
XGBoost algorithm and logistic regression to predict the postoperative 5-year outcome in patients with glioma
Introduction
Gliomas originate from brain glial cells and ranks the first in primary intracranial tumor (1). The World Health Organization (WHO) classifies gliomas into low grade (grades I and II) and high grade (grades III and IV) tumors. In clinical practice, patients with grade I glioma are often misdiagnosed. More than 80% of brain malignancies are gliomas (2). Glioblastoma (GBM), which is classified as WHO grade IV, accounts for about 50% of primary malignant central nervous system gliomas (3). The annual incidence of gliomas and malignant gliomas in China is 3.0–6.4 per 100,000 and 5.8 per 100,000, respectively, with 7,000–10,000 new cases diagnosed each year (4,5). The incidence of glioma has only increased slightly in the past 20 years, especially among the elderly, possibly due to advances in imaging diagnostic techniques, improved quality of life, and increased knowledge and awareness of diseases and demand for medical services. Malignant gliomas are 40% more common in men than women, and twice as common in Caucasians and people of African descent. The median age of patients when first diagnosed with GBM and anaplastic glioma is 64 and 45 years, respectively (6). The 5-year mortality rate of glioma patients is very high and only rank after pancreatic cancer and lung cancer, and it is one of the malignant tumors with the worst prognosis. While the pathogenesis remains unclear, high-dose ionizing radiation exposure is believed to be a major risk factor. Previous studies have mostly used multivariate logistic regression models or Cox proportional hazard models to analyze factors related to end-point events (7,8). These models have high requirements on the sample size and related parameters of the study, which greatly limits the development of clinical research. The XGBoost algorithm is based on gradient boosting decision-making, has low data requirements, fast training speed, and accurate training results. It has been widely used in artificial intelligence and the data analysis fields, and has been increasingly used in clinical research (9,10). This study retrospectively analyzed patients who received surgical treatment for glioma for the first time. The XGBoost algorithm and logistic regression model were used to identify the factors associated with death within 5 years after glioma surgery. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3384/rc).
Methods
Study population
This is a retrospective study. Consecutive patients who received surgical treatment for glioma for the first time in our hospital from January 2006 to April 2017 were retrospectively enrolled. The following inclusion criteria were applied: (I) adult patients; (II) with definite diagnosis of primary glioma of grade II or above; (III) no previous surgery for glioma. Exclusion criteria: (I) presentation brain tumors of other types or cancer in other organs and tissues; (II) co-existence of heart dysfunction, renal dysfunction, liver dysfunction, etc.; and (III) co-existence of rheumatic diseases. According to these criteria, a total of 638 patients were included in the statistical analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xijing Hospital (Approval No. KY20222151) and individual consent for this retrospective analysis was waived.
Treatment methods
All patients received surgical resection, and the operations were formulated and completed by the same glioma surgical team. A total of 291 (45.6%) patients underwent extended tumor resection or total resection, and 347 (54.4%) patients had partial resection, including 289 (45.3%) patients who underwent subtotal resection and 58 (9.1%) patients who had local excision. There were 426 patients (66.8%) who received radiotherapy after surgery, 434 patients (68.0%) who received chemotherapy, 337 patients (52.8%) who received both radiotherapy and chemotherapy, and 115patients (18.0%) who did not receive radiotherapy nor chemotherapy.
Observation indicators
The baseline data was collated for each patient prior to their first operation. The demographic data included age, gender, family income, and medical history included underlying diseases, smoking and drinking history, blood pressure, heart rate, weight, and height. Laboratory indicators included blood cell count, level of hemoglobin, indicators of liver function, and renal function, and levels of electrolyte. Information of imaging examination included that obtained at the first diagnosis of glioma and the latest preoperative imaging examination results, including the location and diameter of glioma. Surgical information and Karnofsy performance score (KPS) were also collated.
Follow-up
All the last follow-ups were conducted during this study, and the time from the baseline operation to the latest follow-up was not less than 5 years. Follow-up data included survival (including tumor-related death and all-cause death) within 5 years after baseline surgery, rehospitalization for glioma (due to recurrence or progression), or any other treatment.
Statistical analysis
The SPSS 22.0 statistical software was used for statistical processing. Quantitative data were tested for normal homogeneity. Data that conformed to the normal distribution are expressed as the mean ± standard deviation (SD), and the comparison between groups was conducted using the Student’s t-test. Data that did not conform to normal distribution are expressed as the median (percentile). The rank sum test was used for comparison between groups. Qualitative data are expressed as numerical values and percentages, and comparison between groups was performed using the χ2 test or Fisher’s exact test. The XGBoost algorithm and multivariate logistic regression model were used to analyze the factors associated with death within 5 years after surgery. The receiver operating characteristic (ROC) was used to analyze the predictive value of different models. The area under the ROC curve (AUC) and the Youden index for the two models was compared. When performing XGBoost analysis, qualitative data was converted into numerical data, that is, “yes” and “no” are converted into “1” and “0”. A two-sided P value <0.05 was considered statistically significant.
Results
Follow-up results
At the last follow-up, there were 336 cases of all-cause deaths within 5 years after glioma surgery, with a mortality rate of 52.7%, and 295 cases (46.2%) of tumor-related deaths. Among all patients, 189 (29.6%) underwent reoperation due to tumor recurrence or progression, and 88 (13.8%) received non-surgical treatment. According to the postoperative 5-year survival, the patients were divided into a deceased group (n=336) and a survival group (n=302). The comparison of baseline data between the two groups is shown in Tables 1,2. There were statistically significant differences in multiple indicators between the two groups at baseline, including age, sex, body mass index (BMI), proportion of smoker and drinker, tumor diameter, WHO classification, postoperative radiotherapy and chemotherapy ratios, and surgical method.
Table 1
| Characteristics | Deceased (n=336) | Survived (n=302) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 44.6±10.3 | 38.5±9.9 | 7.607 | <0.001 |
| Male, n (%) | 141 (64.4) | 215 (51.3) | 9.963 | 0.002 |
| BMI (kg/m2), mean ± SD | 23.6±2.9 | 22.7±2.6 | 4.109 | <0.001 |
| Smoking, n (%) | 112 (33.3) | 77 (25.5) | 4.685 | 0.030 |
| Alcohol, n (%) | 124 (36.9) | 76 (25.2) | 10.185 | 0.001 |
| WBC (×109/L), mean ± SD | 6.5±2.2 | 6.3±2.1 | 1.171 | 0.242 |
| NEU (×109/L), mean ± SD | 3.8±1.7 | 3.7±1.6 | 0.763 | 0.446 |
| LYM (×109/L), mean ± SD | 2.0±0.7 | 2.1±0.6 | 1.927 | 0.055 |
| RBC (×1012/L), mean ± SD | 4.7±0.9 | 4.8±0.8 | 1.477 | 0.143 |
| Hb (g/L), mean ± SD | 143.4±13.9 | 144.1±13.2 | 0.650 | 0.516 |
| PLT (×109/L), mean ± SD | 176.7±31.5 | 173.9±30.2 | 1.143 | 0.253 |
| Cr (μmol/L), mean ± SD | 68.7±18.2 | 70.1±19.8 | 0.931 | 0.352 |
| UA (μmol/L), mean ± SD | 242.6±70.3 | 251.5±61.6 | 1.692 | 0.091 |
| ALT (U/L), mean ± SD | 25.3±6.8 | 26.1±6.3 | 1.536 | 0.125 |
| AST (U/L), mean ± SD | 21.8±4.7 | 22.4±4.4 | 1.659 | 0.098 |
| TBIL (μmol/L), mean ± SD | 12.1±4.3 | 12.6±4.7 | 1.403 | 0.161 |
| DBIL (μmol/L), mean ± SD | 5.7±1.9 | 5.8±1.7 | 0.697 | 0.486 |
| BUN (mmol/L), mean ± SD | 7.2±2.3 | 7.0±2.0 | 1.166 | 0.244 |
| K (mmol/L), mean ± SD | 4.54±0.36 | 4.55±0.33 | 0.364 | 0.716 |
| Na (mmol/L), mean ± SD | 143.5±11.2 | 144.0±10.7 | 0.575 | 0.566 |
| Cl (mmol/L), mean ± SD | 106.8±12.3 | 107.5±12.1 | 0.723 | 0.470 |
| Ca (mmol/L), mean ± SD | 2.2±0.7 | 2.1±0.6 | 1.927 | 0.055 |
| Hypertension, n (%) | 23 (6.8) | 20 (6.6) | 0.013 | 0.911 |
| Hyperlipidemia, n (%) | 31 (9.2) | 27 (8.9) | 0.016 | 0.900 |
| Diabetes, n (%) | 11 (3.3) | 9 (3.0) | 0.045 | 0.832 |
| CAD, n (%) | 9 (2.7) | 7 (2.3) | 0.085 | 0.771 |
| COPD, n (%) | 5 (1.7) | 4 (1.3) | 0.026 | 0.872 |
| Aspirin, n (%) | 16 (4.8) | 11 (3.6) | 0.526 | 0.468 |
| Statins, n (%) | 15 (4.5) | 12 (4.0) | 0.095 | 0.759 |
| ADDs, n (%) | 11 (3.3) | 9 (3.0) | 0.045 | 0.832 |
| KPS, mean ± SD | 64.8±2.6 | 68.3±2.3 | 17.924 | <0.001 |
SD, standard deviation; BMI, body mass index; WBC, white blood cell; NEU, neutrophils; LYM, lymphocyte; RBC, red blood cell; Hb, hemoglobin; PLT, platelet; Cr, creatine; UA, uric acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; BUN, blood urea nitrogen; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; ADD, anti-diabetic drug; KPS, Karnofsy performance score.
Table 2
| Characteristics | Deceased (n=336) | Survived (n=302) | t/χ2 value | P value |
|---|---|---|---|---|
| Location, n (%) | 21.352 | 0.0001 | ||
| Upper lobe (n=484) | 252 (75.0) | 232 (76.8) | ||
| Upper brain midline (n=76) | 55 (16.4) | 21 (7.0) | ||
| Cerebellum (n=63) | 21 (6.3) | 42 (13.9) | ||
| Brain stem (n=15) | 8 (2.4) | 7 (2.3) | ||
| Diameter (mm), mean ± SD | 32.7±9.3 | 29.4±8.8 | 4.590 | <0.001 |
| WHO class, n (%) | 378.245 | <0.001 | ||
| II | 25 (7.4) | 248 (82.1) | ||
| III | 86 (25.6) | 36 (11.9) | ||
| IV | 225 (67.0) | 18 (6.0) | ||
| Surgery, n (%) | 171.477 | <0.001 | ||
| Partial resection | 265 (78.9) | 82 (27.2) | ||
| Total/extensive resection | 71 (21.1) | 220 (72.8) | ||
| Radiotherapy, n (%) | 8.532 | 0.004 | ||
| Yes | 207 (61.6) | 219 (72.5) | ||
| No | 129 (38.4) | 83 (27.5) | ||
| Chemotherapy, n (%) | 9.962 | 0.002 | ||
| Yes | 210 (62.5) | 224 (74.2) | ||
| No | 126 (37.5) | 78 (25.8) |
SD, standard deviation; WHO, World Health Organization.
Multivariate logistic regression analysis related to death within 5 years after surgery
Univariate and multivariate analysis showed that age, gender, WHO grade, extent of tumor resection, KPS, tumor diameter, and postoperative radiotherapy and chemotherapy were closely related to death during the 5-year follow-up period (Table 3).
Table 3
| Factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age | 1.09 | 1.04–1.15 | <0.001 | 1.07 | 1.02–1.11 | <0.001 | |
| Male | 1.12 | 1.06–1.29 | <0.001 | 1.08 | 1.03–1.22 | <0.001 | |
| WHO class | 1.37 | 1.23–1.96 | <0.001 | 1.34 | 1.20–1.85 | <0.001 | |
| Extent of resection | 1.40 | 1.19–2.27 | <0.001 | 1.36 | 1.17–2.06 | <0.001 | |
| KPS | 0.94 | 0.88–0.98 | 0.001 | 0.95 | 0.90–0.99 | 0.017 | |
| Diameter | 1.66 | 1.31–3.42 | <0.001 | 1.63 | 1.39–3.34 | <0.001 | |
| Chemotherapy/radiotherapy | 0.84 | 0.57–0.96 | <0.001 | 0.80 | 0.62–0.94 | <0.001 | |
WHO, World Health Organization; KPS, Karnofsy performance score; OR, odds ratio; CI, confidence interval.
Risk prediction model of death within 5 years after operation based on the XGBoost algorithm
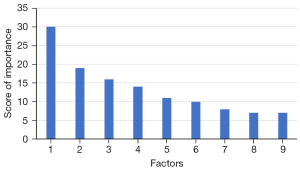
The XGBoost model demonstrated that multiple factors were related to the death of glioma patients within 5 years after operation. The top 5 factors were WHO classification (30 points), extent of tumor resection (19 points), postoperative radiotherapy and chemotherapy (16 points), KPS (14 points), and age (11 points) (Figure 1).
A comparison of the predictive ability between the XGBoost algorithm and the logistic regression model
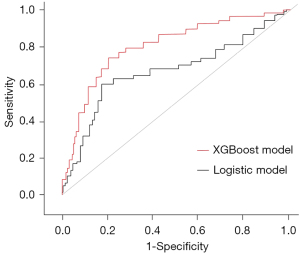
The AUC of the multivariate logistic regression model was 0.738 [95% confidence interval (CI): 0.704–0.781], with sensitivity and specificity of 0.785 and 0.632, respectively, and a Youden index of 0.417. The AUC of the XGBoost model for predicting death within 5 years after surgery in glioma patients was 0.803 (95% CI: 0.718–0.832), with sensitivity and specificity of 0.894 and 0.581, respectively, and a Youden index of 0.475. The AUC of the XGBoost model increased by 0.065 compared with that of the logistic model (Figure 2), and the Youden index increased by 0.058.
Discussion
This study retrospectively analyzed the clinical data and follow-up results of glioma patients. Logistic multivariate regression analysis revealed that the factors associated with the death of glioma patients within 5 years after surgery included age, gender, WHO grade, extent of tumor resection, preoperative KPS, tumor diameter, and postoperative radiotherapy and chemotherapy. The XGBoost model analysis showed that the top factors related to the death of glioma patients within 5 years after surgery are WHO grade, extent of tumor resection, postoperative radiotherapy and chemotherapy, preoperative KPS, and age. A comparison of the two models demonstrated that the area under ROC was larger with the XGBoost model compared to the logistic model, and the Youden index was also greater.
Brain gliomas usually occur between the ages of 20 and 40 years. Clinical presentations vary with the location and volume of glioma and are largely related to the compressive effects of the tumor. The most often seen clinical presentation is seizure, which can be found in about 60–80% of patients. Other often seen symptoms include headaches, cognitive dysfunction, abnormal behavior, motor dysfunction, and sensory dysfunction. In most patients, seizures can be treated effectively with antiepileptic drugs. Symptoms usually can be improved following the resection of tumor in most patients. In previous study conducted by Robe et al., the results showed that early wide resection improved outcomes in patients with glioma (11). If surgical treatment is delayed or the tumor tissue is unresectable, the growth of tumor should be intensively monitored with imaging [especially magnetic resonance imaging (MRI)] within 6 months after the first time of glioma diagnosis.
The prognosis of gliomas with different WHO grades varies greatly. Gliomas of WHO grade I–II gliomas are usually high-differentiated with slow progression. These gliomas are usually clinically stable over a long period of time, their slow progression can only be detected by imaging examination at longer intervals (12). A significant proportion of WHO grades I–II gliomas eventually progress into advanced stage at some point in the disease process if not treated with timely and effective surgical treatment (13). Standard treatments include surgical resection and radiation therapy. Radiation therapy is often used in patients with symptomatic and/or progressive disease or in patients with poor prognosis (14). Although the early role of surgery and extent of resection have not been demonstrated in randomized studies, retrospective studies have suggested that wide resection improves outcomes (15). However, in clinical practice, the operation time and surgery strategy are often decided according to the characteristics of the patient’s condition, and some patients eventually will relapse and quickly progress after the surgery.
Some previous studies outlined the predictors of glioma prognosis (16-18), including patient’s history of diseases, such as the results of preoperative laboratory test results, the patient’s living habits, co-existing diseases, and other often-used information (including age and gender, etc.); preoperative information of imaging examination, including the location and volume of tumor, and its relationship with adjacent tissues; and results of pathological examination, such as pathological type. In recent years, a large number of research have investigated the correlation between glioma and some genetic biomarkers such as isocitrate dehydrogenase 1 (IDH1) and IDH2 genotypes, microRNAs, and protein biomarkers. These investigations have brought the clinicians with various new perspectives on gliomas, and evoked novel ideas for the development of effective and timely clinical diagnosis and treatments. However, some of these studies focused on all glioma patients without distinguishing between WHO grades, while others focused only on patients at advanced stages. In addition, for example, a previous report did not take the surgical outcomes into consideration (16). The few studies with postoperative follow-up of patients with lower-grade gliomas often demonstrated a satisfactory surgical outcome. Clinical diagnosis of grade I gliomas are relatively rare, and surgical outcomes for WHO grades III–IV gliomas are often unsatisfactory. However, even the glioma was only at WHO grade II, a considerable number of patients showed poor outcomes after postoperative follow-up. Thus, it is clinically critical to find patients at high-risk early, so as to provide intensive treatment and close monitoring in a timely manner. Previous research have demonstrated that age over 40 years, partial tumor resection, negative IDH1 R132H expression, and positive RTEL1 expression are independent risk factors for progression-free survival in patients with glioma (19). Findings of our present study are partly consistent with this latter study. However, our report suggested that a advanced age is strongly associated with poor prognosis. Although there were differences existed between groups of difference age, the general trend suggested that older patients had worse postoperative outcome. For elderly patients, strict follow-up and monitoring should be provided. In this current study, relevant genetic testing has not been conducted, and also other clinically often-used blood biomarkers have not been tested. The major underlying reason is that although these parameters have high predictive value, it is temporarily difficult to popularize in clinical practice. In clinical practice, simple, convenient, and cheap biomarkers are essential to provide clinical value of implication.
In recent years, there have been many studies examining the effectiveness of some drug therapy on the prognosis of patients with glioma. According to some previous studies, statins have received extensive attention in clinical practice. In many previous clinical studies, statins have been shown to have favorable effect on the reduction of the risk of cancers (20-22) and the risk of deaths due to cancers (23). However, there have also been reports of increased tumor risk (24,25) and others showing no definite relationship between statins and the occurrence, progression and long-term outcome (26). Cote et al. conducted a meta-analysis based on three large studies including the Nurses’ Health Study (NHS, n=114,419), the Nurses’ Health Study II (NHSII, n=115,813), and the Men’s Health Professionals Follow-up Study (HPFS, n=50,223). They found that patients who were treated with statins had a significantly higher risk of developing glioma when compared with patients who have never used statins [hazard ratio (HR) =1.43; 95% CI: 1.10–1.86], and the risk was higher in patients with longer time of statin treatment (HR =1.72; 95% CI: 1.21–2.45, for >8 years of use; P-trend =0.003). Further subgroup analysis revealed that water-soluble statins (e.g., rosuvastatin and pravastatin) were closely associated with the risk of gliomas, while fat-soluble statins (such as simvastatin and atorvastatin) did not show statistical significance (27). However, a meta-analysis combining the three earlier studies found that taking statins was associated with a lower risk of developing gliomas during follow-up [odds ratio (OR) =0.75; 95% CI: 0.62–0.90; P=0.0016] (28). In contrast, a study by Seliger et al. found that there was no definite relationship between the statins treatment and the risk of occurrence or progression of glioma (29). This study included 2,469 glioma patients and 24,690 control patients, which is a large sample size and has good representation (29). The 1,734 (70.2%) glioma patients in this study were over the age of 50, and nearly half of the patients were elderly. Some previous studies did not distinguish the grades of gliomas, while the developmental biology and prognosis of gliomas with different grades vary widely (27-29). At the same time, surgery also has a certain impact on the biological behavior of tumor cells (30) and statins may have a protective effect on this process. In addition, statins may have a certain impact on the local tumor microenvironment. Therefore, the results of studies on the risk of developing gliomas with statins remain inconclusive and this may be related to the significant differences in the included patients. Moreover, the vast majority of patients enrolled in the above-mentioned studies were non-Asian patients, and there may be differences between patients with different human race (27-29). Our present study examined glioma patients who received surgical treatment. These patients are relatively inoperable and may have a positive response to drugs. However, since most of the patients in this study are young and middle-aged, there were few patients taking statins. Therefore, the benefit of statins could not be analyzed. The effect of tumor size and the extent of tumor resection on death within 5 years after surgery in glioma patients was consistent with another study (19), and the difference lies in the specific numerical range. In general, the larger the volume of tumor, the poorer the postoperative prognosis, and the more extension the tumor resection, the better the postoperative outcome.
Our present study had some limitations. First, this was a single-center, retrospective study conducted over a large time span, and there may be different treatment tendencies for the patients over that period of time, and the surgical experience of the surgical team may also change over time, resulting in certain differences or bias between cases. Second, the findings of this study could not be validated in additional case cohorts. In particular, risk prediction models that produce results in one case cohort should be validated with a different cohort to demonstrate model fitness. Third, this study used common clinical indicators, and failed to include gene parameters and molecular parameters which may be more related to the prognosis of glioma patients. The results of this study should be further validated using a prospective cohort, and genetic and molecular testing of patients should be performed to increase the accuracy of the risk prediction models.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3384/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3384/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3384/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xijing Hospital (Approval No. KY20222151) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molinaro AM, Taylor JW, Wiencke JK, et al. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 2019;15:405-17. [Crossref] [PubMed]
- Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2014;16:896-913. [Crossref] [PubMed]
- McKinnon C, Nandhabalan M, Murray SA, et al. Glioblastoma: clinical presentation, diagnosis, and management. BMJ 2021;374: [PubMed]
- Wu JS, Zhang J, Zhuang DX, et al. Current status of cerebral glioma surgery in China. Chin Med J (Engl) 2011;124:2569-77. [PubMed]
- Liang S, Fan X, Zhao M, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med 2019;8:4527-35. [Crossref] [PubMed]
- Davis ME. Epidemiology and Overview of Gliomas. Semin Oncol Nurs 2018;34:420-9. [Crossref] [PubMed]
- Ding X, Zhao Y, Yuan H, et al. Role of PVT1 polymorphisms in the glioma susceptibility and prognosis. Eur J Cancer Prev 2021;30:400-8. [Crossref] [PubMed]
- Gittleman H, Sloan AE, Barnholtz-Sloan JS. An independently validated survival nomogram for lower-grade glioma. Neuro Oncol 2020;22:665-74. [Crossref] [PubMed]
- Kandemirli SG, Kocak B, Naganawa S, et al. Machine Learning-Based Multiparametric Magnetic Resonance Imaging Radiomics for Prediction of H3K27M Mutation in Midline Gliomas. World Neurosurg 2021;151:e78-85. [Crossref] [PubMed]
- Sakai Y, Yang C, Kihira S, et al. MRI Radiomic Features to Predict IDH1 Mutation Status in Gliomas: A Machine Learning Approach using Gradient Tree Boosting. Int J Mol Sci 2020;21:8004. [Crossref] [PubMed]
- Robe PA, Rados M, Spliet WG, et al. Early Surgery Prolongs Professional Activity in IDH Mutant Low-Grade Glioma Patients: A Policy Change Analysis. Front Oncol 2022;12:851803. [Crossref] [PubMed]
- Özcan H, Emiroğlu BG, Sabuncuoğlu H, et al. A comparative study for glioma classification using deep convolutional neural networks. Math Biosci Eng 2021;18:1550-72. [Crossref] [PubMed]
- Dono A, Ballester LY, Primdahl D, et al. IDH-Mutant Low-grade Glioma: Advances in Molecular Diagnosis, Management, and Future Directions. Curr Oncol Rep 2021;23:20. [Crossref] [PubMed]
- Fleischmann DF, Schön R, Corradini S, et al. Multifocal high-grade glioma radiotherapy safety and efficacy. Radiat Oncol 2021;16:165. [Crossref] [PubMed]
- Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer 2021;149:23-33. [Crossref] [PubMed]
- Tunthanathip T, Ratanalert S, Sae-Heng S, et al. Prognostic factors and clinical nomogram predicting survival in high-grade glioma. J Cancer Res Ther 2021;17:1052-8. [Crossref] [PubMed]
- Corr F, Grimm D, Saß B, et al. Radiogenomic Predictors of Recurrence in Glioblastoma-A Systematic Review. J Pers Med 2022;12:402. [Crossref] [PubMed]
- van Kessel E, Schuit E, Huenges Wajer IMC, et al. Added Value of Cognition in the Prediction of Survival in Low and High Grade Glioma. Front Neurol 2021;12:773908. [Crossref] [PubMed]
- Zhang DX, Qu YM, Zhang MS, et al. Analysis of factors influencing progression-free survival in patients with WHO grade II glioma. Chinese Journal of Neurosurgery 2019;35:469-73.
- Rodríguez-Miguel A, Fernández-Antón E, Barreira-Hernández D, et al. Statins and Colorectal Cancer Risk: A Population-Based Case-Control Study and Synthesis of the Epidemiological Evidence. J Clin Med 2022;11:1528. [Crossref] [PubMed]
- Harshman LC, Wang X, Nakabayashi M, et al. Statin Use at the Time of Initiation of Androgen Deprivation Therapy and Time to Progression in Patients With Hormone-Sensitive Prostate Cancer. JAMA Oncol 2015;1:495-504. [Crossref] [PubMed]
- Ren QW, Yu SY, Teng TK, et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J 2021;42:3049-59. [Crossref] [PubMed]
- Pourlotfi A, Bass GA, Ahl Hulme R, et al. Statin Use and Long-Term Mortality after Rectal Cancer Surgery. Cancers (Basel) 2021;13:4288. [Crossref] [PubMed]
- Lin BM, Li WQ, Cho E, et al. Statin use and risk of skin cancer. J Am Acad Dermatol 2018;78:682-93. [Crossref] [PubMed]
- Fujimoto M, Higuchi T, Hosomi K, et al. Association between statin use and cancer: data mining of a spontaneous reporting database and a claims database. Int J Med Sci 2015;12:223-33. [Crossref] [PubMed]
- Emilsson L, García-Albéniz X, Logan RW, et al. Examining Bias in Studies of Statin Treatment and Survival in Patients With Cancer. JAMA Oncol 2018;4:63-70. [Crossref] [PubMed]
- Cote DJ, Rosner BA, Smith-Warner SA, et al. Statin use, hyperlipidemia, and risk of glioma. Eur J Epidemiol 2019;34:997-1011. [Crossref] [PubMed]
- Greenland S. A serious misinterpretation of a consistent inverse association of statin use with glioma across 3 case-control studies. Eur J Epidemiol 2017;32:87-8. [Crossref] [PubMed]
- Seliger C, Meier CR, Becker C, et al. Statin use and risk of glioma: population-based case-control analysis. Eur J Epidemiol 2016;31:947-52. [Crossref] [PubMed]
- Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018;15:205-18. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


