
Cone-beam CT in lung biopsy: a clinical practice review on lessons learned and future perspectives
Introduction
The vast majority of screening detected peripheral pulmonary nodules (PN) will raise the question if the nodule is malignant. Once identified, malignancy risk calculators based on imaging and patient characteristics are used to assess the risk of malignancy and guide subsequent steps (1-3). In case of a low risk of malignancy (i.e., <10%) follow-up of PN is indicated. PN with intermediate to high risk of malignancy (i.e., >10%) should preferably be diagnosed with image guided minimally invasive biopsy before treatment (3-5). However, multiple studies show that stereotactic ablative radiotherapy is often considered without pathological confirmation of lung cancer (6,7). Other studies on surgical lung resections without a pre-procedural pathological confirmation of lung cancer furthermore reveal that benign resection rates vary from approximately 10% (8-10) to as much as 86% (3,11). Contrarily, lung cancer patients with an initial calculated low risk of malignancy may unintentionally suffer from a reduced survival outcome, due to the treatment delay caused by watchful waiting, while minimally invasive diagnostics might have provided a diagnosis in an earlier stage. The International Association for the Study of Lung Cancer (IASLC) lung cancer staging project showed that even within stage I disease, survival is significantly related to size (12). Even when there is no evidence of metastatic spread, a tumour of <1 cm size has a 5-year survival of 92% while a single 2–3 cm sized T1c tumour has a 5-year survival of 77%. To improve survival, it is essential to diagnose as early and as safe as possible and with the lowest possible burden for these symptomless patients. Ideally, an approach that is easily accessible and has a high diagnostic accuracy would provide a means for early diagnostics. This approach might become increasingly more important when the number of detected small PN rises as a result of implemented screening programs increasing the burden on diagnostic test availability. However, it must be considered whether the currently available approaches are able to fulfil this emerging demand.
Historically, the gold standard for diagnosing pulmonary lesions has been image guided trans-thoracic needle biopsy (TTNB), with a diagnostic yield of approximately 90% (13). Yet, passing the pleura is associated with a high pneumothorax rate of 18.8–25.3%, of which 6–7% require a chest tube placement (14). However, while it is considered the gold standard, patients are frequently treated without a diagnosis. Possible explanations could be the relatively high complication rates in an often already vulnerable group with comorbidities like chronic obstructive pulmonary disease (COPD) and emphysema, or because of variation in operator skill across centres. Furthermore, two studies recently hypothesized that traversing the thoracic wall may increase ipsilateral disease recurrence through seeding, and may affect overall survival (15,16).
A conventional bronchoscopy where the bronchoscope is led up until the segmental bronchus after which biopsy tools are advanced further under C-arm fluoroscopy has been reported as an alternative to reach small peripheral nodules. This technique showed a 34% sensitivity in a meta-analysis but a sensitivity lower than 14% with a negative predictive value (NPV) of 47% in lesions as found in analysis of a subset of patients identified through the NELSON computed tomography (CT) screening study (3,17). Potential major advantages of an endobronchial approach is the pooled chance of pneumothorax of 1.5% (of which only 0.6% requires chest tube placement) and the possibility to perform the intervention in patients with severe co-morbidities. However, the conventional ‘blind’ C-arm guided trans-bronchial biopsy certainly does not have the accuracy needed to be a valid alternative to TTNB.
Technological innovations
To address the clinical need for minimal invasive diagnostics, several technological innovations have been developed/implemented that can help the physician to endobronchially navigate more peripheral and more accurate than ever before. The introduction of a radial endobronchial ultrasound (EBUS) mini probe imaging was first reported two decades ago as one of first technologies in this area (18), followed by electromagnetic navigation technology (EMN), virtual bronchoscopy (VB) and ultrathin bronchoscopes (UTB) not many years later. As the landmark meta-analysis of Wang Memoli et al. [2011] showed, all of these technologies indeed increased diagnostic yield to the often encountered limit of 70%, although a minority of studies have reported a slightly higher yield (19). In part, based on the same data, guidelines subsequently recommended the use of radial endobronchial ultrasound (rEBUS) mini probe imaging but also EMN if available (3,20).
However, both technology and our routine clinical practice have evolved since then. High-resolution CT has become the standard and the radiologist routinely can define small nodules as suspicious while they previously would have gone unnoticed. And on top of updates in pre-existing technologies such as EMN, several novel navigation bronchoscopy technologies have been introduced with the potential to further improve diagnostic yield. New kids on the block that aim to help diagnose peripheral pulmonary nodules now include specialized navigation instruments (21), robotics (22-26), cone beam CT (CBCT) (27-30), mobile 3D C-arm imaging (31,32) and hybrid methods that combine modalities for navigation guidance and confirmation (33,34).
Technology implementation
Biopsy of peripheral PN using conventional bronchoscopy has such a low diagnostic accuracy because the samples are taken without clear navigation assistance and because there is a lack of confirmation that the sampling instruments are at the correct target lesion. The navigation bronchoscopy procedure addresses these problems and consists of three basic steps: navigation, positioning confirmation and tissue acquisition.
From this perspective, an optimal navigation bronchoscopy setting that is able to target peripheral pulmonary lesions of all origins must help physicians in all three procedure steps.
A short overview of technology and their clinical outcomes will allow putting these requirements into a more general perspective before we focus on CBCT.
Technology overview
Navigation tools
UTB and virtual bronchoscopy navigation (VBN)
Most familiar to any endoscopist is likely the utility of ultrathin bronchoscopy. It relies on conventional video-endoscopy, but in a smaller package. Therapeutic scopes of >5.0 mm outer diameter often become wedged at the segmental bronchi. The newest scopes now have a 3.0 mm outer diameter whilst facilitating a 1.7 mm working channel that can be used in combination with the smallest of rEBUS mini probes and sampling instruments (for example: Olympus BF-MP190F and UM-S20-17S). Consequently, it can be navigated 3–4 bronchial generations further than conventional bronchoscopes. In selected patients, this is sufficient to directly visualize the lesion. Oki et al. [2019] showed that the ultrathin bronchoscope in combination with tools such as rEBUS, VBN and fluoroscopy had an excellent diagnostic yield of 70.1% in a group of 177 patients with a median largest tumor diameter of only 18.9 mm (35).
While UTB facilitates visualization of the navigation pathway and lesion and can help guide tissue acquisition, there is no meta-analysis or systematic review available on the applicability as a stand-alone device for navigation bronchoscopy. Surely, this modality will evolve and will foremostly be used as means of navigation to the lesion. Combining UTB with rEBUS seems logical, as rEBUS facilitates visualization beyond the bronchial wall and once vision has become obscured due to i.e., mucus or bleeding after the first biopsy. A rEBUS mini probe, with an outer diameter of 1.4 mm, is also able to advance more peripherally once the UTB becomes wedged.
When navigating the UTB through the small peripheral airways, that differ per patient, VBN software is a valuable addition. VBN utilizes a preprocedural CT to segment and plan a path towards the lesion based on the bronchial anatomy which is correlated per-procedurally with the live video-bronchoscope imaging. To help reconstruct the smaller airways for VBN software, CT quality should have slices with a maximum of 1.0 mm thickness. A major limitation of VBN/UTB is that in a significant portion of cases there will not be a bronchus leading to the lesion at all. As the study by Oki et al. showed in their multi-modal approach, a lack of bronchus sign (on 0.5 mm CT-slices) had significant impact on diagnostic yield (74.6% vs. 57.4%) (35). A randomized trial by Ishida et al. [2011] showed that diagnostic yield was higher in cases where VBN was used (80%) than where it did not (67%). However, the added value of VBN goes beyond only increasing diagnostic yield (DY), as they also showed that procedure time was significantly shorter (36).
In the near future, we will likely see redesigned UTB’s that are smaller and facilitate more tools, while simultaneously improving reconstruction of the bronchial tree by VBN to include smaller, more distal airways. An important aspect in that quest is the trade-off between going further out and/or reducing sampling instrument diameter. What Gildea for example already mentioned in 2016 but remains apparent is that all studies report a discrepancy between getting there and getting a diagnosis (37). Theoretically, this could be caused by mispositioning (38), yet on the other hand, it could also be caused by a lack of sufficient volume of tissue for analysis. A means for the UTB to facilitate accurate trans-parenchymal navigation in cases without a bronchus sign is another design challenge.
EMN
EMN technology guides the physician to the target location by electromagnetic sensors on- and within the patient, based on a pre-procedural CT scan. A weak electromagnetic field in the vicinity of the patient allows tracking of the sensors on the patient (for reference position) and the sensor within the special extended working channel or tools that go through the bronchoscope. The catheter used for navigation in combination with the sensor is torsional stiff and pre-angulated at the distal tip. These properties allow navigation through the bronchial tree beyond what is feasible with the conventional bronchoscope, into the most distal segments of the airways. It has been extensively studied and recent data shows that it is often used in combination with imaging confirmation modalities such as rEBUS and fluoroscopy (39). While a systematic review and meta-analysis by Folch et al. [2020] showed EMN had a pooled sensitivity of 77% and specificity of 100% in 3,342 study patients with an average lesion size of 23.2 mm (40), a recent update of the NAVIGATE trial’s (n=1,388) reported a diagnostic yield of 67.8% overall and 62% for <20 mm nodules (41).
Over the years, it became apparent that EMN in clinical practice was often influenced by significant CT to body divergence. CT to body divergence is a definition used to describe the difference between pre-procedural and intra-procedural lesion positioning, which is multi-factorial (42). It is caused by breathing movement (43,44), instrument and bronchoscope movement (45), atelectasis (42,46,47), cardiovascular motion (48) and system measurement error (49). These errors are most outspoken in the lower lobes. Breathing motion alone has shown to cause a divergence of up to 17.6 mm on average (43,44) while system registration error can also be several millimetres (49). As a result, more recent updates of EMN systems are primarily aimed at reducing or compensating for this problem. To reduce CT to body divergence, updates of commercial EMN systems have led to the introduction of using both in- and expiratory CT scans for planning the procedure (43,44) as well as implementing a custom digital tomosynthesis algorithm that can be used in combination with C-arm fluoroscopy (34).
Considering the intuitive means with which the commercial EMN platforms guide the physician toward the target lesion, it is a great help for every physician in need for navigation guidance. EMN is a navigation guidance tool, but cannot confirm location positioning in real-time. Bigger and solid lesions might be targeted in combination with fluoroscopy or with artificial tomosynthesis such as provided by the platforms while smaller lesions with bronchus sign might be viable options when further assisted by rEBUS. If no bronchus sign is present based on high resolution CT, or if precise positioning is necessary, EMN is best used in combination with a system allowing detailed 3D visualization and confirmation of the catheter position (such as CBCT). CBCT allows precise intra-procedural 3D assessment of catheter position in relation to the target lesion and can be used to determine the optimal trans-parenchymal route, if needed (45,50).
Advanced multi-modality reconstruction
Novel hybrid technological systems such as Lung Vision (Body Vision Medical) but in-part also the tomosynthesis feature of the Illumisite™ EMN platform integrate multiple information sources for reconstructing and/or confirming a navigation pathway and lesion position (33,34,51). These systems rely on using advanced image processing that integrates fluoroscopy along with additional information (i.e., a pre-procedural CT-scan) for providing a coarse 3D tomographic image or augmented fluoroscopy image. Being compatible with most other technologies and available with specialistic tools, these systems can be a valuable addition to the toolbox of the physician. As Pritchett showed with Lung Vision technology in combination with a bronchoscope and pre-angulated catheters for navigation, this technique can help guide experienced physicians to the lesion in 96% of cases and can reduce CT to body divergence to as little as 5.9 mm (range, 2.1–10.0 mm) in a cohort of 51 patients (51). CBCT verification allowed Pritchett to obtain a diagnostic accuracy as high as 88.2%. As technology and the navigation bronchoscopy procedure evolves, it becomes increasingly clear that not one technique will be a do-it-all. These multi-modality reconstruction technologies that are compatible and integrate with other methodologies are surely here to stay. While they currently do not provide millimetre accuracy by themselves, they are sufficiently accurate and easily combined with secondary technologies to help obtain diagnoses for all peripheral lesions referred for diagnostic biopsy. A second example showing the added value of this multi-modality approach is the study by Aboudara et al. [2020]. Using the Illumisite™ EMN platform and it’s digital tomography algorithm that reconstructs 3D imaging information out of conventional C-arm fluoroscopy showed to significantly improve diagnostic yield from 59% when using EMN alone to 79% when used in combination (34).
Robotic endoscopy
Robotic assisted bronchoscopy (RAB) is a technology that has gained much attention since the introduction of the first system in the US in 2018. However, since robotics is the subject of a dedicated issue of this series we would like to recommend readers towards this article. In brief, there are currently two systems available in the US market: the Auris Monarch® system (52) and Intuitive Surgical’s Ion® system (53). A third company called Noah medical is developing a platform called the Galaxy system (26). Currently, studies using the two commercially available robotic systems (with or without using additional technology) have shown diagnostic yields of 69–82% (22-25,54). Their major advantage when compared to other technologies is their integrative design; combining multiple technologies into a singular easy-and-intuitive platform. The steady distal tip furthermore allows the advantage of active steering and a stable repeatable exit point for sampling tools which might close the gap between getting there and getting a diagnosis. However, the robotic aspect does show to be in need of secondary imaging confirmation; RAB is therefore routinely combined with rEBUS and fluoroscopic imaging techniques (23,32,55).
Confirmation tools: rEBUS
rEBUS miniprobe
rEBUS has been around for more than two decades and has been a valuable tool for the endoscopist as method for position confirmation. It does not however, provide any navigation support nor real-time tissue acquisition guidance. The currently available commercial systems are sideways looking ultrasound systems with a maximum depth of around 15–20 mm. Wang-Memoli [2011] and more recently Ali [2018] have shown that the diagnostic yield of rEBUS in navigation bronchoscopy is approximately 71%. As not much has changed in its design over time, these numbers are most likely still accurate (19,56). Considering that it is a relatively low-cost device that can be re-used some 40–100 times before it breaks, rEBUS is a cost-effective addition in navigation bronchoscopy. The limited range of application needs to be accounted for when selecting a nodule or using additional tools. Lesions with a lack of bronchus-sign on high resolution CT, or ground glass opacities are best not targeted by rEBUS alone. In the remainder of cases, it is however a useful instrument in confirming correct positioning and gives good indication about chances of adequate tissue sampling. Since it provides local and detailed imaging, it can additionally be used to (repeatedly) measure contact area length and positioning to guide subsequent sampling tool positioning after rEBUS removal. rEBUS can also provide information on pulsating structures that require caution such as in Figure 1. But, interpretation of the rEBUS image can be difficult. Bleeding caused by prior biopsy, remaining fluid after a lavage, or atelectasis (especially in posterior lung fields) will give a similar image as the targeted lesion on rEBUS-imaging. Increased heterogeneity of the rEBUS image might allow differentiation of false positive imaging versus the image as obtained in a solid tumor, but this is not always easy to distinguish.
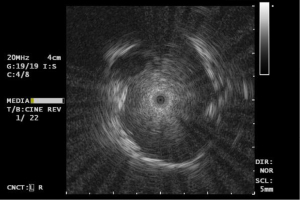
Nakai et al. (57) and Ikezawa et al. (58,59) showed that rEBUS can be used in ground glass opacities (GGO) and part solid lesions, as these lesions provide a relative increase in echogeneity when compared to the surrounding lung parenchyma. This is also our experience, but similarly, we also have experienced that putting the rEBUS under friction or minor bleedings may result in ostensible GGO or part solid lesion imaging.
Tissue sampling
Ultimately, getting the correct diagnosis is the goal. And while navigation success is often around ~90%, the diagnostic accuracy remains at 70–75% in the majority of studies (19,23,33,40,56), although some centres do seem to have closed the gap [i.e., 82–95% (24,29,32,51,60)]. As mentioned before, navigation bronchoscopy procedure can be divided into three different steps. The third step—tissue acquisition—seems to be the area with the highest need for improvement as there often is more than a 10% difference between getting there (e.g., navigation success) and getting a diagnosis (e.g., diagnostic accuracy). The limited literature on tool efficacy shows that a set of sampling tools combined with technology that helps position these tools is likely needed. Even with 3D-imaging confirmed correct positioning, a diagnosis sometimes remains uncertain (37,61) and this is most likely multifactorial. First: precise position is essential in obtaining representative samples, for which real-time 3D-guidance is an important asset. When using navigation tools such as robot bronchoscopy or EMN without additional guidance, it can be difficult to precisely determine where to biopsy in small lesions. Second: when using increasingly smaller sampling tools, the biopsy samples can become too small for optimal evaluation. Third: when using pre-angulated catheters without distal tip manipulation, (as commonly used in EMN and CBCT guided navigation) the rigidity of the sampling tools will change the angulation of the catheter which increases uncertainty. Oki et al. and de Ruiter et al. both showed that the angulation of catheter/bronchoscope can alter significantly based on instruments used and material interaction (62,63). Lastly: even biopsies of endobronchially visible tumours do not always result in a diagnosis if non-vital tissue is sampled (64). When a fluorodeoxyglucose (FDG)-PET-scan is available, diagnostic yield could be optimized by aiming for regions with a high FDG-avidity.
There is no uniform way to overcome all above mentioned hurdles, but as we recently reported, taking more (multimodality) samples than is thought necessary for a diagnosis, might close the gap between getting there (‘navigation success’) and getting a diagnosis (‘diagnostic accuracy’) (29,61). This is corroborated by the in-depth analysis of the NAVIGATE trial which showed that a multi-modal approach for sampling increases yield (65).
Cone-beam CT and augmented fluoroscopy in pulmonary nodule biopsy
Conventional transbronchial biopsy is often performed under guidance of mobile or fixed C-arm fluoroscopy. In larger lesions, fluoroscopy offers gross lesion positioning and provides coarse guidance and confirmation. Smaller PN however are often not visible on fluoroscopy. To allow for real-time guidance during navigation and biopsy, more detailed and precise 3D-imaging such as provided by cone beam CT imaging systems is essential. It is further relevant when looking at future developments of endobronchial treatments.
CBCT and augmented fluoroscopy (CBCT-AF) have long been implemented in almost every hospital’s interventional radiology or cardiology suites and is found in the newly implemented hybrid operating room (OR) environment. It has however only recently become of more interest to the field of interventional pulmonology (27,28). The CBCT system knows the exact patient-table positioning and it can obtain a 3D scan of patient anatomy with high (soft-tissue) contrast within 3 to 8 seconds. Such a high-powered and calibrated system is not just an improved version of a mobile (3D) C-arm. With a reconstructed 3D CBCT scan under breath-hold, the physician obtains slice reconstructions of around 0.65 mm thickness that allow for detailed evaluation of navigation pathways and instrument to lesion positioning. Complementary software can subsequently be used to outline the lesion and navigation pathway intra-procedurally (Figure 2). In cases where sampling very near the pleura is required, it can be useful to outline the pleural edges as well as margin reference. Based on the availability of the software, the delineated pathway and segmented lesion can be projected over the fluoroscopy images under different angles to support real-time augmented fluoroscopy in the hybrid OR.

The overlays on fluoroscopy help the physician to determine navigation progress, where every bifurcation of a bronchus can be found, or, the position at which one needs to create a trans-parenchymal pathway. Without software, these overlays are not possible and its use is limited to reviewing obtained 3D imaging and normal fluoroscopy images. Repeated CBCT scanning allows for detailed confirmation that the target has been reached (or the need for repositioning). If needed, segmentation of the lesion can be performed again to overlay new pathways or provide positioning updates. The CBCT system and augmented fluoroscopy can also provide 3D confirmation of instrument positioning (for biopsy), which is closest to real-time guidance when no endobronchial visualization can be provided.
In summary, CBCT- and AF-imaging systems combine navigation and confirmation of instrument positioning. The entire workflow of a patient undergoing a CBCT guided navigation bronchoscopy is visualized and explained in Figure 3. When compared to the mentioned navigation tools, CBCT-AF provides the essential aspects of intra-procedural and (near-) real-time image guidance needed by the physician. Since it provides this information from a global perspective rather than a local perspective (such as rEBUS/video-endoscopy) it has advantages as well as disadvantages. The major advantage of having a complete and actual 3D-image of the patient compared to technology such as EMN is that it addresses the CT-to-body divergence concerns, and thus allows for a more accurate trans-parenchymal navigation, and allows creating a pathway when there is no bronchus sign. This is a valuable addition in patients with nodules smaller than 20 mm or nodules without a bronchus sign and thus for approximately 40–60% of patient cohort. Especially in these small nodules, CT-to-body divergences can be larger than the maximal diameter of the target nodules (29,42-44,60). Relating 3D-positioning to previous PET/CT-scans also allows the physician to target and verify the most FDG-avid region of a lesion has been reached, increasing the chance of obtaining vital tissue. Having intra- and post-procedural proof of adequate positioning can also help in the in-room decision-making process with the pathologist for the rapid on-site cytology evaluation (ROSE). The 3D-imaging capability is furthermore an essential component in future endobronchial treatments like transbronchial microwave ablation that requires precise catheter positioning confirmation as well as the need to determine the ablation zone to fully cover the tumour in three dimensions with an additional safety margin of 5 to 10 mm.
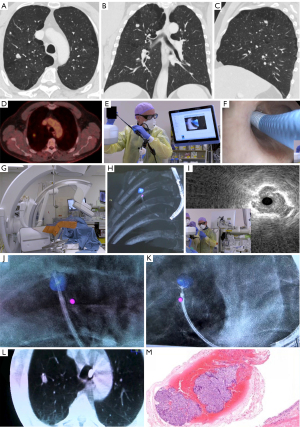
A major disadvantage of current CBCT-AF systems is the learning curve that must be overcome to be able to interpret and translate the global perspective into local navigation, that results in suboptimal results during this learning process (29). This translation is often of lesser concern in lesions of >2 cm size with a central bronchus sign. Small PN’s however, require refined movements and have a small margin of error for biopsy-instrument positioning. Combining CBCT-AF with an additional navigation technique such as EMN or robotic bronchoscopy may reduce this problem, but increases the financial burden of the procedure. A second often discussed disadvantage of CBCT over other navigation methods is the inherent use of radiation (66). The entire team should rigorously adhere to the As Low As Reasonably Achievable (ALARA) principle and thus use shielding and aggressively collimate. While applying ALARA, continuously monitoring dose and adjusting imaging settings, we have been able to significantly reduce radiation dose from a dose area product (DAP) of 47.5 to 28.4 Gy∙cm2, which translated to an estimated effective dose of 5.8 mSv (29). While there has been variation in procedural dosing estimates as well as different reconstruction calculation parameters (29,66), even the worst estimations seem to not exceed a dose obtained by a whole body PET/CT-scan. The vast majority of dose herein comes from the CBCT, which is performed while the staff has left the room.
A potential disadvantage of CBCT-AF for navigation bronchoscopy guided biopsy can be the lack of (allowed) access for the interventional pulmonologist to a hybrid OR, or an interventional radiology room. This requires collaboration with fellow specialties, as purchasing a CBCT only for performing navigation bronchoscopy procedures will be a significant investment. When putting different systems side by side, some aspects might be mentioned. For navigation bronchoscopy, ceiling mounted systems are generally easier to use than floor mounted systems, as these leave more room at the head of the patient and provide a more predictable rotation during CBCT scanning (less likely to hit the endoscope). The user interface of the workstations also slightly differ per vendor, in particular when it comes to delineating the lesion and navigation pathway. Whereas one system for example allows lesion delineation by clicking and pulling a region of interest within the nodule in only one orientation, the other requires defining the lesion in two directions and a paint brush technique. There is furthermore a potential difference in spin time, but this will greatly depend on the imaging protocol that the system has installed and if it is optimized for navigation bronchoscopy. In systems where no specific navigation bronchoscopy protocols have been installed, we recommend consulting to your clinical physicist or system representative as they may be able to allow for an optimization of the system protocol to reduce radiation dose and optimize image quality by tailoring the filtering, hardness and contrast.
When there is no access to a hybrid OR with a fixed CBCT-AF system, a possible alternative solutions might be the integration of mobile 3D imaging systems or tomosynthesis-based software programs. Similar to CBCT, these systems allow intraprocedural determination of lesion-to-instrument positioning. Mobile 3D-imaging systems can—as with CBCT—additionally be used at different locations and across specialities (24). A major limitation is the lower imaging quality obtained with these systems due to system power/sensitivity and resulting higher scan time. Latest technologies have already improved image distortion, spatial resolution and noise of mobile 3D-imaging systems (67), but image quality is still not comparable to CBCT imaging. Images are considered of less quality due to poorer contrast-to-noise ratio (CNR) and therewith also a lesser ability for soft tissue imaging. When looking at the average radiation dose of mobile 3D imaging systems, the currently reported average radiation dose (DAP 37.1–50.3 Gy∙cm2) is comparable or exceeds the radiation dose of CBCT (24,32). This might however decrease over time, as learning curves and radiation safety improve. Another barrier in mobile system application is the lacking software tools for outlining the lesion and navigation trajectory intra-procedurally as well as the overlay during navigation, as are available in CBCT systems. Furthermore, because of longer scan times also longer inspiratory breath-holds are required. Currently, one rotation of the gantry takes 30 seconds (CIOS 3D Spin Mobile, Siemens Healthineers), compared to 3–8 seconds with routine CBCT systems. This might however be a smaller problem in procedures under general anaesthesia with inspiratory breath-hold during scanning. Alternatively to dedicated 3D mobile C-arms, tomosynthesis-based software algorithms (i.e., the Lung Vision platform) used in conjunction with existing mobile 2D C-arms can be used. Emerging robotic innovations are also being developed to integrate these tomosynthesis-based imaging techniques (68). Major advantage of this technology is the lower cost and higher availability of C-arms (31). However, the low CNR of the C-arm based tomography produces images of lower quality than conventional (CB)CT, which could remain a major disadvantage. This reduced image quality in both mobile 3D-imaging and tomosynthesis-based software systems can be problematic in cases with small lesions, ground glass opacities, atelectasis and local bleeding. Small GGO’s will be barely visible on 3D-images produced by a mobile C-arm, while they will be more easily visualized in CBCT imaging (31). Both mobile 3D-imaging and tomosynthesis-based software systems also experience smaller 3D volume recordings that require extra attention on focusing the centre of the C-arm rotation on the lung nodule. It also remains to be seen if the lower image quality is sufficient enough to be utilized in endobronchial treatments such as microwave ablation (31).
Future perspectives
As physicians we tend to rely on decade’s old data, but technology is constantly updated and improved. The outcomes and usability of technology are valid for a limited time. For example, commercially available EMN systems have different technological features across systems and have now been integrated with mobile c-arms for generating tomosynthesis images to compensate for CT-to-body divergence. Whereas we tend to look and discuss a specific technique such as EMN, we are increasingly moving to multi-modality approaches and integrations where one specific system might have a significantly different approach than another.
There is a growing body of literature on navigation bronchoscopy for lung biopsy, but one modality does not seem to achieve the same diagnostic accuracy in every study. At current, we have to acknowledge it is very difficult to compare technologies head-to-head based on reported study outcomes. Most, if not all—studies have at least some sort of selection bias. Every physician will refer the patient for navigation bronchoscopy using slightly different inclusion criteria. To date, for small peripheral pulmonary nodules, the highest results in terms of diagnostic accuracy (defined as the total of true positive and true negative results with adequately long follow-up time for non-malignant lesions, divided by the total number of procedures) is CBCT-AF. Robotic assisted bronchoscopy is runner up, but also here, position confirmation seems to become an integral part to meet the high standards of diagnostic yield/accuracy we want to achieve. No single device seems to perform best and a multi-modality approach combined with experience seems to be the only option for achieving a diagnostic accuracy of >85% at current.
In experienced centers, coarse navigation guidance seems of lesser concern and fine positioning and optimal tissue sampling are the biggest problems to be overcome. One should therefore look beyond the reported diagnostic yields when deciding on choosing for a specific technology. Not only does the definition seem different per report, it is also highly affected by inclusion criteria such as size and bronchus sign. In a multi-modality diagnostic approach, we should look for techniques that are most complementary and within cost-effective range. When considering moving to locally applied, minimal invasive therapies, it will become essential to integrate detailed imaging into the workflow.
The WHO predicts the global lung cancer incidence to double in the next 20 years, and with screening programs being implemented, the demand for minimal invasive, safe biopsy procedures will likely increase exponentially. These patients, when diagnosed with early-stage lung cancer, will most likely be referred for minimal invasive treatment options. To improve diagnostic and treatment procedures, this could even be directed into a one-stop-shop setting. However, when considering local curative treatment, we must address potential local spread in order to make curation feasible. Current combined EBUS/EUS staging procedures cannot evaluate any lymph nodes beyond station 12, while in reality the pathologist will routinely report finding additional lymph nodes in the further peripherals. In breast cancer and melanoma, this is currently tackled by performing a sentinel lymph node (SLN) procedure, where these lymph nodes are imaged, biopsied and pathologically assessed as part of routine workup. In lung cancer we have recently seen that more than 23.1% of clinical N0 patients are upstaged to N1 or N2 disease after surgical resection and pathological evaluation (69). SLN evaluation in lung cancer has been attempted over the last decades, but has shown difficult to implement in routine clinical practice (70,71). Further development in this field will be necessary to help improve survival of early-stage lung cancer.
When looking at local treatment options, microwave ablation seems to be most advanced in its scientific evaluation and is therefore subject of a dedicated manuscript in this series. For current and future treatment options, high-resolution 3D-imaging for precise positioning seems essential and at current CBCT is the best available technology in this area.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Calvin S. H. Ng) for the series “Lung Cancer Management—The Next Decade” published in Annals of Translational Medicine. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2845/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2845/coif). The series “Lung Cancer Management—The Next Decade” was commissioned by the editorial office without any funding or sponsorship. RLJV reports that his institution has received unrestricted funding from Philips, AstraZeneca, Johnson & Johnson, Siemens, Pentax, Galvanize Therapeutics, and Bioncise. Besides, his institution has received funding for consultancy from Johnson & Johnson, compensation for lecturing at educational events from Medtronic. He and his institution have patents planned, issued and pending. His institution has obtained compensation for travel fees for attending meetings from Pentax. He’s an unpaid board member of the Dutch Society of Technical Physicians. SEPK reports that his institution has received unrestricted research funding from Philips, AstraZeneca, Johnson & Johnson, Pentax, and Siemens. Besides, his institution has received fees for consultancy from Johnson & Johnson. INW and DKMW report that the institution has received research funding from Philips, AstraZeneca, Johnson & Johnson, and Pentax. EHFMH reports that his institution has received unrestricted research funding from Philips, AstraZeneca, Johnson&Johnson, Pentax, Galvanize Therapeutics, and Bioncise. His institution has received fees for consultancy from Johnson & Johnson and Philips, speaker’s fees from Janssen-Cilag and Pentax, and travel support from Pentax. He and his institution have patents planned, issued and pending. He’s an unpaid board member of the WABIP and EABIP. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-ii54. Correction appears in Thorax 2015;70:1188.
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Louie AV, Senan S, Patel P, et al. When is a biopsy-proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer?: A decision analysis. Chest 2014;146:1021-8. [Crossref] [PubMed]
- IJsseldijk MA, Shoni M, Siegert C, et al. Survival After Stereotactic Body Radiation Therapy for Clinically Diagnosed or Biopsy-Proven Early-Stage NSCLC: A Systematic Review and Meta-Analysis. J Thorac Oncol 2019;14:583-95. [Crossref] [PubMed]
- Flores R, Bauer T, Aye R, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619-26. [Crossref] [PubMed]
- Kuo E, Bharat A, Bontumasi N, et al. Impact of video-assisted thoracoscopic surgery on benign resections for solitary pulmonary nodules. Ann Thorac Surg 2012;93:266-72; discussion 272-3. [Crossref] [PubMed]
- Petersen RH, Hansen HJ, Dirksen A, et al. Lung cancer screening and video-assisted thoracic surgery. J Thorac Oncol 2012;7:1026-31. [Crossref] [PubMed]
- Cardillo G, Regal M, Sera F, et al. Videothoracoscopic management of the solitary pulmonary nodule: a single-institution study on 429 cases. Ann Thorac Surg 2003;75:1607-11; discussion 1611-2. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Hong H, Hahn S, Matsuguma H, et al. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis. Thorax 2021;76:582-90. [Crossref] [PubMed]
- Moon SM, Lee DG, Hwang NY, et al. Ipsilateral pleural recurrence after diagnostic transthoracic needle biopsy in pathological stage I lung cancer patients who underwent curative resection. Lung Cancer 2017;111:69-74. [Crossref] [PubMed]
- van 't Westeinde SC, Horeweg N, Vernhout RM, et al. The role of conventional bronchoscopy in the workup of suspicious CT scan screen-detected pulmonary nodules. Chest 2012;142:377-84. [Crossref] [PubMed]
- Herth F, Becker HD, Manegold C, et al. Endobronchial ultrasound (EBUS)--assessment of a new diagnostic tool in bronchoscopy for staging of lung cancer. Onkologie 2001;24:151-4. [PubMed]
- Wang Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011;140:1550-6. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCNN guidelines for Treatment of Non-Small Cell Lung Cancer. J Natl Compr Cancer Netw. 2016. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- Sun J, Criner GJ, Dibardino D, et al. Efficacy and safety of virtual bronchoscopic navigation with fused fluoroscopy and vessel mapping for access of pulmonary lesions. Respirology 2022;27:357-65. [Crossref] [PubMed]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021;159:845-52. [Crossref] [PubMed]
- Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. [Crossref] [PubMed]
- Kalchiem-Dekel O, Connolly JG, Lin IH, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022;161:572-82. [Crossref] [PubMed]
- Simoff MJ, Pritchett MA, Reisenauer JS, et al. Shape-sensing robotic-assisted bronchoscopy for pulmonary nodules: initial multicenter experience using the IonTM Endoluminal System. BMC Pulm Med 2021;21:322. [Crossref] [PubMed]
- Noahmed.com. The Galaxy robotic system [Internet]. Available online: https://www.noahmed.com/
- Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018;10:6950-9. [Crossref] [PubMed]
- Pritchett M, Radaelli A, Schampaert S, et al. Cone Beam CT-Guided Endobronchial Biopsy Assisted by Augmented Fluoroscopy. Chest 2017;152:A887. [Crossref]
- Verhoeven RLJ, van der Sterren W, Kong W, et al. Cone-beam CT and Augmented Fluoroscopy-guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J Bronchology Interv Pulmonol 2021;28:262-71. [Crossref] [PubMed]
- Kheir F, Thakore SR, Uribe Becerra JP, et al. Cone-Beam Computed Tomography-Guided Electromagnetic Navigation for Peripheral Lung Nodules. Respiration 2021;100:44-51. [Crossref] [PubMed]
- Chan JWY, Lau RWH, Chu CM, et al. Expanding the scope of electromagnetic navigation bronchoscopy-guided transbronchial biopsy and ablation with mobile 3D C-arm Machine Cios Spin®-feasibility and challenges. Transl Lung Cancer Res 2021;10:4043-6. [Crossref] [PubMed]
- Reisenauer J, Duke JD, Kern R, et al. Combining Shape-Sensing Robotic Bronchoscopy With Mobile Three-Dimensional Imaging to Verify Tool-in-Lesion and Overcome Divergence: A Pilot Study. Mayo Clin Proc Innov Qual Outcomes 2022;6:177-85. [Crossref] [PubMed]
- Cicenia J, Bhadra K, Sethi S, et al. Augmented Fluoroscopy: A New and Novel Navigation Platform for Peripheral Bronchoscopy. J Bronchology Interv Pulmonol 2021;28:116-23. [Crossref] [PubMed]
- Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020;25:206-13. [Crossref] [PubMed]
- Oki M, Saka H, Asano F, et al. Use of an Ultrathin vs Thin Bronchoscope for Peripheral Pulmonary Lesions: A Randomized Trial. Chest 2019;156:954-64. [Crossref] [PubMed]
- Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011;66:1072-7. [Crossref] [PubMed]
- Gildea TR. Lung Lesion Localization and the Diagnostic Drop. Ann Am Thorac Soc 2016;13:1450-2. [Crossref] [PubMed]
- Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020;157:694-701. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Folch EE, Labarca G, Ospina-Delgado D, et al. Sensitivity and Safety of Electromagnetic Navigation Bronchoscopy for Lung Cancer Diagnosis: Systematic Review and Meta-analysis. Chest 2020;158:1753-69. [Crossref] [PubMed]
- Folch EE, Bowling MR, Pritchett MA, et al. NAVIGATE 24-Month Results: Electromagnetic Navigation Bronchoscopy for Pulmonary Lesions at 37 Centers in Europe and the United States. J Thorac Oncol 2022;17:519-31. [Crossref] [PubMed]
- Pritchett MA, Bhadra K, Calcutt M, et al. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis 2020;12:1595-1611. Correction appears in J Thorac Dis 2020;12:4593-4595.
- Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015;147:1275-81. [Crossref] [PubMed]
- Furukawa BS, Pastis NJ, Tanner NT, et al. Comparing Pulmonary Nodule Location During Electromagnetic Bronchoscopy With Predicted Location on the Basis of Two Virtual Airway Maps at Different Phases of Respiration. Chest 2018;153:181-6. [Crossref] [PubMed]
- Verhoeven RLJ, Fütterer JJ, Hoefsloot W, et al. Cone-Beam CT Image Guidance With and Without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:60-9. [Crossref] [PubMed]
- Sagar AS, Sabath BF, Eapen GA, et al. Incidence and Location of Atelectasis Developed During Bronchoscopy Under General Anesthesia: The I-LOCATE Trial. Chest 2020;158:2658-66. [Crossref] [PubMed]
- Bhadra K, Setser RM, Condra W, et al. Lung Navigation Ventilation Protocol to Optimize Biopsy of Peripheral Lung Lesions. J Bronchology Interv Pulmonol 2022;29:7-17. [Crossref] [PubMed]
- White BM, Santhanam A, Thomas D, et al. Modeling and incorporating cardiac-induced lung tissue motion in a breathing motion model. Med Phys 2014;41:043501. [Crossref] [PubMed]
- Veran versus Superdimension; a direct comparison [Internet]. Available online: https://www.veranmedical.com/spin-system/comparison/
- Sobieszczyk MJ, Yuan Z, Li W, et al. Biopsy of peripheral lung nodules utilizing cone beam computer tomography with and without trans bronchial access tool: a retrospective analysis. J Thorac Dis 2018;10:5953-9. [Crossref] [PubMed]
- Pritchett MA. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2021;28:107-15. [Crossref] [PubMed]
- The Monarch robotic endoscopy platform. Available online: https://www.aurishealth.com/monarch-platform.
- The Ion robotic endoluminal platform for peripheral pulmonary lesions biopsy. Available online: https://www.intuitive.com/en-us/products-and-services/ion.
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Murgu SD. Robotic assisted-bronchoscopy: technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm Med 2019;19:89. [Crossref] [PubMed]
- Ali MS, Sethi J, Taneja A, et al. Computed Tomography Bronchus Sign and the Diagnostic Yield of Guided Bronchoscopy for Peripheral Pulmonary Lesions. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2018;15:978-87. [Crossref] [PubMed]
- Nakai T, Matsumoto Y, Suzuk F, et al. Predictive factors for a successful diagnostic bronchoscopy of ground-glass nodules. Ann Thorac Med 2017;12:171-6. [Crossref] [PubMed]
- Ikezawa Y, Sukoh N, Shinagawa N, et al. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014;88:137-43. [Crossref] [PubMed]
- Ikezawa Y, Shinagawa N, Sukoh N, et al. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann Thorac Surg 2017;103:470-5. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Verhoeven RLJ, Vos S, van der Heijden EHFM. Multi-modal tissue sampling in cone beam CT guided navigation bronchoscopy: comparative accuracy of different sampling tools and rapid on-site evaluation of cytopathology. J Thorac Dis 2021;13:4396-406. [Crossref] [PubMed]
- Fielding D, Oki M. Technologies for targeting the peripheral pulmonary nodule including robotics. Respirology 2020;25:914-23. [Crossref] [PubMed]
- de Ruiter QMB, Fontana JR, Pritchard WF, et al. Endovascular steerable and endobronchial precurved guiding sheaths for transbronchial needle delivery under augmented fluoroscopy and cone beam CT image guidance. Transl Lung Cancer Res 2021;10:3627-44. [Crossref] [PubMed]
- Coghlin CL, Smith LJ, Bakar S, et al. Quantitative analysis of tumor in bronchial biopsy specimens. J Thorac Oncol 2010;5:448-52. [Crossref] [PubMed]
- Gildea TR, Folch EE, Khandhar SJ, et al. The Impact of Biopsy Tool Choice and Rapid On-Site Evaluation on Diagnostic Accuracy for Malignant Lesions in the Prospective: Multicenter NAVIGATE Study. J Bronchology Interv Pulmonol 2021;28:174-83. [Crossref] [PubMed]
- Casal RF. Cone Beam CT-Guided Bronchoscopy: Here to Stay?. J Bronchology Interv Pulmonol 2018;25:255-6. [Crossref] [PubMed]
- Sheth NM, De Silva T, Uneri A, et al. A mobile isocentric C-arm for intraoperative cone-beam CT: Technical assessment of dose and 3D imaging performance. Med Phys 2020;47:958-74. [Crossref] [PubMed]
- Hogarth, K, Cicenia, J, Bhadra K. Endoluminal Robotics & Lung Navigation, Where Do We Go From Here? Available online: https://sabronchoscopy.org/endoluminal-robotics-lung-navigation/
- Beyaz F, Verhoeven RLJ, Schuurbiers OCJ, et al. Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 2020;150:186-94. [Crossref] [PubMed]
- Sun WYL, Dang JT, Modasi A, et al. Diagnostic accuracy of sentinel lymph node biopsy using indocyanine green in lung cancer: a systematic review and meta-analysis. Gen Thorac Cardiovasc Surg 2020;68:905-13. [Crossref] [PubMed]
- Gregor A, Ujiie H, Yasufuku K. Sentinel lymph node biopsy for lung cancer. Gen Thorac Cardiovasc Surg 2020;68:1061-78. [Crossref] [PubMed]


