
Itaconic acid facilitates inflammation abatement and alleviates liver ischemia-reperfusion injury by inhibiting NF-κB/NLRP3/caspase-1 inflammasome axis
Introduction
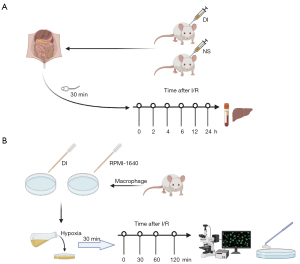
Conservative treatment and surgical resection are no longer therapeutic options for many patients with end-stage and acute liver disease, and liver transplantation provides the only remaining opportunity. However, ischaemia-reperfusion injury (IRI) during surgery not only limits the utilization of many donor livers, but also severely restricts the prognosis of liver transplant patients (1-3). IRI is caused by the accumulation of inflammatory cytokines in the donor liver due to ischemia and hypoxia during perfusion via organ preservation fluid after isolation. Accumulated inflammatory cytokines then enter the body with the blood, resulting in a violent inflammatory factor storm and serious damage to the recipient body (Figure 1A). IRI not only affects the safety of transplantation, but also determines the recovery of donor liver function after transplantation (4,5).

Marginal donor livers have been used increasingly in recent years to alleviate the shortage of donor livers. Marginal donor livers are mainly from elderly, obese, and cardiac arrest donors, and have prolonged cold and heat ischemia which causes more severe IRI because the liver itself is already potentially damaged. Data show that 60% of donor livers from potential cardiac death are abandoned due to irreversible IRI, which further aggravates the shortage of donor livers (6,7). Consequently, the prevention and treatment of IRI in liver transplantation is the key to improving outcomes and alleviating the shortage of donor livers.
The inflammatory immune response is the core mechanism of IRI, of which there are three main phases: Initiation, progression, and regression. Although most current research on IRI in liver transplantation has focused on “anti-inflammatory” therapeutic agents that prevent the initiation and progression of inflammation, such as steroid hormones, gadolinium chloride, and TNF-α antagonists, their efficacy is unsatisfactory (1,6,8). Since the immune system is extensively involved in the normal physiological functions of the body, interventions with “anti-inflammatory” therapeutic agents may cause major disruptions to immune homeostasis and lead to adverse reactions (9). As an example, although TNF-α antagonists reduce IRI, they also interfere with the beneficial effects of TNF-α in promoting liver regeneration and fail to achieve clinical benefit (10). Currently, emphasis in research on inflammatory diseases is shifting to the receding phase of inflammation. Studies have shown impaired inflammatory regression is the basis for the pathogenesis of many immune diseases and promoting regression can facilitate the restoration of immune homeostasis and mitigate the progression of inflammatory disease, providing better results than traditional “anti-inflammatory” treatment (3,9,11).
The same phenomenon of inflammatory regression is observed during IRI in liver transplantation. Clinical and animal studies have demonstrated 10 min of ischemic preconditioning prior to donor liver acquisition can effectively mitigate subsequent IRI and reduce the risk of associated complications (12,13). This suggests the liver may generate substances capable of promoting the regression of inflammation during ischemia-reperfusion.
The regression of inflammation involves complex biological processes that are incompletely understood, and current research suggests immune metabolites may play an important role in this process (8,14). During the initiation and progression of inflammation, the metabolic pathways of many immune cells change significantly, resulting in the accumulation of many metabolites. Itaconate is one of the most significantly upregulated metabolites in the tricarboxylic acid cycle and is increasingly coming to the attention of researchers (Figure 1B).
Itaconic acid was first identified as an antibacterial agent in fungi such as Aspergillus by inhibiting isocitrate lyase and was widely used as an industrial raw material in the chemical industry. With the development of metabolomics, research has revealed itaconic acid is not only present in microorganisms such as fungi but also widely present in mammalian immune cells (especially macrophages), and that it is produced in large quantities in response to external stimuli of inflammation (15). Recent years have also seen an explosion in the study of itaconic acid in macrophages (16-19).
As a metabolite significantly up-regulated by macrophages in the inflammatory response, itaconic acid has a powerful function in promoting the regression of inflammation and links cellular metabolism to the innate immune response. Current studies have also shown it may alleviate a wide range of immune diseases (20-22). Itaconic acid also mediates the killing of pathogens such as bacteria and viruses, promotes macrophage M2 polarization to regulate blood supply to ischemic muscles, and plays a regulatory role in STING-related infantile-onset vasculopathy (23-28).
Recently, serum expression of itaconic acid was found to be upregulated in some pregnant women who were at high risk of developing gestational diabetes mellitus. This phenomenon suggests that as a metabolite that is upregulated in inflammatory states, it may have potential clinical value in the diagnosis and prognosis of many inflammatory-related diseases (29). These studies illustrate itaconic acid can be secreted in vitro for a wide range of biological effects in addition to intracellular effects, suggesting it may have strong potential as an exogenous drug for clinical application. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3388/rc).
Methods
Experimental animals
Male C57BL/6J mice aged 6–8 weeks and weighing 20–24 g were obtained from the SLAC Laboratory Animal Corporation (Shanghai, China). Mice were raised in a specific pathogen-free (SPF) room and were not fasted prior to the experiment.
Since itaconic acid has two carboxylic groups, the polarity is stronger, which makes it more difficult to permeate the cell membrane and not conducive to the experiment. In contrast, its derivative, dimethyl itaconate (DI) has good membrane permeability and was used in subsequent investigations.
Random digital labeling was performed on the mice, which were subsequently divided equally into DI and control groups, and only experimenters were aware of the group differences. Experiments were performed under a project license (No. 2020 JS-357) granted by the ethics board of Huashan Hospital, in compliance with Chinese national guidelines for the care and use of animals. A protocol was prepared before the study without registration.
To simulate the liver transplantation process the mice were divided into two groups and each group was subdivided into seven subgroups, including ischemia 0 min and ischemia 30 min, and reperfusion 2, 4, 6, 12, and 24 h. The DI group was injected intraperitoneally with an equal amount of DI (dissolved in saline) and the control group was injected with an equal amount of saline daily for 4 days before the operation. Subsequently, the liver ischemia-reperfusion model was constructed by blocking the blood flow into the liver. Except for the pre-ischemic subgroup, the vascular clamps were removed 30 min after liver ischemia in all subgroups, and the blood and liver tissue samples were collected after the mice were sequentially euthanized at each pre-set time point. Each subgroup had eight mice, the obtained results were averaged for each subgroup, and the ANOVA degree of freedom (E) was 15, which met the general guideline of taking values between 10 and 20. Since there were seven subgroups per group, the control and DI groups each had 56 mice, with a total of 118 mice participating in the experiment.
The liver tissue samples were subjected to HE staining to detect liver injury (Figure 2A).
Extraction of peritoneal macrophages
- 6–8-week-old C57BL/6J mice were injected intraperitoneally with 2 mL of 3% sterile sodium mercaptoethanolate solution and their abdomens were gently rubbed.
- Mice were euthanized by cervical dislocation after 3 days and subsequently immersed in 75% alcohol for 5 min to kill bacteria on the body surface.
- A disposable syringe was used to aspirate 3 mL of DMEM high sugar medium into the abdominal cavity, and the abdomen gently massaged by hand to facilitate the acquisition of more macrophages.
- After massaging for 2 min, ascites in the abdominal cavity of the mice was extracted with a disposable syringe, centrifuged at 1,500 r/min for 5 min, the supernatant removed, and the obtained macrophages resuspended with complete culture medium and spread on the plate.
After extraction, cells were divided into DI and control groups, then each group was divided into five subgroups, including hypoxia 0 min and hypoxia 30 min, and reoxygenation 30, 60, and 120 min. Except for the hypoxia 0 min subgroup, all others were removed from sterile paraffin oil after 30 min of hypoxia, and the cells were removed and lysed to collect proteins at each pre-set time point (Figure 2B).
Exploration of itaconic acid pretreatment concentration and time
Pharmaceutical configuration: DI; molecular weight: 158.1519, DMSO was configured into 200 mM mother liquor.
MTT assay: MTT was added and incubated away from light. The supernatant was then discarded and DMSO added. The OD value was measured at 492 nm by enzyme standardizer.
Immunofluorescence assay
For rinsing with PBS and paraformaldehyde fixation, 0.1% Triton treatment, PBS rinsing, and endogenous peroxidase inactivation were used.
Incubation with 3% H2O2 at room temperature was performed before rinsing with PBS. The solution was closed with 5% FBS at 37 ℃ for 30 min then incubated with primary antibody at 4 ℃ overnight. Rinsing with PBS followed before incubation with CY3-labeled secondary antibody at 37 ℃ and rinsing with PBS. The solution was then incubated with Hoechst at room temperature for 15 min before being rinsed with PBS and observed under microscope.
Statistical analysis
SPSS 23.0 (SPSS Inc., Chicago) and GraphPad Prism 7.0 (GraphPad Software, California) were used for all statistical analyses. The t-test was used for normality and chi-squared data, otherwise the non-parametric test was used. Differences were considered statistically significant at P<0.05.
Results
The degree of liver damage in the mice in the itaconic acid group was significantly alleviated compared to the control group
As revealed by HE staining of liver tissue samples, the liver injury in control mice showed a fluctuating trend during ischemia-reperfusion, and gradually increased with the opening of blood flow. At 0 min ischemia, 30 min ischemia, and 2 h and 4 h ischemia 30 min reperfusion, there was no significant difference in liver injury between the DI and control mice, but at 6, 12, and 24 h ischemia 30 min reperfusion, a significant difference in liver injury between the two groups was observed. The control mice showed extensive hepatocyte death at this time point, especially at 12 h reperfusion, while mice in the DI group showed a significant decrease in liver injury and the hepatocyte morphology was significantly improved (Figure 3).
The above results indicated itaconic acid could reduce tissue damage in the liver during ischemia-reperfusion.
Itaconic acid can reduce the degree of hepatic impairment and inflammation caused by IRI
ELISA of TNF-α and IL-1β concentrations of means of eight mice per subgroup in the serum of DI and control showed the concentrations of these two inflammatory factors were significantly lower in the DI group compared with the control group at all time points (Figure 4). This indicated itaconic acid could reduce the inflammation caused by ischemia-reperfusion.

In addition, advanced biochemical assays revealed ALT and AST concentrations of means of eight mice per subgroup were lower in the DI group compared to the control group at all time points (Figure 4).
The above in vivo studies confirmed itaconic acid treatment significantly reduced the extent of liver injury.
Concentration and time screening of itaconic acid pretreatment in macrophages
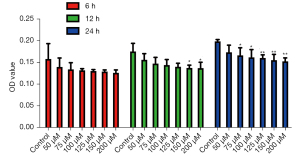
To screen the appropriate DI treatment concentration and time at the cellular level, the cell viability was examined at different DI concentrations and treatment times using MTT assay. The results showed that at 6 h, there was no significant difference in OD values between the DI group and the control group, indicating there was little difference in the activity of the cells in each group under this pretreatment condition. However, at 12 h, there was a significant decrease in cell viability at 150 and 200 µM compared with the control group, and when the pretreatment time was increased to 24 h, starting from 75 µM, there was a significant decrease in cell viability in the DI group compared with the control group. The above results indicated 125 µM DI treatment for 12 h did not cause any change in cell viability (Figure 5), and this condition was used in subsequent experiments to treat the cells.
Activity of the NF-κB signaling pathway during hypoxia-reoxygenation was significantly inhibited by itaconic acid
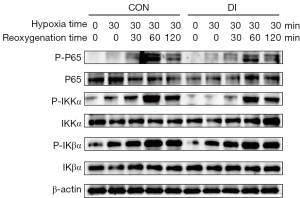
The NF-κB pathway is crucial in regulating the inflammatory response, and to explore the mechanism of itaconic acid to reduce the inflammatory response in the liver, its effect on activation of the NF-κB pathway was explored. Western blot results showed that in the five time points of hypoxia 0 min, hypoxia 30 min, hypoxia 30 min followed by reoxygenation 30, 60, and 120 min, the expression levels of P-P65 and P-IKKα, which are important factors in the NF-κB pathway, showed a tendency to change from feeble to intense, suggesting the pathway was activated. The protein levels of P-P65 and P-IKKα also began to decay with prolongation of the reoxygenation time. Of interest, the expression levels of P-P65 and P-IKKα peaked after 30 min of hypoxia and 60 min of reoxygenation, but their protein levels in the DI-treated group were significantly lower than in the control group at this time (Figure 6). This indicates DI treatment significantly inhibited activity of the NF-κB signaling pathway during hypoxia-reoxygenation.
Itaconic acid alleviates the entry of p-p65 into the nucleus during hypoxia-reoxygenation
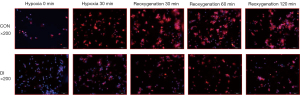
Phosphorylation of transcription factor P65 protein to form P-P65 into the nucleus is an essential step for initiating downstream gene expression during activation of the NF-κB pathway, and immunofluorescence assay can visualize the accumulation of P-P65 in the nucleus. Our results showed the percentage of P-P65 entering the nucleus was low in both DI and control groups before hypoxia, and the protein content of P-P65 in the nucleus increased significantly after experiencing hypoxia and reoxygenation. However, the P-P65 protein signal in the nucleus was significantly reduced in the DI group compared to the control group (Figure 7). This is consistent with the above-mentioned Western blot results, further confirming DI can inhibit activation of the NF-κB pathway by reducing the accumulation of transcription factor P-P65 in the nucleus.
Itaconic acid attenuates the expression of proteins related to downstream of the NF-κB signaling pathway
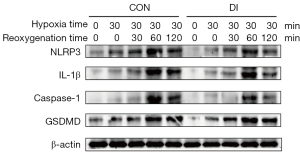
Based on the above findings, we further examined the expression levels of inflammation-related protein factors downstream of the NF-κB pathway. Western blot results showed the expression of NF-κB downstream related proteins NLRP3, GSDMD, caspase-1, and IL-1β showed a weak and strong trend at five time points; namely, 0 min of hypoxia, 30 min of hypoxia, and 30 min of hypoxia followed by 30, 60, and 120 min of reoxygenation. The expression of NLRP3, GSDMD, caspase-1, and IL-1β showed a trend from feeble to intense but weakened at 120 min of reoxygenation. Comparing the DI group with the control group, the levels of NLRP3, caspase-1, and IL-1β were weaker in the DI group at 30 min of hypoxia and 60 min of reoxygenation than in the control group (Figure 8). This indicates DI can reduce the expression of relevant inflammatory factors by inhibiting the NF-κB pathway activation, which may be an important mechanism for DI to reduce liver injury and inflammatory status.
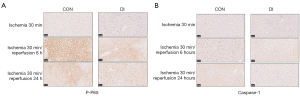
Immunohistochemistry further confirms DI attenuates expression of the NF-κB pathway and its downstream molecules during ischemia-reperfusion
Immunohistochemical staining of liver samples from the DI and control groups showed P-P65 expression was lower before ischemia, but after 6 h of reperfusion, its coloration in the nucleus deepened, indicating an increase in the accumulation of P-P65 protein in the nucleus. Further, after 24 h of ischemia and reperfusion, coloration of the nucleus gradually decreased, suggesting activation of the NF-κB pathway gradually decreased, while the expression of P-P65 in the DI group was significantly weaker compared with the control group (Figure 9A). The expression of P-P65 was significantly lower in the DI group compared with the control group (Figure 9A), and the expression of caspase-1 was also lower before ischemia. However, after 6 h of reperfusion, coloration in the cytoplasm was significantly deepened, and after 24 h of reperfusion, the coloration was gradually reduced, while the expression of caspase-1 was significantly lower in the DI group compared with the control group in this process (Figure 9B). In addition, the expression of caspase-1 was significantly lighter in the DI group compared with the control group during this process (Figure 9B). The above results further confirmed in vivo that DI treatment reduced the activity of the NF-κB pathway and the expression of downstream inflammatory factors during ischemia-reperfusion.
Discussion
IRI in organ transplantation is a significant scientific challenge for clinical medicine and serves as a key constraint on the prognosis of transplant patients and the source of donor organs. The essential mechanism of IRI experienced in organ transplantation lies in imbalance of the inflammatory state.
The process of mouse liver ischemia-reperfusion simulates the pathophysiological state of human liver transplantation after the disruption and revascularization of donor liver blood flow. Inflammation regression is a rising topic in immune disease research, and itaconic acid, a metabolite produced by immune cells such as macrophages in an inflammatory state, has only recently been discovered as having a powerful ability to promote inflammation regression. However, its role and mechanism in IRI in liver transplantation have been rarely reported.
NF-κB, a transcription factor first identified in the nucleus of lymphocytes at the end of the last century, has been found to be involved in a wide range of biological functions and consistently or excessively activated during the development of various acute and chronic inflammatory diseases. Therefore, blockade of the NF-κB pathway may be an attractive target for the treatment of inflammatory disease (30).
The addition or removal of phosphate groups (dephosphorylation) acts as a biological “on/off” for many reactions and controls many biological processes. NF-κB proteins usually form homo/heterodimers from P65 and P50, which are inactivated in the cytoplasm by binding to the inhibitory protein IkB to form a trimeric complex. When the upstream signal binds to the cell membrane surface receptor, the receptor conformation changes and transmits the signal to IKK kinase, which in turn phosphorylates IkB protein and dissociates it from the trimer. In turn, phosphorylation of P65, a molecule in NF-κB protein, can cause it to translocate to the nucleus and bind to the promoters of pro-inflammatory genes, leading to enhanced gene expression and amplification of the inflammatory response, ultimately leading to tissue inflammatory damage (31,32).
Activation of the NF-κB signaling pathway promotes the expression of its downstream inflammation-related genes (33,34). It has been reported that NF-κB pathway activation induces the production of NLRP3, which in turn triggers the recruitment of the bridging protein ASC and caspase-1 to form a macromolecular complex in which caspase-1 is activated. The activated caspase-1 directly cleaves GSDMD and the precursor cytokine pro-IL-1β to produce IL-1β, and the cleaved GSDMD forms pores in the cell membrane and mediates the release of cytoplasmic contents to cause inflammation (35-37).
It was found that the systemic inflammatory state of mice pretreated with itaconic acid was significantly reduced after experiencing IRI, with significant decreases in liver function indexes and inflammatory factor concentrations, as well as significant reductions in liver injury and significant improvements in hepatocyte morphology. It was also confirmed that itaconic acid treatment could inhibit NF-κB pathway activity and reduce the expression level of downstream inflammation-related factors, which in turn reduced liver injury and inflammation status.
Firstly, we confirmed the liver function indexes ALT and AST, and inflammatory factors TNF-α and IL-1β, were significantly reduced in mice pretreated with DI compared with the control mice at all time points of ischemia-reperfusion. The HE staining of liver tissues also confirmed the injury was reduced in the DI group compared with the control group, and the morphology of hepatocytes was normal. Subsequently, by extracting mouse peritoneal macrophages and constructing a hypoxia-reoxygenation model it was confirmed that itaconic acid could inhibit activity of the NF-κB signaling pathway and reduce cellular inflammation during the process. In vivo experiments have demonstrated DI can alleviate IRI-induced inflammation and subsequent liver injury in mice, suggesting itaconic acid, as an intermediate product of the immune cell tricarboxylic acid cycle, links immunity and metabolism in a cascade and has a powerful potential to promote the regression of inflammation. As dysregulation of NF-κB activity has been shown to contribute to inflammation-related diseases, NF-κB may also be a potential target for intervention in disease progression (38). For the first time, this study demonstrated that exogenous provision of DI can alleviate IRI through the NF-κB signaling pathway, reflecting the promising translational value and significance of itaconic acid in the field of organ transplantation.
Conclusions
Itaconic acid inhibits NF-κB pathway activity, reduces the accumulation of transcription factor P-P65 in the nucleus, and attenuates the expression of inflammatory proteins associated with the NF-κB pathway downstream, thereby reducing liver injury and inflammation during ischemia-reperfusion.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81873874 and 82071797) and Clinical Research Plan of SHDC (No. SHDC2020CR2021B). The funders made no substantive contributions to the article.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3388/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3388/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3388/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2020 JS-357) granted by the ethics board of Huashan Hospital, in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dar WA, Sullivan E, Bynon JS, et al. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int 2019;39:788-801. [Crossref] [PubMed]
- Masior L, Grat M. Primary non-function and early allograft dysfunction after liver transplantation. Dig Dis 2022; Epub ahead of print. [Crossref] [PubMed]
- Zhang H, Li Z, Li W. M2 Macrophages Serve as Critical Executor of Innate Immunity in Chronic Allograft Rejection. Front Immunol 2021;12:648539. [Crossref] [PubMed]
- Nemeth N, Peto K, Magyar Z, et al. Hemorheological and Microcirculatory Factors in Liver Ischemia-Reperfusion Injury-An Update on Pathophysiology, Molecular Mechanisms and Protective Strategies. Int J Mol Sci 2021;22:1864. [Crossref] [PubMed]
- Warmuzinska N, Luczykowski K, Bojko B. A Review of Current and Emerging Trends in Donor Graft-Quality Assessment Techniques. J Clin Med 2022;11:487. [Crossref] [PubMed]
- Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79-89. [Crossref] [PubMed]
- Mao XL, Cai Y, Chen YH, et al. Novel Targets and Therapeutic Strategies to Protect Against Hepatic Ischemia Reperfusion Injury. Front Med (Lausanne) 2021;8:757336. [Crossref] [PubMed]
- Zulpaite R, Miknevicius P, Leber B, et al. Tryptophan Metabolism via Kynurenine Pathway: Role in Solid Organ Transplantation. Int J Mol Sci 2021;22:1921. [Crossref] [PubMed]
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 2016;15:551-67. [Crossref] [PubMed]
- Fingas CD, Beste M, Penndorf V, et al. Liver Regeneration-Related Cytokine Profiles in Donors and Recipients Before and After Living-Donor Liver Transplant. Exp Clin Transplant 2018;16:554-61. [PubMed]
- Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med 2013;5:661-74. [Crossref] [PubMed]
- Patel MS, Abt PL. Current practices in deceased organ donor management. Curr Opin Organ Transplant 2019;24:343-50. [Crossref] [PubMed]
- Lin J, Huang H, Yang S, et al. Protective Effects of Ischemic Preconditioning Protocols on Ischemia-Reperfusion Injury in Rat Liver. J Invest Surg 2020;33:876-83. [Crossref] [PubMed]
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017;356:1026-30. [Crossref] [PubMed]
- Peace CG, O'Neill LA. The role of itaconate in host defense and inflammation. J Clin Invest 2022;132:e148548. [Crossref] [PubMed]
- Diotallevi M, Ayaz F, Nicol T, et al. Itaconate as an inflammatory mediator and therapeutic target in cardiovascular medicine. Biochem Soc Trans 2021;49:2189-98. [Crossref] [PubMed]
- Nassef MZ, Hanke JE, Hiller K. Mitochondrial metabolism in macrophages. Am J Physiol Cell Physiol 2021;321:C1070-81. [Crossref] [PubMed]
- Tomlinson KL, Prince AS, Wong Fok Lung T. Immunometabolites Drive Bacterial Adaptation to the Airway. Front Immunol 2021;12:790574. [Crossref] [PubMed]
- Kim JK, Park EJ, Jo EK. Itaconate, Arginine, and Gamma-Aminobutyric Acid: A Host Metabolite Triad Protective Against Mycobacterial Infection. Front Immunol 2022;13:832015. [Crossref] [PubMed]
- Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 2016;24:158-66. [Crossref] [PubMed]
- Bambouskova M, Gorvel L, Lampropoulou V, et al. Electrophilic properties of itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature 2018;556:501-4. [Crossref] [PubMed]
- Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018;556:113-7. [Crossref] [PubMed]
- Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe 2009;5:593-601. [Crossref] [PubMed]
- Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal 2010;3:re3. [Crossref] [PubMed]
- Fang L, Deng Z, Shatseva T, et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene 2011;30:806-21. [Crossref] [PubMed]
- Naujoks J, Tabeling C, Dill BD, et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog 2016;12:e1005408. [Crossref] [PubMed]
- Ganta VC, Choi MH, Kutateladze A, et al. A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle. Circulation 2017;135:2403-25. [Crossref] [PubMed]
- Daniels BP, Kofman SB, Smith JR, et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity 2019;50:64-76.e4. [Crossref] [PubMed]
- de Seymour JV, Conlon CA, Sulek K, et al. Early pregnancy metabolite profiling discovers a potential biomarker for the subsequent development of gestational diabetes mellitus. Acta Diabetol 2014;51:887-90. [Crossref] [PubMed]
- Barnabei L, Laplantine E, Mbongo W, et al. NF-kappaB: At the Borders of Autoimmunity and Inflammation. Front Immunol 2021;12:716469. [Crossref] [PubMed]
- Yu H, Lin L, Zhang Z, et al. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther 2020;5:209. [Crossref] [PubMed]
- Roy M, Singh R. TRIMs: selective recruitment at different steps of the NF-kappaB pathway-determinant of activation or resolution of inflammation. Cell Mol Life Sci 2021;78:6069-86. [Crossref] [PubMed]
- Meier-Soelch J, Mayr-Buro C, Juli J, et al. Monitoring the Levels of Cellular NF-kappaB Activation States. Cancers (Basel) 2021;13:5351. [Crossref] [PubMed]
- Prescott JA, Mitchell JP, Cook SJ. Inhibitory feedback control of NF-kappaB signalling in health and disease. Biochem J 2021;478:2619-64. [Crossref] [PubMed]
- Afonina IS, Zhong Z, Karin M, et al. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol 2017;18:861-9. [Crossref] [PubMed]
- Reid S, Scholey JW. Recent Approaches to Targeting Canonical NFkappaB Signaling in the Early Inflammatory Response to Renal IRI. J Am Soc Nephrol 2021;32:2117-24. [Crossref] [PubMed]
- Chawla M, Roy P, Basak S. Role of the NF-kappaB system in context-specific tuning of the inflammatory gene response. Curr Opin Immunol 2021;68:21-7. [Crossref] [PubMed]
- Duez H, Pourcet B. Nuclear Receptors in the Control of the NLRP3 Inflammasome Pathway. Front Endocrinol (Lausanne) 2021;12:630536. [Crossref] [PubMed]
(English Language Editor: B. Draper)



