
Construction of a predictive nomogram and bioinformatic investigation of the potential mechanism of postoperative early recurrence of hepatocellular carcinoma meeting the Milan criteria
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors. According to epidemiological statistics, HCC is the seventh most common tumor worldwide and the second leading cause of cancer-related deaths (1). With greater attention being paid to health examination and the development of imaging technologies, several advances have been made for the early diagnosis of HCC. Some cases of HCC diagnosed in the early stage meet the Milan criteria (one lesion ≤5 cm or three lesions all <3 cm without evidence of extrahepatic spread or macrovascular invasion). For this subset of HCC patients, the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) suggest radical methods such as hepatectomy, ablation, and liver transplantation (2,3). Due to the relatively high risk of recurrence following radiofrequency ablation and the rarity of liver donation, hepatectomy is still the main therapeutic option for patients with HCC meeting the Milan criteria.
Unfortunately, a previous study showed that the incidence of recurrence of early-stage HCC (Barcelona Clinic Liver Cancer stage 0/A) was almost 50–70% within 5 years after radical hepatectomy, and most cases of recurrence occur
The Cancer Genome Atlas (TCGA) database contains the gene expression information of HCC and normal liver tissues. The corresponding clinicopathological information and follow-up results are also documented in this database. Herein, data downloaded from TCGA were used to preliminarily explore the mechanisms involved in the PER of HCC patients meeting the Milan criteria. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3390/rc).
Methods
Patients
This retrospective study included patients who underwent hepatectomy in West China Hospital between January 2009 and May 2015 and were pathologically diagnosed with HCC. The study’s selection criteria: (I) cases involving tumors meeting the Milan criteria; (II) cases in which only liver resection was performed before recurrence; and (III) patients whose detailed clinical characteristics were well preserved. The exclusion criteria: (I) cases with incomplete follow-up information; and (II) patients with concomitant cholangiocarcinoma or other malignancies. In this study, all participants were divided into two groups at random: a training set (n=347, 80%) and a validation set (n=86, 20%). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of West China Hospital, Sichuan University, China (No. 2020/8). Individual consent for this retrospective analysis was waived.
Collection of clinical and pathological information
The demographic data including age and sex were collected and all participants in our study underwent comprehensive preoperative tests, including routine blood examination, routine coagulation tests, liver and renal function tests, hepatitis virus screening tests, and the screening of serum tumor markers [such as alpha-fetoprotein (AFP), etc.]. Three-phase enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans were also applied conventionally to evaluate the tumor characteristics. Additionally, intraoperative ultrasonography was performed during surgery to detect additional nodules that may have been missed during the preoperative imaging (11,12). Therefore, the clinical information involving demographics, blood test information, and tumor imaging data could be retrospectively collected.
Histological examination was also performed on the resected specimens. The pathological information included the tumor differentiation grade, cutting edge, satellite lesions, microvascular invasion (MVI), Glisson’s capsule invasion, and Ishak score. The Edmondson-Steiner classification criteria were applied to confirm the differentiation grade. A negative cutting edge implied that no cancer cells were found at the margins of the tumor tissue specimens. Satellite lesions meant that distinct nodules were found within 2 cm both in size and distance from the primary tumor. MVI was defined as the presence of a tumor that was visible only on microscopy in a hepatic vein, portal vein, or a large capsular vessel of the surrounding endothelium-lined hepatic tissue (13). Glisson’s capsule invasion signified that tumor cells invaded but did penetrate the liver capsule.
Follow-up
After discharge, follow-up was performed at 1-month postoperatively and then at 3-month intervals in the first 3 years and every 6 months subsequently. Laboratory examinations including routine blood tests, liver function tests, AFP, abdominal ultrasonography, and chest radiographs were performed. For cases in which suspicious lesions were found or AFP levels were elevated persistently, enhanced abdominal CT/MRI was performed immediately thereafter. Recurrence was diagnosed meeting the combined findings of these clinical examinations. It takes 2 years or more for adenomatous hyperplasia to develop into HCC (14), and therefore, PER was defined as recurrence within 2 years after liver resection. The endpoint of our study was measured until the date of death or the last follow-up visit.
Statistics analysis
The collected clinicopathological information in the training cohort was divided into categorical and continuous variables. SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. Data were reported as mean ± standard deviation or frequency, as appropriate. Categorical variables were assessed by the Chi-square or Fisher exact test (as appropriate), while continuous variables were evaluated by the Student’s t-test, one-way analysis of variance, or the Mann-Whitney test (as appropriate). Furthermore, a univariate analysis was performed to explore the potential risk factors, and factors with a P value <0.05 in the univariate analysis were subsequently included in a stepwise multivariate analysis using a logistics model to identify risk factors that are independently associated with PER.
Receiver operating characteristic (ROC) curve analysis was conducted thereafter. The survival curve was constructed using the Kaplan-Meier method and compared using the log-rank test. The establishment of a nomogram was conducted using the rms package in R software version 4.1.2 (The R Foundation, Vienna, Austria; http://www.r-project.org/) based on the independent risk factors in the training cohort. The total points for each patient were formulated using the above nomogram. Next, the total points were treated as a new risk factor to predict the possibility of PER for each patient. The predictive accuracy of the nomogram was evaluated using Harrell’s C-index; a bootstrap with 1,000 resamples was performed to reduce the biased estimates. The calibration curves were applied to illustrate the agreement between the nomogram-predicted and actual observed probability of recurrence. Validation of the nomogram was performed in the validation cohort using the same methods. Decision curve analysis (DCA) was performed using the rmda package in R software and was validated in the validation cohort. All statistical tests were two-tailed, and a P value <0.05 was considered statistically significant.
Bioinformatics analysis
Data related to HCC were downloaded from TCGA database (https://portal.gdc.cancer.gov/). Although there was no information on HCC conforming to the Milan criteria, the data included HCC within the American Joint Committee on Cancer (AJCC) stage T1 (single tumor lesion with a maximum diameter ≤2 cm or isolated tumor with a maximum diameter >2 cm without vascular invasion). Patients with AJCC stage T1 who received surgical treatment and did not receive any other treatment before surgery were included. The patients were divided into the PER and non-PER groups based on the recurrence time. The differentially-expressed genes (DEGs) between these two groups were then identified using the DESeq2 package in R software (version 4.1.2). Additionally, the potential molecular function and involved pathways of these DEGs were analyzed in KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/).
Results
Patient and tumor characteristics
A total of 433 patients who satisfied our inclusion criteria were analyzed in this study between 2009 and 2015. These patients were randomly assigned to training (n=347) and validation (n=86) cohorts at a ratio of 4:1. The detailed clinicopathological information of the enrolled patients is listed in Table 1. Most patients were male (male/female: 359/74), the median age of the patients was 51±11 years (range, 21–81 years), and only 109 patients were over 60 years of age. As shown in the preoperative image findings, the average diameter of the largest tumor was 3.4±1.0 cm around. The hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) were detected in 389 patients (89.8%) and 78 patients (18.0%), respectively, and only eight patients (1.8%) were hepatitis B c antibody (HBcAb) negative.
Table 1
| Clinical parameter | Total (n=433) | Training group (n=347) | Validation group (n=86) |
|---|---|---|---|
| Gender, male/female | 359/74 | 289/58 | 70/16 |
| Age, years | 51±11 | 51±11 | 50±11 |
| Size, cm | 3.4±1.0 | 3.4±1.0 | 3.2±1.0 |
| Number, single/multiple | 401/32 | 323/24 | 78/8 |
| HBsAg, positive/negative | 389/44 | 311/36 | 78/8 |
| HBeAg, positive/negative | 78/355 | 65/282 | 13/73 |
| HBcAb, positive/negative | 425/8 | 340/7 | 85/1 |
| AFP, ng/mL | 854.3±2,931.5 | 858.6±3,012.5 | 837.1±2,595.3 |
| AFP ≥400 ng/mL, yes/no | 118/315 | 92/255 | 26/60 |
| WBC, 109/L | 5.3±4.2 | 5.5±4.6 | 4.8±1.6 |
| NEU, 109/L | 3.2±3.2 | 3.3±3.6 | 3.0±1.3 |
| LYM, 109/L | 1.5±0.6 | 1.6±0.6 | 1.5±0.6 |
| NLR | 2.4±1.9 | 2.3±1.9 | 2.4±2.2 |
| PLT, 109/L | 117.2±48.8 | 117.8±50.0 | 115.3±44.8 |
| PLR | 84.5±44.9 | 83.5±41.7 | 88.7±56.4 |
| MONO, 109/L | 0.3±0.2 | 0.3±0.2 | 0.3±0.1 |
| PT, s | 12.1±1.2 | 12.1±1.2 | 12.0±1.3 |
| INR | 1.4±7.2 | 1.5±8.0 | 1.1±0.1 |
| Fib, mg/dL | 2.4±0.7 | 2.5±0.8 | 2.4±0.6 |
| TB, μmol/L | 15.5±6.9 | 15.2±6.9 | 16.4±7.2 |
| ALT, U/L | 50.9±50.5 | 49.5±46.7 | 56.9±63.5 |
| AST, U/L | 43.1±32.5 | 42.2±30.5 | 46.6±39.6 |
| TP, g | 69.7±6.8 | 69.4±7.1 | 70.8±5.4 |
| ALB, g | 42.0±4.3 | 41.7±4.4 | 43.1±3.6 |
| GGT, U/L | 62.0±81.4 | 61.8±75.4 | 62.9±102.3 |
| GGT ≥60 U/L, yes/no | 134/299 | 108/239 | 26/60 |
| Creatinine, μmol/L | 76.9±20.6 | 76.9±21.5 | 76.5±17.0 |
| Anatomic resection, yes/no | 218/215 | 173/174 | 45/41 |
| Glisson’s capsule invasion, yes/no | 177/256 | 138/209 | 39/47 |
| Differentiation, I + II/III + IV | 256/177 | 221/126 | 33/53 |
| Cutting edge, positive/negative | 4/429 | 4/344 | 0/86 |
| MVI, yes/no | 77/356 | 66/281 | 11/75 |
| Satellite, yes/no | 31/402 | 24/323 | 7/79 |
| Cirrhosis, yes/no | 329/104 | 255/92 | 74/12 |
Data were reported as mean ± standard deviation or frequency, as appropriate. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcAb, hepatitis B c antibody; AFP, alpha-fetoprotein; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; NLR, neutrophil-lymphocyte ratio; PLT, platelet; PLR, platelet-to-lymphocyte ratio; MONO, monocyte; PT, prothrombin time; INR, international normalized ratio; Fib, fibrinogen; TB, total bilirubin; ALT, alanine transaminase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GGT, γ-glutamyl transpeptidase; MVI, microvascular invasion.
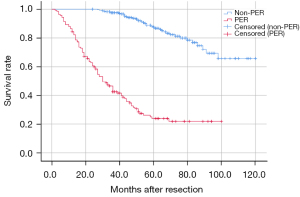
A total of 401 patients had one lesion, while 32 patients had multiple lesions. The AFP levels were significantly increased in 118 patients (26.8%, ≥400 ng/mL) and gamma-glutamyl transpeptidase (GGT) levels are markedly elevated in 134 patients (30.1%, ≥60 U/L). Half of the patients underwent anatomic resection. According to the histological results, Glisson’s capsule invasion was observed in 177 patients (40.1%), and less than half of the patients (40.9%) had poorly differentiated tumors. MVI occurred in 77 patients, and 31 patients (7.2%) had satellite lesions. The survival curve showed that the OS time in the PER group was dramatically shorter than that in the non-PER group (P<0.001) (Figure 1); patients in the non-PER group had a median OS time of 102 months, while patients with PER had a median OS time of 43 months.
Independent risk factors of PER in the training cohort
Univariate analysis showed that tumor size (P=0.018), serum AFP levels ≥400 ng/mL (P<0.001), GGT ≥60 U/L (P=0.016), Glisson’s capsule invasion (P=0.005), tumor differentiation (P=0.018), MVI (P=0.001), and satellite lesions (P=0.002) were significantly related to PER of HCC meeting the Milan criteria (Table 2). The multivariate analysis results showed that AFP ≥400 ng/mL [P=0.001, odds ratio (OR) =2.450, 95% confidence interval (CI): 1.460–4.110], GGT ≥60 U/L (P=0.034, OR =1.735, 95% CI: 1.043–2.885), Glisson’s capsule invasion (P=0.034, OR =1.699, 95% CI: 1.042–2.769), MVI (P=0.006, OR =2.266, 95% CI: 1.270–4.042), and satellite lesions (P=0.012, OR =3.203, 95% CI: 1.286–7.976) were the independent predictors of PER (Table 3).
Table 2
| Factors | OR (95% CI) | P value |
|---|---|---|
| Gender, male/female | 1.109 (0.612–2.011) | 0.733 |
| Age, years | 0.996 (0.977–1.015) | 0.674 |
| Size, cm | 1.298 (1.045–1.613) | 0.018* |
| Number, single/multiple | 2.198 (0.955–5.061) | 0.064 |
| HBsAg, positive/negative | 0.962 (0.462–2.001) | 0.917 |
| HBeAg, positive/negative | 1.378 (0.787–2.411) | 0.262 |
| HBcAb, positive/negative | 0.638 (0.140–2.898) | 0.560 |
| AFP, ng/mL | 1.000 (1.000–1.000) | 0.095 |
| AFP ≥400 ng/mL, yes/no | 2.806 (1.711–4.602) | <0.001* |
| WBC, 109/L | 0.980 (0.907–1.059) | 0.616 |
| NEU, 109/L | 1.045 (0.96–1.137) | 0.307 |
| LYM, 109/L | 0.885 (0.612–1.279) | 0.515 |
| NLR | 1.013 (0.901–1.138) | 0.830 |
| PLT, 109/L | 0.996 (0.992–1.001) | 0.116 |
| PLR | 0.998 (0.992–1.003) | 0.419 |
| MONO, 109/L | 1.217 (0.333–4.448) | 0.766 |
| PT, s | 0.948 (0.769–1.168) | 0.614 |
| INR | 0.963 (0.696–1.332) | 0.818 |
| Fib, mg/dL | 0.937 (0.693–1.267) | 0.671 |
| TB, μmol/L | 0.991 (0.958–1.024) | 0.590 |
| ALT, U/L | 1.000 (0.995–1.005) | 0.988 |
| AST, U/L | 0.999 (0.992–1.007) | 0.860 |
| TP, g | 0.974 (0.943–1.006) | 0.110 |
| ALB, g | 1.002 (0.952–1.054) | 0.945 |
| GGT, U/L | 1.001 (0.999–1.004) | 0.343 |
| GGT ≥60 U/L, yes/no | 1.796 (1.117–2.887) | 0.016* |
| Creatinine, μmol/L | 1.007 (0.997–1.018) | 0.188 |
| Anatomic resection, yes/no | 1.347 (0.858–2.114) | 0.195 |
| Glisson’s capsule invasion, yes/no | 1.923 (1.218–3.035) | 0.005* |
| Differentiation, I + II/III + IV | 1.744 (1.100–2.766) | 0.018* |
| Cutting edge, positive/negative | 2.090 (0.291–15.03) | 0.464 |
| MVI, yes/no | 2.512 (1.453–4.345) | 0.001* |
| Satellite, yes/no | 3.827 (1.620–9.041) | 0.002* |
| Cirrhosis, yes/no | 1.315 (0.780–2.217) | 0.305 |
*, statistical significance. PER, postoperative early recurrence; OR, odds ratio; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcAb, hepatitis B c antibody; AFP, alpha-fetoprotein; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; NLR, neutrophil-lymphocyte ratio; PLT, platelet; PLR, platelet-to-lymphocyte ratio; MONO, monocyte; PT, prothrombin time; INR, international normalized ratio; Fib, fibrinogen; TB, total bilirubin; ALT, alanine transaminase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GGT, γ-glutamyl transpeptidase; MVI, microvascular invasion.
Table 3
| Factors | OR | 95% CI | P value |
|---|---|---|---|
| AFP ≥400 ng/mL | 2.450 | 1.460–4.110 | 0.001* |
| GGT ≥60 U/L | 1.735 | 1.043–2.885 | 0.034* |
| Glisson’s capsule invasion | 1.699 | 1.042–2.769 | 0.034* |
| MVI | 2.266 | 1.270–4.042 | 0.006* |
| Satellite | 3.203 | 1.286–7.976 | 0.012* |
*, statistical significance. PER, postoperative early recurrence; OR, odds ratio; AFP, alpha-fetoprotein; GGT, γ-glutamyl transpeptidase; MVI, microvascular invasion.
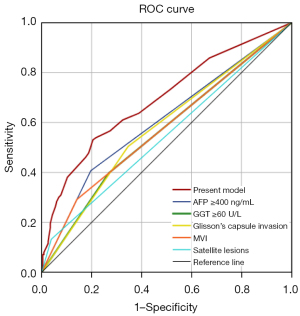
Following the logistics regression analysis, the present model was constructed to predict PER. The area under the curve (AUC) of the present model was greater than any single risk factor, and the AUCs of the present model, AFP ≥400 ng/mL, GGT ≥60 U/L, Glisson’s capsule invasion, MVI, and satellite lesions was 0.693 (P<0.001, 95% CI: 0.631–0.754), 0.605 (P=0.001, 95% CI: 0.54–0.671), 0.564 (P=0.051, 95% CI: 0.499–0.630), 0.579 (P=0.017, 95% CI: 0.515–0.644), 0.576 (P=0.023, 95% CI: 0.509–0.642), and 0.547 (P=0.155, 95% CI: 0.481–0.614), respectively (Figure 2). Maximum joint sensitivity and specificity (sensitivity =0.531, specificity =0.795) was achieved when the optimal cut-off value of the present model was 0.326, which was identified by the Youden index.
Construction and validation of the nomogram for PER
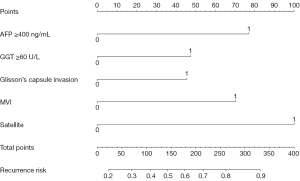
Using the independent risk factors obtained by the multivariate analysis, a novel, easy-to-use, and effective nomogram model was constructed to predict the PER of HCC after hepatectomy meeting the Milan criteria (Figure 3). The elements of the nomogram involved five categorical variables (AFP ≥400 ng/mL, GGT ≥60 U/L, Glisson’s capsule invasion, MVI, and satellite lesions). The assessment was performed internally and measured using the C-index and calibration plots. The bootstrap-corrected C-index of the nomogram in the training cohort was 0.693 (95% CI: 0.632–0.754; P<0.001). The calibration curve of the training cohort demonstrated optimal agreement between the actual observed and nomogram-predicted probability of early recurrence after hepatectomy (Figure 4A).

Furthermore, the performance of this nomogram was validated using an internal validation cohort. The total points for each patient in the validation cohort were formulated using the above nomogram. Thereafter, the total points were treated as a new risk factor, which was used to calculate the C-index and produce the PER calibration curves. The results showed that the C-index for the prediction of PER in the validation cohort was 0.658 (95% CI: 0.529–0.787; P=0.016). The calibration curve also showed favorable consistency between the predicted and observed probability of PER (Figure 4B).
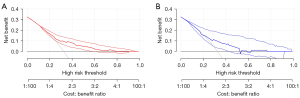
DCA
DCA was used to evaluate the clinical utility of a diagnostic test while taking into account the subjective nature of risk (15,16). In this study, we developed the decision curves of the nomogram for predicting the possibility of early recurrence in the training and validation sets (Figure 5A,5B). The results indicated that the constructed model presented a notable clinical utility.
Bioinformatics analysis
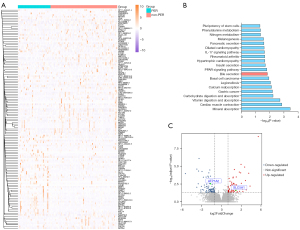
In TCGA database, a total of 131 patients within AJCC T1 were considered to have early-stage HCC. Among them, 43 patients had PER, while the remaining 88 patients did not. The DEGs between these two groups were analyzed, and the results showed that 133 genes were significant DEGs (log2FoldChange ≥1 or ≤−1, P<0.05) and the expression of these genes is shown in the heatmap (Figure 6A), including 57 up-regulated and 76 down-regulated genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of these DEGs was performed (Figure 6B). As expected, multiple cancer-related pathways were enriched, such as the peroxisome proliferator-activated receptor (PPAR) signaling pathways, gastric cancer, and basal cell carcinoma. Notably, the bile secretion pathway, which contains ATP1A2 and SLC5A1, was also enriched. Also, bile secretion was closely associated with the GGT levels. As shown in Figure 6C, ATP1A2 was significantly down-regulated in the PER group, whereas SLC5A1 was significantly up-regulated.
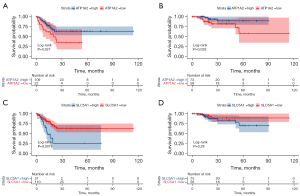
Moreover, patients in TCGA database were stratified into different groups according to the median expression values of ATP1A2 or SLC5A1. Survival analysis showed that patients with a high expression of ATP1A2 had worse disease-free survival (DFS) and OS rates following surgical resection (DFS, P=0.027, HR =2.086, 95% CI: 0.916–4.749; OS, P=0.028, HR =3.380, 95% CI: 1.172–9.752, Figure 7A,7B). Furthermore, low expression of SLC5A1 was significantly associated with DFS (P=0.0016; HR =0.361, 95% CI: 0.145–0.898) rather than OS (P=0.28; HR =0.536, 95% CI: 0.186–1.544) (Figure 7C,7D).
Discussion
This study retrospectively analyzed the risk factors associated with PER in HCC patients meeting the Milan criteria. The univariate and multivariate analyses showed that the factors associated with PER included AFP ≥400 ng/mL, GGT ≥60 U/L, Glisson’s capsule invasion, MVI, and satellite lesions. Based on these factors, a novel predictive model was constructed for PER in HCC patients meeting the Milan criteria. The C-index of the predictive model in the training and validation groups were 0.693 and 0.658, respectively, which represents a good predictive ability. Additionally, based on the independent risk factors, clinical DCA was conducted and can better help clinicians to develop treatment strategies and carry out individual follow-ups after surgery.
AFP is a glycoprotein that is synthesized mainly by the yolk sac and embryonic liver and exists at very low levels in adult serum. Although AFP is closely related to the occurrence and development of a variety of tumors, including liver cancer or non-cancerous liver disease, it is still regarded as a significant marker for the screening and diagnosis of liver cancer (17). However, the mechanism remains unclear. A study has reported that AFP and AFP receptors are only expressed in AFP-positive HCC tissues but are not expressed in normal liver tissue and the serum of patients with AFP-negative HCC (18). Furthermore, another study has shown that AFP-positive HCC cells are in rich organelles, particularly the rough endoplasmic reticulum and mitochondria, and the Golgi’s complex can be observed in the cytoplasm (19), which is likely associated with the synthesis of proteins that promote metastasis and recurrence. Multiple previous studies have pointed out that an increase in the AFP value is a risk factor for postoperative recurrence and have shown that a high level of serum AFP is positively correlated with the recurrence and metastasis of human HCC (20-22). Patients whose preoperative AFP levels are ≥400 ng/mL may have a bad prognosis, and this could be an early signal for clinicians to enact measures to prevent early recurrence.
The Glisson’s capsule is the connective collagenous layer surrounding the liver parenchyma (23) and is a powerful barrier that protects against invasion by tumor cells or tissues. In numerous solid tumors, organ capsule invasion is associated with poor outcomes. In thyroid cancer, microscopic invasion of the thyroid capsule is associated with frequent vascular invasion, poor tumor size, and unsatisfactory prognosis. In lung cancer, invasion of the visceral pleural is an independent risk factor for worse prognosis in each tumor-node-metastasis (TNM) stage. Similarly, invasion of the liver capsule suggests that the tumor is likely to infiltrate into surrounding tissues, and indicates that the tumor has an excellent ability to invade, which likely leads to a high rate of PER and poor OS.
MVI is defined as the microscopic presence of tumor emboli in the hepatic vein, portal vein system, or lymphatic vessels. Many previous studies have found that the presence of MVI is closely related to the recurrence of HCC after hepatectomy (21,22,24). In this study, MVI was also found to be an independent risk factor for PER in patients with liver cancer according to the Milan criteria. This may be related to the fact that a tumor thrombus can easily metastasize through the portal vein system, leading to intrahepatic recurrence (25).
The high postoperative recurrence rate of HCC is partly attributable to the untreated satellite lesions that are too small to be detected on pretreatment imaging. Furthermore, the presence of satellite lesions may suggest a more aggressive biological feature of liver cancer, thus leading to PER. A previous study demonstrated that tumor differentiation was significantly associated with the prevalence of satellite lesions (26). Therefore, it is crucial to improve the preoperative detection rate of satellite lesions and to clarify the tumor stage, which could assist clinicians to take measures as soon as possible to reduce the incidence of PER.
Increased GGT levels are closely related to tumor occurrence, development, and prognosis. As reported in previous studies, GGT is associated with various tumors, including renal cell carcinoma, ovarian cancer, endometrial carcinoma, and esophageal squamous cell carcinoma (27-29). In this study, GGT ≥60 U/L was also found to be closely associated with the PER of HCC (P=0.033). With the incidence of liver cancer, intrahepatic obstruction leads to cholestasis, which can induce the liver to produce GGT. At the same time, the liver cancer cells themselves also synthesize GGT, and thus, the plasma GGT is significantly increased. Faber et al. found that increased preoperative GGT levels can reduce the cumulative survival rate of patients and is an independent risk factor for PER (30), and to the best of our knowledge, its specific mechanism has been rarely investigated.
Using the clinical information and gene expression of patients with HCC in TCGA database, we identified two bile secretion-related genes, SLC5A1 and ATP1A2, which were significantly aberrantly expressed in the PER group. Sodium/glucose cotransporter 1 (SGLT1), which is encoded by SLC5A1, is usually expressed in intrahepatic bile duct cells and is involved in bile secretion. When HCC occurs, the SGLT1 level in cancer cells is increased, which may be related to improving the tolerance of HCC to low glucose (31). The protein encoded by ATP1A2 belongs to the subfamily of sodium-potassium-ATPases (Na+/K+-ATPases). Na+/K+-ATPase is an integral membrane protein that is responsible for establishing and maintaining the electrochemical gradients of Na+ and K+ ions across the plasma membrane (28,32). Bile acid synthesis, transport, secretion, and enterohepatic circulation require a variety of Na+-dependent transporters (NTCP/mEH) (33-35). The significantly low expression of this gene in HCC cells affects the concentration of sodium and potassium ions both inside and outside of the cells, thereby affecting the functions of various transporters, which could result in the accumulation of bile acids in liver cells and bile ducts, and could result in a further increase in GGT (33-35). GGT is a glycosylated protein that is partially embedded in the surface of the plasma membrane and is involved in the synthesis and transport of intracellular glutathione (GSH) (32,33,36,37). The synthesized GSH can protect cells from the damage caused by oxidants in the metabolic process, thereby playing an important antioxidant and anti-inflammatory role. However, overexpression of GGT may break the balance between oxidation and anti-oxidation, which is responsible for promoting the development of tumors (38). Studies have shown that in HCC, increased GGT can promote the increase of GSH in liver cells, which is related to the drug resistance of HCC (39-41). In addition, GGT also plays a role in regulating cell proliferation and apoptosis, as well as cancer progression and invasion (39). Therefore, a significant increase in preoperative GGT may be a poor prognostic factor for HCC. Consequently, the low expression of ATP1A2 in liver cancer cells may increase GGT by affecting bile acid secretion, and then affect the antioxidant, drug resistance, progression, and invasion processes of tumor cells, ultimately resulting in a poor prognosis.
The present study had some limitations that should be noted. Firstly, this was a retrospective study, which may have involved some selective bias. Also, there was no comparison between the training and validation groups, and the baseline consistency between the two groups may affect the accuracy and effectiveness of the predictive model. Also, AJCC T1 stage HCC was not completely consistent with HCC according to the Milan criteria, and thus, the initially explored recurrence mechanism had certain limitations.
Conclusions
In this study, we identified that AFP ≥400 ng/mL, GGT ≥60 U/L, Glisson’s capsule invasion, MVI, and satellite lesions were independent risk factors for PER of HCC meeting the Milan criteria. We constructed a novel predictive model, which showed fair accuracy, calibration, and clinical utility. Furthermore, we came to a preliminary conclusion that the aberrant expression of two bile secretion-related genes (SLC5A1 and ATP1A2) likely promoted the PER of HCC according to the Milan criteria by increasing the level of GGT.
Acknowledgments
Funding: This study was supported by the Key Technology Research and Development Program of the Sichuan Province (Nos. 2021YFSY0009 and 2021YFS0106), and the Post-Doctor Research Project, West China Hospital, Sichuan University (No. 2020HXBH076).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3390/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3390/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3390/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of West China Hospital, Sichuan University, China (No. 2020/8). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Yao LQ, Chen ZL, Feng ZH, et al. Clinical Features of Recurrence After Hepatic Resection for Early-Stage Hepatocellular Carcinoma and Long-Term Survival Outcomes of Patients with Recurrence: A Multi-institutional Analysis. Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016;25:24-9. [Crossref] [PubMed]
- Hong YM, Cho M, Yoon KT, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol 2017;39:1010428317720863. [Crossref] [PubMed]
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330-9. [Crossref] [PubMed]
- Zeng J, Zeng J, Lin K, et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr 2022;11:176-87. [Crossref] [PubMed]
- Lee IC, Huang JY, Chen TC, et al. Evolutionary Learning-Derived Clinical-Radiomic Models for Predicting Early Recurrence of Hepatocellular Carcinoma after Resection. Liver Cancer 2021;10:572-82. [Crossref] [PubMed]
- Zhang K, Tao C, Siqin T, et al. Establishment, validation and evaluation of predictive model for early relapse after R0 resection in hepatocellular carcinoma patients with microvascular invasion. J Transl Med 2021;19:293. [Crossref] [PubMed]
- Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-50. [PubMed]
- Makuuchi M, Torzilli G, Machi J. History of intraoperative ultrasound. Ultrasound Med Biol 1998;24:1229-42. [Crossref] [PubMed]
- Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. [Crossref] [PubMed]
- Kobayashi A, Miyagawa S, Miwa S, et al. Prognostic impact of anatomical resection on early and late intrahepatic recurrence in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2008;15:515-21. [Crossref] [PubMed]
- Hernandez JM, Tsalatsanis A, Humphries LA, et al. Defining optimum treatment of patients with pancreatic adenocarcinoma using regret-based decision curve analysis. Ann Surg 2014;259:1208-14. [Crossref] [PubMed]
- Wynants L, Timmerman D, Verbakel JY, et al. Clinical Utility of Risk Models to Refer Patients with Adnexal Masses to Specialized Oncology Care: Multicenter External Validation Using Decision Curve Analysis. Clin Cancer Res 2017;23:5082-90. [Crossref] [PubMed]
- Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol 2013;11:212. [Crossref] [PubMed]
- Li P, Wang SS, Liu H, et al. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol 2011;17:4563-71. [Crossref] [PubMed]
- Zheng M, Ruan Y, Yang M, et al. The comparative study on ultrastructure and immunohistochemistry in AFP negative and positive hepatocellular carcinoma. J Huazhong Univ Sci Technolog Med Sci 2004;24:547-9, 559. [Crossref] [PubMed]
- Park JH, Koh KC, Choi MS, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg 2006;192:29-33. [Crossref] [PubMed]
- Hayashi M, Shimizu T, Hirokawa F, et al. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am Surg 2011;77:572-8. [Crossref] [PubMed]
- Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275-83. [Crossref] [PubMed]
- Xu S, Kang CH, Gou X, et al. Quantification of liver fibrosis via second harmonic imaging of the Glisson's capsule from liver surface. J Biophotonics 2016;9:351-63. [Crossref] [PubMed]
- Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832-42. [Crossref] [PubMed]
- Toyosaka A, Okamoto E, Mitsunobu M, et al. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. HPB Surg 1996;10:97-103; discussion 103-4. [Crossref] [PubMed]
- Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931-7. [Crossref] [PubMed]
- Hofbauer SL, Stangl KI, de Martino M, et al. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer 2014;111:1526-31. [Crossref] [PubMed]
- Takemura K, Fukushima H, Ito M, et al. Prognostic significance of serum γ-glutamyltransferase in patients with advanced urothelial carcinoma. Urol Oncol 2019;37:108-15. [Crossref] [PubMed]
- Yang F, Zhang S, Yang H, et al. Prognostic significance of gamma-glutamyltransferase in patients with resectable esophageal squamous cell carcinoma. Dis Esophagus 2015;28:496-504. [Crossref] [PubMed]
- Faber W, Sharafi S, Stockmann M, et al. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery 2013;153:510-7. [Crossref] [PubMed]
- Lei S, Yang J, Chen C, et al. FLIP(L) is critical for aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res 2016;35:79. [Crossref] [PubMed]
- Huang W, Zhang Y, Xu Y, et al. Comprehensive analysis of the expression of sodium/potassium-ATPase α subunits and prognosis of ovarian serous cystadenocarcinoma. Cancer Cell Int 2020;20:309. [Crossref] [PubMed]
- Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med 2003;9:558-64. [Crossref] [PubMed]
- Zollner G, Fickert P, Zenz R, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology 2001;33:633-46. [Crossref] [PubMed]
- Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica 2008;38:1043-71. [Crossref] [PubMed]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263-355. [Crossref] [PubMed]
- Pastore A, Federici G, Bertini E, et al. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003;333:19-39. [Crossref] [PubMed]
- Yang JG, He XF, Huang B, et al. Rule of changes in serum GGT levels and GGT/ALT and AST/ALT ratios in primary hepatic carcinoma patients with different AFP levels. Cancer Biomark 2018;21:743-6. [Crossref] [PubMed]
- Zhu Y, Zhang AJ, Wu DB, et al. Prognostic significance of the pretreatment serum gamma-glutamyltransferase levels in Chinese patients with non-metastatic cervical cancer. Oncotarget 2017;8:115701-8. [Crossref] [PubMed]
- Godwin AK, Meister A, O'Dwyer PJ, et al. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A 1992;89:3070-4. [Crossref] [PubMed]
- Daubeuf S, Balin D, Leroy P, et al. Different mechanisms for gamma-glutamyltransferase-dependent resistance to carboplatin and cisplatin. Biochem Pharmacol 2003;66:595-604. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


