
Efficacy and safety of anti-PD-1 antibody plus regorafenib in refractory microsatellite stable metastatic colorectal cancer: a retrospective single-arm cohort study
Introduction
Colorectal cancer is the 3rd most common cancer and the 2nd most deadly cancer worldwide (1). There have been significant advances in its treatment in recent years; however, the prognosis for patients with metastatic colorectal cancer (mCRC) remains poor, and the 5-year overall survival (OS) rate of such patients is <15% (2). Anti-programmed cell death protein 1 (PD-1) antibody is more effective than conventional treatments of mCRC with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H). The KEYNOTE-016 study showed that the objective response rate (ORR) of dMMR/MSI-H mCRC patients treated with anti-PD-1 antibody was as high as 40%, while the ORR of refractory proficient mismatch repair (pMMR)/microsatellite stable (MSS) mCRC was 0% (3).
However, dMMR/MSI-H cases account for only 5% of patients with mCRC. Approximately 95% of mCRC patients are MSS; thus, the majority of mCRC patients do not benefit from immune monotherapy (4). Various immune combination methods are being actively explored. In the REGONIVO study, the mCRC cohort of 24 patients with pMMR/MSS had an ORR of 33%, a medical progression-free survival (mPFS) of 7.9 months (5). With the addition of regorafenib, the efficacy of nivolumab was shown to increase significantly (6,7). The mechanism was explored in preclinical colorectal cancer models. Regorafenib is an anti-angiogenic multitargeted tyrosine kinase inhibitor (TKI). It plays a synergistic role in the process of immunotherapy by changing the immune microenvironment of the tumor. Its mechanism is as follows: (I) it reduces tumor-associated macrophages (TAMs) in tumor models by inhibiting the colony-stimulating factor 1 receptor; (II) it reduces regulatory T cells by inhibiting vascular endothelial growth factor receptor 2; (III) it normalizes tumor vasculature and increases the infiltration of CD8 T cells; and (IV) it enhances immune activity by inhibiting the p38 kinase/CREB1/KLF4 axis of TAMs (6,7).
However, several subsequent studies of immune checkpoints inhibitors (ICIs) combined with small molecule TKIs have not replicated REGONIVO’s excellent results in pMMR/MSS mCRC patients. For example, Cousin and Ren et al. (8,9), found an ORR of 0%, and a mPFS of 3.6 and 1.8 months, and a median OS (mOS) of 10.8 and 7.8 months in their single-arm and phase-II trials, respectively (8,9). Notably, the efficacy of the combination treatment of regorafenib plus nivolumab in a North American population was not consistent with the results for a Japanese population. Indeed, the ORR in the North American population was only 7%.
So far, there are no large phase III randomized controlled studies to confirm the efficacy of regorafenib plus anti-PD-1 antibodies. The sample size of previous studies was relatively small, and different studies combined with different anti-PD-1 antibodies. It is unknown which anti-PD-1 antibody plus regorafenib may be more efficacious. In our study, regorafenib combined with 1 of 5 different types of anti-PD-1 antibodies. We sought to investigate the efficacy and safety of anti-PD-1 antibody plus regorafenib in refractory pMMR/MSS mCRC in real world and which anti-PD-1 antibody may be more efficacious combined with regorafenib. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3690/rc).
Methods
Study design
The primary end point was OS. The secondary end points were median mPFS, the incidence of treatment-related adverse events (TRAEs) and the ORR. We performed a retrospective analysis to examine the efficacy and safety in 103 patients treated with anti-PD-1 antibodies (i.e., nivolumab, pembrolizumab, camrelizumab, sintilimab, and toripalimab) plus regorafenib until disease progression or i
Patients
The eligibility criteria for our study were at least 2 lines of standard chemotherapy, including fluorouracil, oxaliplatin, and irinotecan with or without targeted drugs, such as bevacizumab and cetuximab, for disease progression in pMMR/MSS mCRC patients. MMR or MSI testing was accomplished by examining either the loss of protein expression via an immunohistochemistry analysis of the 4 MMR enzymes (i.e., MLH1, MSH2, MSH6, and PMS2) or a MMR gene analysis by polymerase chain reaction or next generation sequencing panel validation using formalin-fixed paraffin-embedded tissue specimens. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Hunan Cancer Hospital (No. 2022-69) and informed consent was taken from all individual participants.
Treatment
The patients were treated orally with regorafenib (80 mg once daily
Assessment
The patients were evaluated by computed tomography every 2 or 3 treatment cycles until disease progression or being lost to follow-up. Tumor response was evaluated as per the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). ORR was defined as the proportion of complete responses (CRs) and partial responses (PRs). Disease control rate was defined as the addition of (CR + PR) rate and stable disease (SD) rate. PFS was defined from the beginning of treatment to the date of disease progression or death due to any cause. OS was defined from the beginning of treatment to the date of death. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events 5.0 (NCI-CTCAE 5.0).
Statistical analysis
The analysis population included all the patients who received their 1st dose of regorafenib plus an anti-PD-1 antibody before June 2021, and for whom clinical data for at least 1 subsequent day were available. PFS and OS in the regorafenib plus anti-PD-1 antibodies cohort were examined by a Kaplan-Meier analysis. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were calculated using a Cox proportional-hazards model. The proportional-hazards assumption of OS was examined by both graphical and analytical methods. Additionally, this analysis included adjustments for varying confounding and risk factors. The results are reported as point estimates and 95% CIs. All the statistical tests were two-sided. The analyses were performed with the use of R software (version 3.6.0).
Results
Patient characteristics
A total of 103 pMMR/MSS mCRC patients were included in this study. Their baseline characteristics are listed in Table 1. The patients had a median age of 56.0 (range, 20.0–79.0) years, and a median of 2 (range, 1–19) cycles of regorafenib plus PD-1 administration. All the patients received ≥2 previous lines of chemotherapy with or without targeted drugs, such as bevacizumab and cetuximab, 45 (43.7%) patients received at least 3 lines of treatment, and 65 (63.1%) patients did not receive any treatment after regorafenib plus PD-1 administration. A total of 59 (57.3%) patients had liver metastases, and 45 (43.7%) had lung metastases. Among the 103 patients, 66 (64.0%) received sintilimab, 18 (17.5%) received toripalimab, 10 (9.7%) received nivolumab, 8 (7.8%) received camrelizumab, and 1 (1.0%) received pembrolizumab combined with regorafenib.
Table 1
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 56 (54.4) |
| Female | 47 (45.6) |
| Age (years) | |
| Median (range) | 56.0 (20.0–79.0) |
| <60 | 76 (73.8) |
| ≥60 | 27 (26.2) |
| ECOG PS | |
| 0 | 36 (35.0) |
| 1 | 61 (59.2) |
| 2 | 5 (4.8) |
| 3 | 1 (1.0) |
| Primary tumor location | |
| Right colon | 24 (23.3) |
| Left colon | 27 (26.2) |
| Rectum | 52 (50.5) |
| Histological type of primary | |
| Adenocarcinoma | 97 (94.2) |
| Mucinous adenocarcinoma | 6 (5.8) |
| Metastases location | |
| Liver metastases | 59 (57.3) |
| Lung metastases | 45 (43.7) |
| Lymph node metastases | 36 (34.9) |
| Bone metastases | 12 (11.6) |
| Peritoneal metastases | 14 (13.5) |
| Adrenal metastases | 2 (1.9) |
| Ovarian metastases | 4 (3.8) |
| Numbers of metastatic sites | |
| 1 site | 37 (35.9) |
| ≥2 sites | 66 (64.1) |
| Previous treatment agents | |
| 5-fluorouracil | 103 (100.0) |
| Oxaliplatin | 100 (97.1) |
| Irinotecan | 101 (98.1) |
| Bevacizumab | 87 (84.5) |
| Cetuximab | 35 (34.0) |
| Regorafenib | 8 (7.8) |
| PD-1 | 3 (2.9) |
| Fruquintinib | 11 (10.7) |
| Radiotherapy | |
| Yes | 27 (26.2) |
| No | 76 (73.8) |
| Surgery | |
| Yes | 77 (74.8) |
| No | 26 (25.2) |
| Previous lines of chemotherapy | |
| 2 lines | 58 (56.3) |
| ≥3 lines | 45 (43.7) |
| Gene mutation status | |
| RAS wild type | 31 (30.1) |
| RAS mutant type | 40 (38.8) |
| BRAF mutant type | 6 (5.8) |
| Unknown | 26 (25.3) |
| PD-L1 expression level | |
| PD-L1 CPS <1 | 9 (8.7) |
| PD-L1 CPS ≥1 | 3 (2.9) |
| Unknown | 91 (88.4) |
| PD-1 | |
| Sintilimab | 66 (64.0) |
| Nivolumab | 10 (9.7) |
| Toripalimab | 18 (17.5) |
| Camrelizumab | 8 (7.8) |
| Pembrolizumab | 1 (1.0) |
| Cycles of regorafenib plus PD-1 | |
| 1 | 48 (46.6) |
| ≥2 | 55 (53.4) |
| MMR or MSI status | |
| pMMR or MSS | 103 (100.0) |
| dMMR or MSI-H | 0 (0.0) |
| Lines of treatment after regorafenib plus PD-1 | |
| No | 65 (63.1) |
| 1 line | 32 (31.0) |
| ≥2 lines | 6 (5.9) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PD-1, programmed cell death 1; PD-L1, programmed cell death-ligand 1; CPS, combined positive score; MMR, mismatch repair; MSI, microsatellite instability; pMMR, proficient MMR; MSS, microsatellite stable; dMMR, deficient MMR; MSI-H, MSI-high.
Efficacy
The mOS of for the entire 103 patients was 8.40 months (Figure 1A). The mOS was 16.07 months for patients who received >1 cycle and 4.37 months for patients who received only 1 cycle (P<0.0001; Figure 1B). No difference was found in terms of OS between patients in the sintilimab group vs. patients in the other anti-PD-1 antibody groups (8.97 vs. 6.67 months, P=0.92; Figure 1C). The mOS were also comparable in patients with or without liver metastases (6.40 vs. 9.93 months, P=0.36; Figure 1D).

PFS and ORR were evaluated in 55 patients, as the remaining 48 patients lost radiological assessment. The mPFS was 2.50 months for all patients (Figure 2A). The mPFS was significantly longer in patients who received >1 cycle than that in patients who received only 1 cycle (3.10 vs. 1.11 months, P<0.0001; Figure 2B). Similarly, PFS was improved from 1.61 to 3.30 months patients received sintilimab, compared with those receiving other anti-PD-1 antibodies (P=0.0089; Figure 2C). Whereas in those with or without liver metastases, the mPFS was comparable (2.37 vs. 3.13 months, P=0.12; Figure 2D). Seven of the 55 patients (12.7%) were confirmed to have a PR, and another 16 (29.1%) had SD, but none had a CR. Thus, the ORR was 12.7%, and the disease control rate was 41.8%. The ORR of patients without liver metastases was higher than the ORR of those with liver metastases, but the difference was not significant (17.4% vs. 9.4%, P=0.355; Table 2).
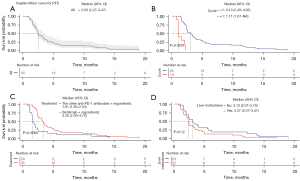
Table 2
| Response | Evaluable patients (n=55) | Patients with liver metastases (n=32) | Patients without liver metastases (n=23) |
|---|---|---|---|
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR | 7 (12.7) | 3 (9.4) | 4 (17.4) |
| SD | 16 (29.1) | 8 (25.0) | 8 (34.8) |
| Progressive disease | 32 (58.2) | 21 (65.6) | 11 (47.8) |
| ORR | 7 (12.7) | 3 (9.3) | 4 (17.4) |
| Disease control rate | 23 (41.8) | 11 (34.4) | 12 (52.2) |
Data are presented as n (%). RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; ORR, objective response rate.
Univariate and multivariate analysis
As shown in Table 3, univariate analysis found that patients who received >1 cycle of regorafenib plus PD-1, previously undergone surgery and with only one metastatic site had longer OS (all P<0.05). There were no significant differences in OS in terms of sex, Eastern Cooperative Oncology Group (ECOG), radiotherapy, primary tumor location, pathological type, liver metastases, lung metastases, gene mutation status, different types of PD-1, and previous lines of chemotherapy (P>0.05). Considering it has been well-established that radiotherapy was significantly associated with immune response and based on previous univariate analysis, we included significant variables (all P<0.05) in the above univariate analysis and radiotherapy for multivariate analysis. Further multivariate analysis confirmed that only receiving >1 cycle of regorafenib plus PD-1 (HR: 0.21; 95% CI: 0.12–0.38; P<0.001) and previously undergoing surgery (HR: 0.51; 95% CI: 0.27–0.93; P=0.029) were independent predictors for OS (Table 4).
Table 3
| Characteristics | HR | 95% CI | P |
|---|---|---|---|
| Sex | 0.059 | ||
| Male | Reference | ||
| Female | 1.68 | (0.98, 2.89) | |
| ECOG PS | 0.72 | ||
| 0–1 | Reference | ||
| ≥2 | 0.81 | (0.25, 2.61) | |
| Cycles of regorafenib plus PD-1 | <0.001 | ||
| 1 | Reference | ||
| >1 | 0.19 | (0.11, 0.35) | |
| Surgery | 0.0074 | ||
| No | Reference | ||
| Yes | 0.45 | (0.25, 0.81) | |
| Radiotherapy | 0.093 | ||
| No | Reference | ||
| Yes | 0.55 | (0.28, 1.1) | |
| Primary tumor location | 0.1 | ||
| Left side | Reference | ||
| Right side | 1.64 | (0.91, 2.97) | |
| Pathological type | 1 | ||
| Adenocarcinoma | Reference | ||
| Mucinous adenocarcinoma | 1 | (0.31, 3.22) | |
| Liver metastases | 0.39 | ||
| No | Reference | ||
| Yes | 1.27 | (0.74, 2.19) | |
| Lung metastases | 0.14 | ||
| No | Reference | ||
| Yes | 0.66 | (0.38, 1.15) | |
| Gene mutation status | 0.44 | ||
| RAS mutant type | Reference | (0.4, 1.49) | |
| RAS wild type | 0.77 | ||
| Numbers of metastatic sites | 0.035 | ||
| 1 site | Reference | ||
| ≥2 sites | 1.89 | (1.05, 3.42) | |
| Treatment | 0.73 | ||
| The other PD-1 | Reference | ||
| Sintilimab | 0.9 | (0.51, 1.6) | |
| Previous lines of chemotherapy | 0.75 | ||
| ≥3 | Reference | ||
| 2 | 0.91 | (0.53, 1.58) | |
OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-1, programmed cell death protein 1; HR, hazard ratio; CI, confidence interval.
Table 4
| Characteristics | HR | 95% CI | P |
|---|---|---|---|
| Cycles of regorafenib plus PD-1 | <0.001 | ||
| 1 | Reference | ||
| >1 | 0.21 | (0.12, 0.38) | |
| Surgery | 0.029 | ||
| No | Reference | ||
| Yes | 0.51 | (0.27, 0.93) | |
| Numbers of metastatic sites | 0.051 | ||
| 1 site | Reference | ||
| ≥2 sites | 1.89 | (1, 3.57) | |
| Treatment | 0.92 | ||
| The other PD-1 | Reference | ||
| Sintilimab | 0.97 | (0.54, 1.74) | |
| Radiotherapy | 0.43 | ||
| No | Reference | ||
| Yes | 0.74 | (0.34, 1.58) | |
OS, overall survival; PD-1, programmed cell death protein 1; HR, hazard ratio; CI, confidence interval.
With respect to PFS, multivariate analysis which enrolled all the variables in Table 5, confirmed that receiving >1 cycle of regorafenib plus PD-1 (HR: 0.12; 95% CI: 0.05–0.31; P<0.001) and sintilimab (HR: 0.55; 95% CI: 0.31–0.99; P=0.044) were significantly associated with PFS (Table 5).
Table 5
| Characteristics | HR | 95% CI | P |
|---|---|---|---|
| Cycles of regorafenib plus PD-1 | <0.001 | ||
| 1 | Reference | ||
| >1 | 0.12 | (0.05, 0.31) | |
| Treatment | 0.044 | ||
| The other PD-1 | Reference | ||
| Sintilimab | 0.55 | (0.31, 0.99) | |
| Sex | 0.77 | ||
| Male | Reference | ||
| Female | 0.91 | (0.51, 1.65) | |
| Radiotherapy | 0.86 | ||
| No | Reference | ||
| Yes | 0.94 | (0.49, 1.81) | |
| Previous lines of chemotherapy | 0.99 | ||
| ≥3 lines | Reference | ||
| 2 line | 1 | (0.56, 1.76) | |
PFS, progression-free survival; PD-1, programmed cell death protein 1; HR, hazard ratio; CI, confidence interval.
Safety
Of the 103 patients assessed for toxicity, 88 (85.4%) had a grade TRAEs, of whom the majority had grade 1 or 2 TRAEs (72.9%). The most common severe adverse events (≥ grade 3) were an aspartate aminotransferase (AST) increase (4 patients, 3.8%), an alanine aminotransferase (ALT) increase (3 patients, 2.9%), hypothyroidism (3 patients, 2.9%), a hemoglobin decrease (3 patients. 2.9%), and palmar-plantar erythrodysesthesia (2 patients, 1.9%). The results are set out in Table 6.
Table 6
| Adverse events | Any grade | Grade 1 | Grade 2 | ≥ Grade 3 |
|---|---|---|---|---|
| All | 88 (85.4) | 46 (44.7) | 29 (28.2) | 13 (12.6) |
| Palmar-plantar erythrodysesthesia | 14 (13.6) | 7 (6.7) | 5 (4.8) | 2 (1.9) |
| Hypertension | 4 (3.8) | 1 (0.9) | 3 (2.9) | 0 (0.0) |
| Rash | 8 (7.8) | 5 (4.8) | 3 (2.9) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (0.9) | 3 (2.9) | 0 (0.0) |
| Mucositis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 19 (0.9) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Vomiting | 1 (0.9) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Leukopenia | 7 (6.7) | 6 (5.8) | 0 (0.0) | 1 (0.9) |
| Neutropenia | 4 (3.8) | 3 (2.9) | 1 (0.9) | 0 (0) |
| Hemoglobin decreased | 15 (14.6) | 7 (6.7) | 5 (4.8) | 3 (2.9) |
| Platelet count decreased | 3 (2.9) | 3 (2.9) | 0 (0) | 0 (0) |
| AST increase | 28 (27.2) | 21 (20.3) | 3 (2.9) | 4 (3.8) |
| ALT increase | 26 (25.2) | 19 (18.4) | 4 (3.8) | 3 (2.9) |
| TBIL increase | 23 (22.3) | 18 (17.4) | 4 (3.8) | 1 (0.9) |
| Hyperthyroidism | 69 (5.8) | 3 (2.9) | 3 (2.9) | 0 (0) |
| Hypothyroidism | 13 (12.6) | 4 (3.8) | 6 (5.8) | 3 (2.9) |
Data are presented as n (%). TRAEs, treatment-related adverse events; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBIL, total bilirubin.
Discussion
In recent years, immunotherapy has significantly improved the ORR, PFS, and OS of patients with dMMR/MSI-H mCRC, which is characterized by a high tumor mutation burden (TMB), robust Th1-type immune infiltration, and the high expression of multiple immune checkpoints, such as PD-1, programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). PMMR/MSS mCRC is characterized by a lower TMB and less immune infiltration (10). Some studies have reported that dMMR/MSI-H patients possess higher tumor infiltration levels of CD3+, CD8+ and CD45RO+ T cells than pMMR/MSS patients (11,12). Thus, pMMR/MSS mCRC, which accounts for 95% of mCRC, has been considered a biomarker of resistance to checkpoint inhibitors (13,14). Recently, regorafenib plus anti-PD-1 antibodies have shown encouraging anti-tumor activities in pMMR/MSS mCRC patients (5,15). Regorafenib can modulate the tumor immune microenvironment via the polarization of antigen-presenting cells, particularly macrophages, and induce the p38MAPK/Creb1/Klf4 signaling pathway, which can activate TAMs, and in turn, the subsequent production of pro-inflammatory cytokines [i.e., interleukin (IL)-10, IL-12, and IL-23] can activate cytotoxic T cells (7,16). Thus, regorafenib may enhance the anti-tumor activity of anti-PD-1 antibody.
Conventional treatment options for chemotherapy refractory mCRC include regorafenib, single-agent fruquintinib, and TAS-102 (trifuridine/tipiracil) for patients whose disease has progressed after antiangiogenic treatment. Such patients have ORRs of 1–5%, an mPFS of around 2 months, and an mOS of 6–8 months (17-20). Our study demonstrated that patients who received anti-PD-1 antibody plus regorafenib had a better ORR (12.7%) than those who received regorafenib alone (ORR: 1–4%) as a standard 3rd-line regimen for mCRC, especially those without liver metastases (ORR 17.4%) (18,19). In our study, mPFS and mOS were not significantly prolonged, and only 55 of the 103 patients received ≥2 cycles of treatment. Among those who received ≥2 cycles of treatment, their mPFS and mOS were significantly prolonged to 3.10 and 16.07 months, respectively. These results are consistent with those of REGOTORI study (21). In the REGONIVO trial, the ORR of the mCRC cohort of 24 patients with pMMR/MSS was 33%, and the mPFS of 7.9 months was better than that of other ICI combined TKI studies, including those in our study.
There are a number of possible explanations for the conflicting results. First, the ICIs included PD-1, PD-L1, and CTLA-4 inhibitors; however, there are many types of PD-1 and PD-L1 inhibitors, and the differences between them have not yet been compared. Different studies also used different ICIs. In our study, patients received 5 types of anti-PD-1 antibodies (i.e., nivolumab, pembrolizumab, camrelizumab, sintilimab, and toripalimab), among which sintilimab prolonged mPFS more than the other anti-PD-1 antibodies. The baseline characteristics of the patients also differed.
Second, there were inconsistencies in the patient inclusion criteria among the studies. In the REGONIVO study, all patients in the mCRC cohort had an ECOG performance status (PS) of 0, and most patients did not have RAS mutations. These patients had a good prognosis, but they do not reflect populations in routine clinical practice (5). Third, in the REGONIVO trial, only 1 patient (2%) discontinued the treatment because of TRAEs. However, in a North American trial of regorafenib plus nivolumab, the initial dose of regorafenib of 80 mg once daily for 3 weeks on, 1 week off, was increased to 120 mg for 3 weeks on 1 week off from the 2nd cycle, and 8 patients (11%) discontinued the treatment. It may be for these reasons that the efficacy of nivolumab combined with regorafenib in the Japanese population was better than that of other ICI combined regorafenib studies, including those used in our study.
In our study, the ORR was higher in the non-liver metastatic subgroup than the liver metastatic subgroup (17.4% vs. 9.4%), which is consistent with the findings of the study on regorafenib plus nivolumab in the North American population (22% vs. 0%). One study reported that liver metastasis is associated with resistance to checkpoint inhibitor therapy, as the liver has a relatively high fraction of immunosuppressive cells (22). That study also showed that the PFS of the sintilimab group was significantly increased compared to the PFS of other anti-PD-1 antibody groups. A phase 1b study of fruquintinib plus sintilimab showed encouraging anti-tumor activity in refractory mCRC. The ORR was 27.3% and the mPFS was 6.9 months in the 5-mg intermittent cohort. Sintilimab may be more suitably combined with TKIs than other anti-PD-1 antibodies, but further prospective studies need to be conducted to confirm this.
Despite some interesting findings, our study had some limitations. First, it was a retrospective, single-center study, and lacking of controlled group. As a result, there was no way to fully record the adverse events of patients outside the hospital and the incidence of adverse events may be underestimated. In addition, the exact reasons for termination of further treatment and evaluation could not been investigated, possible reasons including the COVID-19 pandemic, different medical insurance, and TRAEs, which might affect OS and PFS. Second, there were 5 different types of anti-PD-1 antibodies used in our study, the price and related adverse events might be different, which might also impact the patients’ compliance with treatments. Third, we did not compare the combination of regorafenib plus anti-PD-1 antibody with regorafenib alone, so it’s uncertain how much they contributed to efficacy and adverse events. Last but not least, only about half of the entire patients (55/103, 53.3%) could been assessed by radiological evaluation, which might impair the second end point of the present study, such as ORR and mPFS.
To date, all the studies conducted on ICIs combined with TKIs for refractory mCRC have been phase Ib/II clinical or retrospective studies, and the results of these studies are inconsistent. Large randomized controlled trials need to be conducted to confirm the findings. In our study, we found that more cycles of combined therapy led to a greater survival benefit, and sintilimab appeared to have better anti-tumor activity than the other anti-PD-1 antibodies. Further studies need to be conducted to confirm these findings and a dominant population needs to be selected for ICI combined with TKI therapy.
In conclusion, in this retrospective cohort study including the largest sample size so far investigating efficacy and safety of anti-PD-1 antibodies plus regorafenib in refractory pMMR/MSS mCRC, we revealed that this combination treatments had a manageable safety profile and could improve prognosis of those patients, especially in those receiving >1 cycle. Compared to the other anti-PD-1 antibodies, sintilimab appears to be more efficacious, but further prospective studies need to be conducted.
Acknowledgments
The authors acknowledge the patients who participated in this study. The authors also appreciate the academic support from the AME Colorectal Cancer Collaborative Group. The abstract has been published on the 2022 ASCO Annual Meeting I (https://ascopubs.org/doi/10.1200/JCO.2022.40.16_suppl.e15582).
Funding: The study was supported by Climbing Foundation of the National Cancer Center (No. NCC201909B02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3690/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3690/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3690/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Hunan Cancer Hospital (No. 2022-69) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Milano AF, Singer RB. The Cancer Mortality Risk Project - Cancer Mortality Risks by Anatomic Site: Part 1 - Introductory Overview; Part II - Carcinoma of the Colon: 20-Year Mortality Follow-up Derived from 1973-2013 (NCI) SEER*Stat Survival Database. J Insur Med 2017;47:65-94. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Gelsomino F, Barbolini M, Spallanzani A, et al. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev 2016;51:19-26. [Crossref] [PubMed]
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol 2020;38:2053-61. [Crossref] [PubMed]
- Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer 2020;8:e001435. [Crossref] [PubMed]
- Ou DL, Chen CW, Hsu CL, et al. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer 2021;9:e001657. [Crossref] [PubMed]
- Ren C, Mai ZJ, Jin Y, et al. Anti-PD-1 antibody SHR-1210 plus apatinib for metastatic colorectal cancer: a prospective, single-arm, open-label, phase II trial. Am J Cancer Res 2020;10:2946-54. [PubMed]
- Cousin S, Cantarel C, Guegan JP, et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin Cancer Res 2021;27:2139-47. [Crossref] [PubMed]
- Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43-51. [Crossref] [PubMed]
- Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222:350-66. [Crossref] [PubMed]
- Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48-61. [Crossref] [PubMed]
- Chen EX, Jonker DJ, Loree JM, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol 2020;6:831-8. [Crossref] [PubMed]
- O'Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 2017;12:e0189848. [Crossref] [PubMed]
- Wang C, Chevalier D, Saluja J, et al. Regorafenib and Nivolumab or Pembrolizumab Combination and Circulating Tumor DNA Response Assessment in Refractory Microsatellite Stable Colorectal Cancer. Oncologist 2020;25:e1188-94. [Crossref] [PubMed]
- Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019;12:76. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018;319:2486-96. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Xu J, Kim TW, Shen L, et al. Results of a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients With Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J Clin Oncol 2018;36:350-8. [Crossref] [PubMed]
- Wang F, He MM, Yao YC, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med 2021;2:100383. [Crossref] [PubMed]
- Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res 2016;22:5971-82. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


