
Effect of recombinant human brain natriuretic peptide on acute kidney injury after coronary artery bypass grafting: a retrospective comparative cohort study
Introduction
With an incidence of 20–30%, acute kidney injury (AKI) is one of the most frequent complications of cardiac surgery (1-3). Postoperative AKI can adversely affect patient outcomes in terms of increased short- and long-term mortality, prolonged intensive care unit (ICU) and hospital stays, and increased costs (4-7). It can also increase the risk of dialysis and end-stage renal disease (8,9). Thus, the prevention and treatment of AKI following coronary artery bypass grafting (CABG) are of great significance to the short-term and long-term prognosis of patients.
Recombinant human brain natriuretic peptide (rh-BNP) is a hormone produced using deoxyribonucleic acid recombination technology that has the same chemical structure composition and biological activity as endogenous BNP. It can induce diuresis, dilate blood vessels, inhibit the renin-angiotensin-aldosterone system (RAAS), reduce aldosterone secretion, resist myocardial hypertrophy and myocardial fibrosis, and affect the neuroendocrine system and secretion of inflammatory cytokines (10-13). Human atrial natriuretic peptide and human brain natriuretic peptide belong to the Natriuretic Peptides (NPs) family. Some studies have shown that human atrial natriuretic peptide (ANP), which has similar pharmacological effects with BNP, has achieved good clinical results in improving cardiopulmonary function and reducing acute renal injury after cardiac surgery (14-16). However, to date few studies have reported on the effect of BNP in AKI patients after cardiac surgery. This study sought to evaluate the efficacy of rh-BNP on renal function after CABG. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3727/rc).
Methods
Study design and population
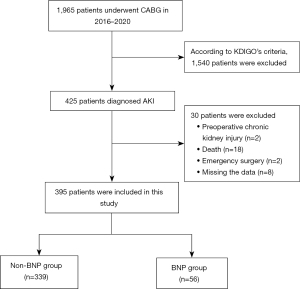
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have undergone a CABG and had postoperative AKI at The Affiliated Hospital of Qingdao University from 2016 to 2020; and (II) be aged >18 years. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a preoperative chronic renal injury; (II) had undergone intraoperative combined valve replacement (molding), macrovascular surgery, or re-exploration after a secondary operation; (III) had undergone an emergency surgery (i.e., surgical treatment within 24 hours of admission); (IV) had died within 7 days of surgery; and/or (V) had missing data. Based on the criteria, a total of 395 patients were included in this study (56 in the BNP group and 339 in the non-BNP group). Rh-BNP was pumped intravenously in patients to observe the trend of serum creatinine levels and changes in urine volume. The screening process is illustrated in Figure 1.
Use of rh-BNP and diuretics
All the patients underwent isolated CABG. Each patient was transferred to the ICU following the operation, after which the physicians administered rh-BNP according to each patient’s specific situation and experience. The intravenous pumping dose was 0.0075–0.01 µg/(kg·min), and only patients who received intravenous pumping of rh-BNP within 4 days of surgery were included in the study. Diuretics were routinely used when patients develop AKI after CABG, and the amount of diuretics was determined based on each patient’s postoperative urine volume and the physicians’ experience.
Data collection
Clinical characteristics, demographic data, preoperative, and operative data were extracted from the database for each patient at The Affiliated Hospital of Qingdao University. All the laboratory data were the last recorded results before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is a single-center retrospective study, and the study protocol was reviewed and approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (No. QYFY WZLL 27256). The requirement of informed consent from participants was waived.
Definition of indicators
This study included a number of indicators. The first indicator was acute renal failure (or AKI), for which the Kidney Disease Improving Global Outcomes diagnostic criteria (17) were adopted. Under the KDIGO criteria, AKI was diagnosed if: (I) the creatinine value within 48 h of surgery increased to 1.3 times that of the baseline value or >26.5 µmol/L; (II) the creatinine value within 7 days of surgery was >1.5 times that of the baseline value; or (III) the urine volume within 6 h of surgery was <0.5 mL/kg/hour. The second indicator was a decrease in creatinine levels, which was defined as: (I) a decrease in postoperative serum creatinine levels that peaked within 4 days of surgery (i.e., peak creatinine − minimum creatinine after peak)/peak creatinine); (II) continuously elevated creatinine levels within 4 days of surgery (i.e., baseline creatinine value − peak creatinine)/peak creatinine; (III) a urine volume that equaled the total urine volume over 4 days after surgery; or (IV) a dosage of diuretics that equaled the total amount used within 4 days of surgery.
Statistical analysis
R4.1 and SPSS 26.0, were used for the data analysis. The continuous variables are expressed as the mean (standard deviation) or median (interquartile range) and were compared using the t-test or Mann-Whitney U test. The classification data are described by proportional counting, and the χ2 test was used for the analysis. Logistic regression was adopted to reduce baseline information differences between the two groups to adjust the confounding effects. The associations between collected variables and rh-BNP were assessed with univariate logistic regression analysis, and variables with a P value <0.05 were entered into a multivariate regression model. Propensity score matching (PSM) was used to perform a sensitivity analysis. Since the use of rh-BNP is related to a patient’s condition after surgery, the variables of the postoperative use of inotropic drugs were included in the PSM to balance the two groups. The control group was selected at a ratio of 1:4 according to the PSM method. When matching, the caliper value was 0.05, and the nearest neighbor method was used. R4.1 was used to verify the matched cohort. A P value <0.05 was considered statistically significant.
Results
A total of 1,965 patients underwent CABG at our hospital between 2016 and 2020, and 425 of those patients developed AKI. Based on the exclusion criteria, 395 patients were included in this study (56 in the BNP group and 339 in the non-BNP group) (see Figure 1). The preoperative, perioperative and postoperative characteristics of patients are presented in Table 1 and Table 2. The preoperative left ventricular ejection fraction (LVEF), usage rate of preoperative angiotensin converting enzyme inhibitors (ACEI), dosages of postoperative adrenaline, perioperative intra-aortic balloon pumps (IABP), decreasing trend of serum creatinine levels and postoperative urine volume were statistically significant difference between BNP group and non-BNP group (P<0.05). All of the characteristics with clinical significance were included in the multivariate analysis for predicting independent correlations of rh-BNP. Multivariable logistic regression showed that the decreasing trend of serum creatinine levels (OR =15.209, 95% CI: 4.692–49.294, P<0.001) and postoperative urine volume (OR =1.0002, 95% CI: 1.0002–1.0003, P=0.001) were independently associated with rh-BNP. Besides, patients in BNP group tend to had lower value of LVEF (P=0.003), more usage rate of ACEI (P=0.005), more perioperative intra-aortic balloon pumps (P=0.009) than non-BNP group (Table 3).
Table 1
| Characteristics | BNP (N=56) | Non-BNP (N=339) | Overall (N=395) | P value |
|---|---|---|---|---|
| Age (years) | 66 (9.2) | 63.8 (8.7) | 64.1 (8.8) | 0.085 |
| Male, n (%) | 43 (76.8) | 238 (70.2) | 281 (71.1) | 0.316 |
| BMI (kg/m2) | 25.7 (3.8) | 25.8 (3.4) | 25.8 (3.5) | 0.297 |
| Smoking, n (%) | 30 (53.6) | 141 (41.6) | 171 (43.3) | 0.096 |
| Drinking, n (%) | 21 (37.5) | 78 (23.0) | 99 (22.5) | 0.058 |
| Hypertension, n (%) | 41 (73.2) | 244 (72.0) | 285 (72.2) | 0.848 |
| Diabetes mellitus, n (%) | 21 (37.5) | 142 (41.9) | 163(41.3) | 0.537 |
| LVEF (%) | 56 [48, 60] | 60 [56, 61] | 56.3 [55, 61] | 0.001 |
| PVD, n (%) | 0 (0) | 8 (2.4) | 8 (2.0) | 0.999 |
| COPD, n (%) | 2 (3.6) | 4 (1.2) | 6 (1.5) | 0.197 |
| HF, n (%) | 4 (7.1) | 14 (4.1) | 18 (4.6) | 0.323 |
| ACS, n (%) | 54 (96.4) | 332 (97.9) | 386 (97.7) | 0.489 |
| Coronary stent, n (%) | 4 (7.1) | 31 (9.1) | 35 (8.9) | 0.626 |
| Stroke, n (%) | 8 (14.3) | 28 (8.3) | 0.152 | |
| OMI, n (%) | 4 (7.1) | 19 (5.6) | 23 (5.8) | 0.65 |
| Preoperative blood results | ||||
| Scr (μmol/L) | 65 [55, 82] | 57 [73, 91] | 71 [57, 90] | 0.438 |
| LDLC (mmol/L) | 2.7 (1.04) | 2.6 (0.9) | 2.6 (0.92) | 0.283 |
| Triglycerides (mmol/L) | 1.4 [1, 2.35] | 1.4 [1.1, 1.9] | 1.4 [1.1, 2] | 0.349 |
| Uric acid (mmol/L) | 331.1 (91.7) | 346.6 (106.0) | 347 (104.2) | 0.219 |
| Hemoglobin (g/L) | 130 [123, 143] | 132 [120, 144] | 132 [120, 144] | 0.752 |
| Platelet (109/L) | 222 [189, 249] | 216 [177, 260] | 216 [179, 258] | 0.854 |
| Preoperative medication, n (%) | ||||
| ACEI | 19 (33.9) | 64 (18.9) | 83 (21) | 0.012 |
| ARB | 17 (30.4) | 126 (37.2) | 143 (36.2) | 0.327 |
| CCB | 18 (32.1) | 120 (35.4) | 138 (34.9) | 0.636 |
| BRB | 42 (75.0) | 240 (70.8) | 282 (71.4) | 0.52 |
| Statin | 43 (76.8) | 258 (76.1) | 301 (76.2) | 0.912 |
| Diuretics | 25 (44.6) | 148 (43.7) | 173 (43.8) | 0.891 |
| Inotropic drugs | 7 (12.5) | 32 (9.4) | 39 (9.9) | 0.478 |
The data are shown as n (%) or as mean (standard deviation) or as median [interquartile range; 25th–75th percentile]. rh-BNP, recombinant human brain natriuretic peptide; CABG, coronary artery bypass grafting; BMI, body mass index; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; ACS, acute coronary syndrome; OMI, old myocardial infarction; Scr, serum creatinine; LDLC, low-density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blockers; BRB, β receptor blocker; BNP, brain natriuretic peptide.
Table 2
| Characteristics | BNP (N=56) | Non-BNP (N=339) | Overall (N=395) | P value |
|---|---|---|---|---|
| IABP, n (%) | 10 (17.9) | 14 (4.1) | 24 (6.1) | <0.001 |
| Operative time (min) | 281.5 (73.5) | 267.2 (83.0) | 269 (81.8) | 0.226 |
| CPB, n (%) | 2 (3.6) | 22 (6.5) | 24 (6.1) | 0.404 |
| Postoperative adrenaline, n (%) | 14 (25.0) | 33 (9.7) | 47 (11.7) | 0.002 |
| Postoperative noradrenaline, n (%) | 55 (98.2) | 314 (92.6) | 369 (93.4) | 0.152 |
| Postoperative dopamine, n (%) | 56 (100.0) | 296 (87.3) | 354 (89.1) | 0.997 |
| Decreasing trend of Scr (%) | 0.02 (0.28) | –0.16 (0.27) | –0.14 (0.28) | <0.001 |
| Urine volume (L) | 11.3 (2.87) | 9.47 (2.96) | 9.73 (3.02) | <0.001 |
| Postoperative diuretic (mg) | 60 [40, 80] | 60 [40, 120] | 60 [40, 96.1] | 0.881 |
The data are shown as n (%) or as mean (standard deviation) or as median [interquartile range; 25th–75th percentile]. rh-BNP, recombinant human brain natriuretic peptide; CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; CPB, cardiopulmonary bypass; Scr, serum creatinine.
Table 3
| Characteristics | β-coefficient | OR | 95% CI | P value |
|---|---|---|---|---|
| LVEF (%) | -0.06 | 0.942 | 0.906–0.979 | 0.003 |
| ACEI, n (%) | 1.021 | 2.755 | 1.366–5.635 | 0.005 |
| IABP, n (%) | 1.373 | 3.948 | 1.418–10.991 | 0.009 |
| Postoperative adrenaline, n (%) | 0.543 | 1.721 | 0.748–3.959 | 0.201 |
| Decreasing trend of Scr (%) | 2.722 | 15.209 | 4.692–49.294 | <0.001 |
| Urine volume (L) | 0.0002 | 1.0002 | 1.0002–1.0003 | 0.001 |
rh-BNP, recombinant human brain natriuretic peptide; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitor; IABP, intra-aortic balloon pump; Scr, serum creatinine.
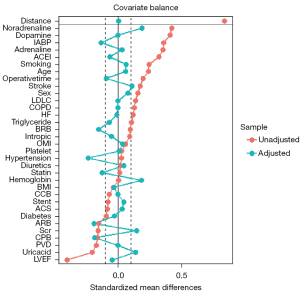
In addition, PSM was applied to have sensitivity analysis (Table 4,5). Before PSM, the preoperative left ventricular ejection fraction of the BNP group was lower than that of the non-BNP group {56 [48, 60] vs. 60 [56, 61], P=0.001}. The dosages of postoperative adrenaline and noradrenaline were higher in the BNP group than the non-BNP group (25% vs. 9.7%, P=0.001 and 98.2% vs. 92.6%, P=0.005, respectively). Additionally, the history of drinking and the usage rate of preoperative angiotensin converting enzyme inhibitors and perioperative intra-aortic balloon pumps were higher in the BNP group than the non-BNP group (33.9% vs. 18.9%, P=0.011; 37.5% vs. 23%, P=0.021; 17.9% vs. 4.1%, P<0.001). The other clinical characteristics or demographic data did not differ significantly. After PSM, the differences in the variables became insignificant.
Table 4
| Characteristics | Patients with AKI before PSM (N=395) | Patients with AKI after PSM (N=175) | |||||
|---|---|---|---|---|---|---|---|
| BNP (N=56) | Non-BNP (N=339) | P value | BNP (N=44) | Non-BNP (N=131) | P value | ||
| Patient characteristics | |||||||
| Age (years) | 66 (9.2) | 63.8 (8.7) | 0.084 | 65.6 (9.2) | 64.3 (7.8) | 0.344 | |
| Male, n (%) | 43 (76.8) | 238 (70.2) | 0.315 | 32 (72.7) | 90 (68.7) | 0.616 | |
| BMI (kg/m2) | 25.7 (3.8) | 25.8 (3.4) | 0.784 | 25.3 (3.7) | 25.6 (3.5) | 0.493 | |
| Smoking, n (%) | 30 (53.6) | 141 (41.6) | 0.094 | 23 (52.3) | 60 (45.8) | 0.458 | |
| Drinking, n (%) | 21 (37.5) | 78 (23.0) | 0.021 | 12 (27.3) | 36 (27.5) | 0.979 | |
| Hypertension, n (%) | 41 (73.2) | 244 (72.0) | 0.848 | 32 (72.7) | 105 (80.2) | 0.303 | |
| Diabetes mellitus, n (%) | 21 (37.5) | 142 (41.9) | 0.537 | 20 (45.5) | 62 (47.3) | 0.83 | |
| LVEF (%) | 56 [48, 60] | 60 [56, 61] | 0.001 | 58 [51, 60] | 58 [55, 60] | 0.27 | |
| PVD, n (%) | 0 (0) | 8 (2.4) | 0.246 | 0 (0) | 0 (0) | – | |
| COPD, n (%) | 2 (3.6) | 4 (1.2) | 0.176 | 1 (2.3) | 1 (0.8) | 0.416 | |
| HF, n (%) | 4 (7.1) | 14 (4.1) | 0.317 | 2 (4.5) | 7 (5.3) | 0.836 | |
| ACS, n (%) | 54 (96.4) | 332 (97.9) | 0.485 | 43 (97.7) | 129 (98.5) | 0.742 | |
| Coronary stent, n (%) | 4 (7.1) | 31 (9.1) | 0.626 | 3 (6.8) | 7 (5.3) | 0.716 | |
| Stroke, n (%) | 8 (14.3) | 28 (8.3) | 0.147 | 5 (11.4) | 10 (7.6) | 0.446 | |
| OMI, n (%) | 4 (7.1) | 19 (5.6) | 0.649 | 3 (6.8) | 9 (6.9) | 0.991 | |
| Preoperative blood results | |||||||
| Scr (μmol/L) | 65 [55, 82] | 57 [73, 91] | 0.085 | 64 [55, 86] | 68 [54, 86] | 0.745 | |
| LDLC (mmol/L) | 2.7 (1.04) | 2.6 (0.9) | 0.284 | 2.7 (0.93) | 2.6 (0.92) | 0.622 | |
| Triglycerides (mmol/L) | 1.4 [1, 2.35] | 1.4 [1.1, 1.9] | 0.649 | 1.39 [1, 2.3] | 1.37 [1.1, 1.9] | 0.83 | |
| Uric acid (mmol/L) | 331.1 (91.7) | 346.6 (106.0) | 0.219 | 334.2 (95.4) | 326.8 (105.0) | 0.68 | |
| Hemoglobin (g/L) | 130 [123, 143] | 132 [120, 144] | 0.992 | 132 [122, 144] | 132 [120, 144] | 0.728 | |
| Platelet (109/L) | 222 [189, 249] | 216 [177, 260] | 0.735 | 230 [196, 255] | 228 [173, 274] | 0.97 | |
| Preoperative medication, n (%) | |||||||
| ACEI | 19 (33.9) | 64 (18.9) | 0.011 | 13 (29.5) | 36 (27.5) | 0.792 | |
| ARB | 17 (30.4) | 126 (37.2) | 0.326 | 13 (29.5) | 51 (38.9) | 0.265 | |
| CCB | 18 (32.1) | 120 (35.4) | 0.636 | 17 (38.6) | 52 (39.7) | 0.901 | |
| BRB | 42 (75.0) | 240 (70.8) | 0.52 | 33 (75.0) | 103 (78.6) | 0.618 | |
| Statin | 43 (76.8) | 258 (76.1) | 0.912 | 33 (75.0) | 107 (81.7) | 0.339 | |
| Diuretics | 25 (44.6) | 148 (43.7) | 0.891 | 19 (43.2) | 54 (1.2) | 0.82 | |
| Inotropic drugs | 7 (12.5) | 32 (9.4) | 0.477 | 5 (11.4) | 16 (12.2) | 0.881 | |
The data are shown as n (%) or as mean (standard deviation) or as median [interquartile range; 25th–75th percentile]. rh-BNP, recombinant human brain natriuretic peptide; BMI, body mass index; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; ACS, acute coronary syndrome; OMI, old myocardial infarction; Scr, serum creatinine; LDLC, low-density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blockers; BRB, β receptor blocker; AKI, acute kidney injury; PSM, propensity score matching; BNP, brain natriuretic peptide.
Table 5
| Characteristics | Patients with AKI before PSM (N=395) | Patients with AKI after PSM (N=175) | |||||
|---|---|---|---|---|---|---|---|
| BNP (N=56) | Non-BNP (N=339) | P value | BNP (N=44) | Non-BNP (N=131) | P value | ||
| IABP, n (%) | 10 (17.9) | 14 (4.1) | <0.001 | 2 (4.5) | 8 (6.1) | 0.7 | |
| Operative time (min) | 281.5 (73.5) | 267.2 (83.0) | 0.226 | 284.3 (76.4) | 275.4 (80.5) | 0.522 | |
| CPB, n (%) | 2 (3.6) | 22 (6.5) | 0.398 | 2 (4.5) | 8 (6.1) | 0.7 | |
| Postoperative adrenaline, n (%) | 14 (25.0) | 33 (9.7) | 0.001 | 8 (18.2) | 15 (11.5) | 0.254 | |
| Postoperative noradrenaline, n (%) | 55 (98.2) | 314 (92.6) | 0.005 | 44 (100.0) | 131 (100.0) | – | |
| Postoperative dopamine, n (%) | 296 (87.3) | 56 (100.0) | 0.119 | 44 (100.0) | 127 (96.9) | 0.242 | |
The data are shown as n (%) or as mean (standard deviation). rh-BNP, recombinant human brain natriuretic peptide; IABP, intra-aortic balloon pump; CPB, cardiopulmonary bypass; AKI, acute kidney injury; PSM, propensity score matching.
A total of 44 and 131 patients were included in the BNP and non-BNP groups, and the data of 12 patients in the BNP group were missing after PSM. Decreasing trends in serum creatinine levels, urine volume, and diuretics usage were the primary outcome indicators within 4 days of CABG. The postoperative serum creatinine of the BNP group decreased by 4% in 4 days, while that of the non-BNP group increased by 16%, and the difference between the two groups was significant (P=0.001). Additionally, within 4 days of surgery, the urine volume of the BNP group was significantly higher than that of the observation group (11.3 vs. 9.11 L, P<0.001), and there was no significant difference in the postoperative diuretics usage between the two groups (P=0.852). Details of the outcomes are presented in Table 6. The study cohort after PSM is shown in Figure 2.
Table 6
| Characteristics | BNP (N=44) | Non-BNP (N=131) | P value |
|---|---|---|---|
| Decreasing trend of Scr (%) | 0.04 (0.28) | –0.16 (0.36) | 0.001 |
| Urine volume (L) | 11.3 (2.80) | 9.11 (2.66) | <0.001 |
| Postoperative diuretic (mg) | 60 [40, 80] | 60 [40, 120] | 0.852 |
The data are shown as mean (standard deviation) or as median [interquartile range; 25th–75th percentile]. rh-BNP, recombinant human brain natriuretic peptide; Scr, serum creatinine.
Discussion
The natriuretic peptide family includes ANP, BNP, and C-type natriuretic peptide (18). Studies have shown that the intravenous injection of recombinant human ANP (rh-ANP) and rh-BNP effectively reduces pulmonary arterial pressure and blood pressure, protects renal function, and decreases the occurrence of arrhythmia (15,19-22). A meta-analysis showed that plasma BNP levels can be used as a diagnostic and predictive factor for pulmonary hypertension, heart failure, myocardial fibrosis, atrial fibrillation, and metabolic diseases (10).
Due to insufficient circulating blood volume during surgery, the activity of humoral factors (e.g., the RAAS system and catecholamines) and the sympathetic nervous system can be enhanced to strengthen the inflammatory response and coagulation function. The kidney contracts its glomerular arterioles to decrease the glomerular filtration rate and reduce urine output, which enables it to supply blood volume to important organs; however, these changes may have adverse effects on the kidneys (23,24). Rh-BNP acts directly on the renal tubules (exhibiting a diuretic effect) to increase urinary sodium excretion, thereby maintaining the electrolyte balance and avoiding renal parenchymal damage caused by high-dose diuretics and may reduce damage to renal function after surgery (25,26).
From clinical reports, it remains unclear whether rh-BNP has a protective function in the kidneys. Several studies have shown that the effect of rh-BNP on renal function is not obvious (27-29). However, a prospective study by Mentzer et al. (30) revealed that the 24-h urine volume of patients in the rh-BNP group was significantly higher than that of patients in the control group. The peak plasma creatinine concentration and absolute decline in glomerular filtration rate in the rh-BNP group decreased significantly within 14 days of hospitalization. Similarly, a meta-analysis showed that the infusion of any peptide increased the urine volume and creatinine clearance or the glomerular filtration rate, and reduced the dose of diuretics and serum creatinine levels (31).
Currently, rh-ANP is widely used in Japan and has effects similar to those of rh-BNP (18,32,33). Some studies have shown that rh-ANP has a positive effect on long-term renal protection. In a randomized controlled trial of 303 patients with chronic kidney disease who underwent CABG, the dialysis-free rate at 1 year was 98.6% in the carperitide group and 91.6% in the placebo group (34). A prospective study in which patients undergoing CABG were divided into two groups (i.e., the intravenous drip of rh-ANP and placebo groups) showed that the non-dialysis rate of the rh-ANP group was significantly higher than that of the placebo group at 1, 5, and 10 years (35). However, Saito et al. (36) found that a low-dose infusion of rh-ANP did not reduce creatinine and increase urine output during hospitalization. However, it should be noted that Saito et al. conducted a retrospective study. To avoid the bias of observation indicators caused by patients’ conditions, numerous creatinine values reveal the efficacy of rh-ANP on renal function better than a single creatinine value. Additionally, the use of diuretics can also affect patients’ urine output during treatment. However, in Saito et al. study, they did not appear to mention this, which may have caused bias and affect the outcome indicators.
Rh-BNP is widely used in patients with acute and chronic heart failure and cardiac insufficiency in China; however, there are relatively few reports on the protective effect of rh-BNP on renal function after CABG. As patients with AKI after bypass surgery were included in this cohort, this experiment was able to observe the prognosis of rh-BNP in patients with postoperative renal dysfunction. Serum creatinine is a significant diagnostic indicator for AKI (37), and has been shown to be positively associated with hospital mortality and lengths of ICU stay, and even a slight increase in serum creatinine can alter patient outcomes (38). Monitoring the dynamic trend of serum creatinine values after CABG better reflects the change in renal function than a single creatinine value index. We found that rh-BNP enhanced the decreasing trend in creatinine levels and improved renal function, which is consistent with the above report.
Additionally, considering that both postoperative diuretics and rh-BNP had an effect on postoperative urine volume, we separately counted the total amount of diuretics in the two groups within 4 days of surgery to avoid bias in the experimental process. The results showed that there was no significant difference in the doses of diuretics administered between the two groups after surgery (P=0.852), and the urine volume of the rh-BNP group was much higher than that of the non-BNP group (P<0.001). Rh-BNP is commonly used clinically in the treatment of patients with heart failure. From this study, we can conclude that intravenous rh-BNP can also increase their urine volume and improve their renal function. These findings may provide clinical guidance for the treatment of patients with cardio-renal syndrome after cardiac surgery.
This study had some limitations. As a retrospective study, it may have had a bias because it was performed at a single center. Thus, future prospective studies are required to further verify our findings. Second, there is still the possibility of insufficient matching information after passing through the PSM to adjust for the difference between the two groups; thus, studies with larger sample sizes and multicenter prospective studies are needed.
Conclusions
Rh-BNP strengthens the rate of creatinine decline and increases urine output in patients with AKI and thus protects their renal function.
Acknowledgments
We would like to thank all the clinical staff involved in this research.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3727/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3727/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3727/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is a single-center retrospective study, and the study protocol was reviewed and approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (No. QYFY WZLL 27256). The requirement of informed consent from participants was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949-64. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800-7. [Crossref] [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
- Rydén L, Ahnve S, Bell M, et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J Cardiol 2014;172:190-5. [Crossref] [PubMed]
- Rydén L, Ahnve S, Bell M, et al. Acute kidney injury following coronary artery bypass grafting: early mortality and postoperative complications. Scand Cardiovasc J 2012;46:114-20. [Crossref] [PubMed]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756-66. [Crossref] [PubMed]
- Sezai A, Nakata K, Iida M, et al. Results of low-dose carperitide infusion in high-risk patients undergoing coronary artery bypass grafting. Ann Thorac Surg 2013;96:119-26. [Crossref] [PubMed]
- Nina VJ, Matias MM, Brito DJ, et al. Acute kidney injury after coronary artery bypass grafting: assessment using RIFLE and AKIN criteria. Rev Bras Cir Cardiovasc 2013;28:231-7. [Crossref] [PubMed]
- Fu HY, Chou NK, Chen YS, et al. Risk factor for acute kidney injury in patients with chronic kidney disease receiving valve surgery with cardiopulmonary bypass. Asian J Surg 2021;44:229-34. [Crossref] [PubMed]
- Goetze JP, Bruneau BG, Ramos HR, et al. Cardiac natriuretic peptides. Nat Rev Cardiol 2020;17:698-717. [Crossref] [PubMed]
- Zhang SM, Zhao HL, Gu XM, et al. A New Chimeric Natriuretic Peptide, CNAAC, for the Treatment of Left Ventricular Dysfunction after Myocardial Infarction. Sci Rep 2017;7:10099. [Crossref] [PubMed]
- Calderone A. Natriuretic peptides and the management of heart failure. Minerva Endocrinol 2004;29:113-27. [PubMed]
- Nogi K, Ueda T, Matsue Y, et al. Effect of carperitide on the 1 year prognosis of patients with acute decompensated heart failure. ESC Heart Fail 2022;9:1061-70. [Crossref] [PubMed]
- Buglioni A, Burnett JC Jr. New Pharmacological Strategies to Increase cGMP. Annu Rev Med 2016;67:229-43. [Crossref] [PubMed]
- Sezai A, Hata M, Niino T, et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J Am Coll Cardiol 2010;55:1844-51. [Crossref] [PubMed]
- McKie PM, Cataliotti A, Huntley BK, et al. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol 2009;54:1024-32. [Crossref] [PubMed]
- Kellum JA, Lameire NKDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 1988;332:78-81. [Crossref] [PubMed]
- Chen HH, Glockner JF, Schirger JA, et al. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol 2012;60:2305-12. [Crossref] [PubMed]
- O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32-43. [Crossref] [PubMed]
- Cataliotti A, Schirger JA, Martin FL, et al. Oral human brain natriuretic peptide activates cyclic guanosine 3',5'-monophosphate and decreases mean arterial pressure. Circulation 2005;112:836-40. [Crossref] [PubMed]
- Soares-da-Silva P, Fernandes MH. Synthesis and metabolism of dopamine in the kidney. Effects of sodium chloride, monoamine oxidase inhibitors and alpha-human atrial natriuretic peptide. Am J Hypertens 1990;3:7S-10S. [Crossref] [PubMed]
- Sezai A, Shiono M. Natriuretic peptides for perioperative management of cardiac surgery. J Cardiol 2016;67:15-21. [Crossref] [PubMed]
- Rahman SN, Kim GE, Mathew AS, et al. Effects of atrial natriuretic peptide in clinical acute renal failure. Kidney Int 1994;45:1731-8. [Crossref] [PubMed]
- He J, Winterstein AG, Beaver TM. Projecting the effect of nesiritide on dialysis and hospital mortality in cardiac surgery patients. Value Health 2010;13:643-8. [Crossref] [PubMed]
- Choi MR, Fernández BE. Protective Renal Effects of Atrial Natriuretic Peptide: Where Are We Now? Front Physiol 2021;12:680213. [Crossref] [PubMed]
- Kristeller JL, Papps H, Stahl RF. Risk of worsening renal function with nesiritide following cardiac surgery. Am J Health Syst Pharm 2006;63:2351-3. [Crossref] [PubMed]
- Ejaz AA, Martin TD, Johnson RJ, et al. Prophylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgery. J Thorac Cardiovasc Surg 2009;138:959-64. [Crossref] [PubMed]
- Beaver TM, Winterstein A, Hess PJ Jr, et al. Nesiritide following maze and mitral valve surgery. J Card Surg 2008;23:431-6. [Crossref] [PubMed]
- Mentzer RM Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol 2007;49:716-26. [Crossref] [PubMed]
- Mitaka C, Kudo T, Haraguchi G, et al. Cardiovascular and renal effects of carperitide and nesiritide in cardiovascular surgery patients: a systematic review and meta-analysis. Crit Care 2011;15:R258. [Crossref] [PubMed]
- Yasoda A, Nakao K. Translational research of C-type natriuretic peptide (CNP) into skeletal dysplasias. Endocr J 2010;57:659-66. [Crossref] [PubMed]
- Saito Y. Roles of atrial natriuretic peptide and its therapeutic use. J Cardiol 2010;56:262-70. [Crossref] [PubMed]
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011;58:897-903. [Crossref] [PubMed]
- Sezai A, Nakata K, Hata M, et al. Long-term results of dialysis patients with chronic kidney disease undergoing coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 2013;19:441-8. [Crossref] [PubMed]
- Saito K, Uchino S, Fujii T, et al. Effect of low-dose atrial natriuretic peptide in critically ill patients with acute kidney injury: a retrospective, single-center study with propensity-score matching. BMC Nephrol 2020;21:31. [Crossref] [PubMed]
- Griffin BR, Bronsert M, Reece TB, et al. Creatinine elevations from baseline at the time of cardiac surgery are associated with postoperative complications. J Thorac Cardiovasc Surg 2022;163:1378-87. [Crossref] [PubMed]
- Hou J, Shang L, Huang S, et al. Postoperative Serum Creatinine Serves as a Prognostic Predictor of Cardiac Surgery Patients. Front Cardiovasc Med 2022;9:740425. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


