossref] [PubMed]
- Komorowski M, Celi LA, Badawi O, et al. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med 2018;24:1716-20. [Crossref] [PubMed]
- Chapalain X, Huet O. Is artificial intelligence (AI) at the doorstep of Intensive Care Units (ICU) and operating room (OR)? Anaesth Crit Care Pain Med 2019;38:337-8. [Crossref] [PubMed]
- Scherpf M, Gräßer F, Malberg H, et al. Predicting sepsis with a recurrent neural network using the MIMIC III database. Comput Biol Med 2019;113:103395. [Crossref] [PubMed]
- Campbell JP, Lee AY, Abràmoff M, et al. Reporting Guidelines for Artificial Intelligence in Medical Research. Ophthalmology 2020;127:1596-9. [Crossref] [PubMed]
- Liu X, Cruz Rivera S, Moher D, et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med 2020;26:1364-74. [Crossref] [PubMed]
- Lal A, Li G, Cubro E, et al. Development and Verification of a Digital Twin Patient Model to Predict Specific Treatment Response During the First 24 Hours of Sepsis. Crit Care Explor 2020;2:e0249. [Crossref] [PubMed]
- Lal A, Pinevich Y, Gajic O, et al. Artificial intelligence and computer simulation models in critical illness. World J Crit Care Med 2020;9:13-9. [Crossref] [PubMed]
- Dang J, Lal A, Flurin L, et al. Predictive modeling in neurocritical care using causal artificial intelligence. World J Crit Care Med 2021;10:112-9. [Crossref] [PubMed]
- Tao L, Zhang H, Zhuo L, et al. A scientificity and feasibility evaluation of COVID-19 clinical studies registered in China. Ann Transl Med 2020;8:817. [Crossref] [PubMed]
- Lau AYS, Staccini P. Section Editors for the IMIA Yearbook Section on Education and Consumer Health Informatics. Artificial Intelligence in Health: New Opportunities, Challenges, and Practical Implications. Yearb Med Inform 2019;28:174-8. [PubMed]
- Liu G, Li N, Chen L, et al. Registered Trials on Artificial Intelligence Conducted in Emergency Department and Intensive Care Unit: A Cross-Sectional Study on ClinicalTrials.gov. Front Med (Lausanne) 2021;8:634197. [Crossref] [PubMed]
- Saheb T, Saheb T, Carpenter DO. Mapping research strands of ethics of artificial intelligence in healthcare: A bibliometric and content analysis. Comput Biol Med 2021;135:104660. [Crossref] [PubMed]
- Wang W, Dong X, Qu J, et al. Bibliometric Analysis of Microtia-Related Publications From 2006 to 2020. Ear Nose Throat J 2021; Epub ahead of print. [Crossref] [PubMed]
- Zhang Y, Chen S, Tian W, et al. Emerging Trends and Hot Spots in Sepsis-Associated Encephalopathy Research From 2001 to 2021: A Bibliometric Analysis. Front Med (Lausanne) 2022;9:817351. [Crossref] [PubMed]
- Brandt JS, Hadaya O, Schuster M, et al. A Bibliometric Analysis of Top-Cited Journal Articles in Obstetrics and Gynecology. JAMA Netw Open 2019;2:e1918007. [Crossref] [PubMed]
- Laher AE, Buchanan SK. Mechanically Ventilating the Severe Asthmatic. J Intensive Care Med 2018;33:491-501. [Crossref] [PubMed]
- Abrams D, Schmidt M, Pham T, et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am J Respir Crit Care Med 2020;201:514-25. [Crossref] [PubMed]
- Chang V, Goble C, Ramachandran M, et al. Editorial on Machine Learning, AI and Big Data Methods and Findings for COVID-19. Inf Syst Front 2021;23:1363-7. [Crossref] [PubMed]
- Winter NR, Hahn T. Big Data, AI and Machine Learning for Precision Psychiatry: How are they changing the clinical practice? Fortschr Neurol Psychiatr 2020;88:786-93. [PubMed]
- Yu Y, Zhu C, Yang L, et al. Identification of risk factors for mortality associated with COVID-19. PeerJ 2020;8:e9885. [Crossref] [PubMed]
- Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Health 2021;3:e166-74. [Crossref] [PubMed]
- Pezoulas VC, Kourou KD, Papaloukas C, et al. A Multimodal Approach for the Risk Prediction of Intensive Care and Mortality in Patients with COVID-19. Diagnostics (Basel) 2021;12:56. [Crossref] [PubMed]
- Wen A, Fu S, Moon S, et al. Desiderata for delivering NLP to accelerate healthcare AI advancement and a Mayo Clinic NLP-as-a-service implementation. NPJ Digit Med 2019;2:130. [Crossref] [PubMed]
- Gore JC. Artificial intelligence in medical imaging. Magn Reson Imaging 2020;68:A1-4. [Crossref] [PubMed]
- Tao Y, Cai Y, Fu H, et al. Automated interpretation and analysis of bronchoalveolar lavage fluid. Int J Med Inform 2022;157:104638. [Crossref] [PubMed]
- Marshall DC, Komorowski M. Is artificial intelligence ready to solve mechanical ventilation? Computer says blow. Br J Anaesth 2022;128:231-3. [Crossref] [PubMed]
- Suri JS, Agarwal S, Gupta S, et al. Systematic Review of Artificial Intelligence in Acute Respiratory Distress Syndrome for COVID-19 Lung Patients: A Biomedical Imaging Perspective. IEEE J Biomed Health Inform 2021;25:4128-39. [Crossref] [PubMed]
- Kamel Boulos MN, Zhang P. Digital Twins: From Personalised Medicine to Precision Public Health. J Pers Med 2021;11:745. [Crossref] [PubMed]
- Tao F, Qi Q. Make more digital twins. Nature 2019;573:490-1. [Crossref] [PubMed]
- Keskinbora KH. Medical ethics considerations on artificial intelligence. J Clin Neurosci 2019;64:277-82. [Crossref] [PubMed]
- Loftus TJ, Tighe PJ, Filiberto AC, et al. Artificial Intelligence and Surgical Decision-making. JAMA Surg 2020;155:148-58. [Crossref] [PubMed]
- Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis 2014;63:771-80. [Crossref] [PubMed]
- Tse T, Williams RJ, Zarin DA. Update on Registration of Clinical Trials in ClinicalTrials.gov. Chest 2009;136:304-5. [Crossref] [PubMed]
- Que Y, Xiao W, Xu BS, et al. The changing landscape of phase II/III metastatic sarcoma clinical trials-analysis of ClinicalTrials.gov. BMC Cancer 2018;18:1251. [Crossref] [PubMed]
(English Language Editors: L. Huleatt and J. Jones)