
Hsa_circ_0099630 knockdown induces the proliferation and osteogenic differentiation and attenuates the apoptosis of porphyromonas gingivalis lipopolysaccharide–induced human periodontal ligament fibroblasts
Introduction
Periodontitis is a chronic inflammatory disease in which host-mediated inflammation destroys the periodontal support tissue, leading to periodontal and alveolar bone resorption, and even tooth loss (1). Dental calculus, dental plaque, and bacterial infection are the main causative factors in the development of periodontitis (2). The development of periodontitis can cause an inflammatory response around the gums, with localized swelling, bleeding and other pathological changes (3). Thus, the ultimate goal of periodontal therapy is to control the inflammation and achieve the complete regeneration of the periodontal tissue (4). Human periodontal ligament fibroblasts (HPLFs) are a class of periodontal cells with multidirectional differentiation capacity (5). The osteogenic differentiation of HPLFs is the focus of research on periodontal tissue regeneration and is of great importance in periodontal therapy (6). Thus, the identification of molecular targets that promote osteogenic differentiation could provide novel ideas for the treatment of periodontitis.
Circular RNAs (circRNAs) are a special class of non-coding RNAs with a circular structure (7). Approximately 15% of circRNAs are derived from transcripts and most contain exonic sequences (8). Compared to linear RNAs, the circular structure of circRNAs is resistant to digestion by ribonuclease R (9). As a result, circRNAs are less susceptible to degradation and have a more stable structure. CircRNAs are also characterized by a wide distribution, high conservation, and tissue expression specificity (10). CircRNAs have been reported to act as microRNA (miRNA) “sponges” and regulate the expression of the parental genes involved in disease processes, including skin damage, osteoarthritis, tumors, cardiovascular diseases, neurological diseases, and endocrine diseases (11,12). Thus, circRNA has become an ideal diagnostic marker and therapeutic target for diseases with critical clinical applications. Recent studies have also shown that circRNAs participate heavily in multiple processes, such as tissue regeneration, stem cell proliferation, and osteogenic differentiation (13-15). Further, the knockdown of circ_0138959 was shown to attenuate the pyroptosis of HPLFs through the miRNA-527/caspase-5 axis. However, the role of circRNAs in regulating the osteogenic differentiation of HPLFs is currently unclear.
In our preliminary experiment, we found that hsa_circ_0099630 was highly expressed in the gingival tissue of patients with periodontitis by detecting the expression of multiple circRNAs. Based on the circbase database, we discovered that hsa_circ_0099630 is located in chr12:97885421-97924637, its best transcript is NR_024037, and its gene symbol is rhabdomyosarcoma 2 associated transcript (RMST). Additionally, a recent microarray screening study showed that hsa_circ_0099630 was upregulated in chronic sinusitis with nasal polyps (16). Thus, we speculated that hsa_circ_0099630 may be relevant to inflammation and may play a crucial role in periodontitis. Illumina sequencing results had showed that the expression levels of hsa_circ_0099630 were increased in periodontitis tissues compared with control periodontium (17). Recently, the expression of hsa_circ_0099630 was found decreased in inflamed periodontal ligament cells (iPDLCs) compared with healthy PDLCs (hPDLCs), and overexpression hsa_circ_0099630 suppressed iPDLCs proliferation and osteogenic differentiation (18). Thus, the role of hsa_circ_0099630 in periodontitis is confusing.
In our study, we explored the possibility that hsa_circ_0099630 might influence the development progression of periodontitis. We first cultured HPLFs and constructed inflammatory models using porphyromonas gingivalis–lipopolysaccharide (Pg-LPS) based on research (19). We then verified the effects of hsa_circ_0099630 overexpression on the proliferation, apoptosis. and osteogenic differentiation of HPLFs, and the effects of hsa_circ_0099630 silencing on these functions in Pg-LPS-induced HPLFs. We also bioinformatically predicted the miRNA/messenger RNA (mRNA) axis associated with hsa_circ_0099630 and analyzed the possible functions and regulatory pathways of the target genes. Thus, our study provides potential research ideas for periodontal therapy. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4209/rc).
Methods
Tissue samples
Normal periodontal tissues were obtained from the extracted interrupted 3rd molars of 20 healthy patients. Equal amounts of inflammatory periodontal tissues were obtained from 60 patients with periodontitis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of the Hospital of Stomatology, Sun Yat-sen University (No. 2020-07-162). All the participants signed a written informed consent form. The tissues were stored at –80 ℃.
Cell culture
The HPLFs were supplied by Procell (Wuhan, China), and were grown in Dulbecco’s Modified Eagle Medium (Sigma, USA) containing 10% fetal bovine serum (Invitrogen, USA) at 37 ℃ with 5% carbon dioxide.
Cell treatment
The HPLFs were 1st processed with Pg-LPS to build the inflammation model (20). The hsa_circ_0099630 overexpression plasmid (OE-circ), empty vector (OE-CTRL), hsa_circ_0099630 shRNAs (shcirc), and control (shCTRL) were all provided by Integrated Biotech Solutions (Shanghai, China). The HPLFs were inoculated in 6-well plates and cultured for 24 h. Lipofectamine 3000 reagent (Invitrogen, USA) was used to transfect the HPLFs with OE-circ and OE-CTRL, respectively. After 48 h, the transfected cells were harvested, and the transfection efficiency was assessed by real-time (RT)-quantitative polymerase chain reaction (qPCR). Similarly, the Pg-LPS-induced HPLFs were transfected with shcirc and shCTRL, respectively.
RT-qPCR
Total RNAs were separated from the ground periodontal tissues or the treated HPLFs using a TRIzol kit (Invitrogen, USA). After the purity assessment, complementary DNA (cDNA) was generated using the BestarTM RT-qPCR kit (DBI Bioscience, China) with 2 µg of RNA. Next, the RT-PCR was conducted with the SYBR Green qPCR Mix kit (Sparkjade, China). Relative gene expression was counted using the 2-ΔΔCt method.
PCR
The divergent primer and convergent primer were designed based on the sequences of hsa_circ_0099630. The genomic DNA (gDNA) of the HPLFs was extracted in accordance with the instructions and stored at –20 ℃. Next, total RNA was extracted from the HPLFs, reverse transcribed into cDNA, and stored at –20 ℃. Glyceraldehyde 3-phosphate dehydrogenase primers and 2 primers for hsa_circ_0099630 were added to the extracted gDNA and cDNA for PCR amplification. After the products were obtained, agarose gel electrophoresis was performed. The PCR amplification product of hsa_circ_0099630 in the above cDNA was subjected to Sanger sequencing.
Cell Counting Kit-8 (CCK-8)
The HPLFs were treated based on the experiment purpose after being inoculated in 96-well plates for 12 h of incubation. After 48 h of treatment, CCK-8 reagent (Dojindo, Japan) was added to the cells (10 µL per well). After continuing the incubation for 2 h, the absorbance of each well at 450 nm was tested by a microplate reader (Bio-Rad, USA).
Flow cytometry
Groups of HPLFs were collected and washed with pre-cooled phosphate buffered solution (PBS). After centrifugation, the HPLFs were suspended in binding buffer (1×) and the concentration was adjusted to 1×106 cells/mL. The cell suspensions were incubated with 5 µL of AnnexinV-fluorescein isothiocyanate (FITC) for 10 min and 5 µL of propidium iodide (PI) for 5 min. PBS (500 µL) was then added to the HPLFs. FITC (515 nm) and PI (560 nm) were evaluated using flow cytometer (BD Biosciences), and cell apoptosis was analyzed using FlowJo (Version 8.8.6, TreeStar, San Carlos, CA, USA).
Alizarin red staining
The groups of HPLFs were cultured in osteogenic induction medium (conventional medium supplemented with 10 mmol/L of sodium β-glycerophosphate, 0.1 µmol/L of dexamethasone, and 0.05 of mmol/L ascorbyl-2-phosphate) for 2 weeks. After PBS washing, the HPLFs were fixed in 4% paraformaldehyde and stained with 0.1% alizarin red solution at 37 ℃ for 30 min. After washing with doble distilled water (ddH2O), red or purplish red stained calcified nodules were observed microscopically to assess the stromal mineralization capacity of the HPLFs.
Alkaline phosphatase (ALP) activity
The groups of HPLFs were inoculated in 24-well plates at 2×104 cells/well. Based on the reagent instructions, 200 µL of cell lysis solution was added to the HPLFs, and the plates were stored at 4 ℃ overnight. The plates were shaken for 10 min to break up the cells into suspension. The suspensions (30 µL per well) were transferred to a 96-well plate. Next, the cells were treated with 50 µL of substrate solution at 37 ℃ for 15 min, and 150 µL of color developer. The absorbance value was assessed at 520 nm by a microplate reader.
Western blot
The proteins were 1st extracted using radio-immunoprecipitation assay (RIPA) lysis buffer from the treated HPLFs. After quantifying the protein concentration, proteins (5 µg) were mixed with loading buffer, denatured, separated on 12% Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE) gels, and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck, Billerca, MA, USA). After blocking, the bands were incubated with primary antibodies (Abcam) overnight at 4 ℃, and a secondary antibody (1:5,000, Abcam) for 1 h. The bands were the processed with electrochemiluminescence (ECL) reagents (Thermo, Waltham, MA, USA), and the results were observed under Tanon-5200CE (Biotanon, Shanghai, China).
Bioinformatics analysis
The hsa_circ_0099630-miRNA interaction was predicted using the RNAhybrid and miRanda databases. The miRNA-mRNA interaction was predicted using miRDB, TargetScan, and miRTarBase databases.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
Functional annotations of the target genes were identified using the GO (21,22) and KEGG databases (22,23).
Statistical analysis
The data are shown as the mean ± standard deviation. The experiments were performed at least 3 times. The statistical analysis was conducted with SPSS 20.0 (SPSS, Chicago, IL) with the Student’s t-test (for 2 group comparisons) or a 1-way analysis of variance (for multiple group comparisons). A P value <0.05 was considered significant.
Results
Identification and expression of hsa_circ_0099630 in the gingival tissue of patients with periodontitis
To identify hsa_circ_0099630, we performed PCR and Sanger sequencing to confirm the back-splicing junction of hsa_circ_0099630. As Figure 1A shows, we discovered that the divergent primers amplified hsa_circ_009963 in the cDNA but not the gDNA, suggesting the stable existence of hsa_circ_0099630. Through sequencing, we found that the splice site of hsa_circ_0099630 was CAGTAAGAAGGAAACCGGC, which was consistent with the database (see Figure 1B). We also found that hsa_circ_0099630 was highly expressed in the gingival tissues of the periodontitis patients relative to that in the normal tissues of the healthy controls (see Figure 1C). Further, we discovered that hsa_circ_0099630 expression was associated with the order of severity and grade of periodontitis in 60 cases of periodontitis patients (see Table 1). Thus, we revealed that hsa_circ_0099630 was upregulated in patients with periodontitis.
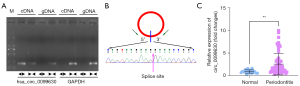
Table 1
| Clinicopathologic characteristics | N | Low expression | High expression | P value |
|---|---|---|---|---|
| Age (years) | 0.1762 | |||
| <60 | 28 | 18 | 10 | |
| ≥60 | 32 | 15 | 17 | |
| Sex | 0.1896 | |||
| Male | 28 | 17 | 11 | |
| Female | 32 | 14 | 18 | |
| BMI (kg/m2) | 0.4951 | |||
| Normal, 18.5–24.99 | 15 | 7 | 8 | |
| Overweight, 25–29.99 | 23 | 11 | 12 | |
| Obesity I, 30–34.99 | 22 | 8 | 14 | |
| Order of severity | 0.0022 | |||
| Slight | 22 | 14 | 8 | |
| Medium | 21 | 5 | 16 | |
| Severe | 17 | 3 | 14 | |
| Grade of periodontitis | 0.0004 | |||
| Level 0 | 21 | 15 | 6 | |
| Level 1 | 25 | 7 | 18 | |
| Level 2 | 14 | 2 | 12 |
Level 0: a healthy periodontium and up to 1 proximal site with loss of attachment ≥3 mm; Level 1: presence of proximal attachment and loss ≥3 mm in ≥2 nonadjacent teeth; and Level 2: presence of proximal attachment loss ≥5 mm in ≥30% of teeth.
Pg-LPS prevents proliferation and osteogenic differentiation, induces apoptosis, and upregulates hsa_circ_0099630 in HPLFs
To further assess the effect of hsa_circ_0099630 on periodontitis, we 1st constructed an inflammation model using Pg-LPS. As Figure 2A shows, the cell viability in the HPLFs was significantly decreased in the Pg-LPS group relative to the control group. Further, the apoptosis of the HPLFs was dramatically enhanced in the Pg-LPS group compared to that of the control group (see Figure 2B). We also discovered that the osteogenic differentiation ability of the HPLFs was markedly diminished after Pg-LPS treatment (see Figure 2C). Additionally, relative to the control group, ALP activity was also notably weakened in the Pg-LPS group (see Figure 2D). The RT-qPCR data showed that Pg-LPS stimulation caused a prominent increase in hsa_circ_0099630 expression in the HPLFs (see Figure 2E). The western blot results showed that RUNX2, Collagen I, osteocalcin, and Osterix expressions were significantly lower in the Pg-LPS group than the control group (see Figure 2F). In general, cell proliferation and osteogenic differentiation were prominently weakened, while apoptosis was enhanced in the Pg-LPS-induced inflammatory model.

Hsa_circ_0099630 silencing accelerates proliferation and attenuates apoptosis of Pg-LPS-induced HPLFs
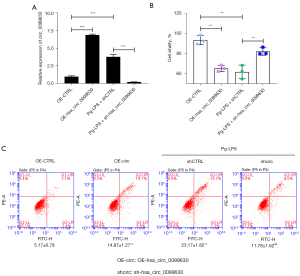
Based on the above results, hsa_circ_0099630 was highly expressed in the Pg-LPS-induced HPLFs. We further overexpressed hsa_circ_0099630 in the HPLFs and silenced hsa_circ_0099630 in the Pg-LPS-induced HPLFs. The RT-qPCR data showed that hsa_circ_0099630 overexpression significantly increased hsa_circ_0099630 expression, and hsa_circ_0099630 silencing significantly decreased hsa_circ_0099630 expression mediated by the. Pg-LPS in HPLFs (see Figure 3A). The CCK-8 results showed that hsa_circ_0099630 overexpression significantly suppressed cell viability in the HPLFs, and hsa_circ_0099630 silencing signally increased cell viability, which were inhibited by the Pg-LPS in the HPLFs (see Figure 3B). We also found that hsa_circ_0099630 overexpression significantly accelerated the apoptosis of HPLFs, and hsa_circ_0099630 silencing notably restrained cell apoptosis, which were induced by Pg-LPS in the HPLFs (see Figure 3C). Overall, we showed that the knockdown of hsa_circ_0099630 induced proliferation and prevented apoptosis in the Pg-LPS-induced HPLFs.
Hsa_circ_0099630 knockdown induces osteogenic differentiation of Pg-LPS-induced HPLFs
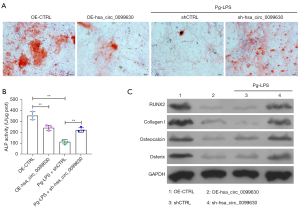
The Alizarin red staining results showed that hsa_circ_0099630 overexpression or Pg-LPS treatment led to a notable decrease in the osteogenic differentiation capacity of HPLFs, and hsa_circ_0099630 silencing caused a notable increase in the osteogenic differentiation capacity, which was attenuated by Pg-LPS (see Figure 4A). Similarly, hsa_circ_0099630 overexpression or Pg-LPS observably reduced ALP activity in the HPLFs, and hsa_circ_0099630 silencing markedly elevated ALP activity in the Pg-LPS-induced HPLFs (see Figure 4B). Additionally, the western blot data showed that hsa_circ_0099630 overexpression or Pg-LPS significantly downregulated RUNX2, Collagen I, osteocalcin, and Osterix in the HPLFs, and hsa_circ_0099630 silencing notably upregulated RUNX2, Collagen I, osteocalcin, and Osterix in the Pg-LPS-induced HPLFs (see Figure 4C). Thus, we further verified that the knockdown of hsa_circ_0099630 also promoted osteogenic differentiation in the Pg-LPS-induced HPLFs.
MiRNA/mRNA axis of hsa_circ_0099630
More importantly, we also investigated the possible miRNA/mRNA axis of hsa_circ_0099630 by a bioinformatics analysis. First, we determined the location of hsa_circ_0099630 in the HPLFs by examining the levels of hsa_circ_0099630 in the cytoplasm and nucleus. Our data showed that hsa_circ_0099630 was mainly expressed in the cytoplasm (see Figure 5A). Second, we explored the potential miRNAs regulated by hsa_circ_0099630. Based on a condition of >2 binding sites to hsa_circ_0099630, we screened 5 eligible miRNAs; that is, miR-182, miR-1200, miR-338, miR-576, and miR-623 (see Figure 5B). Next, we further analyzed the target genes of the 5 miRNAs and obtained the intersection target genes by a Venn diagram. We identified 153 target genes associated with 5 miRNAs (see Figure 5C).
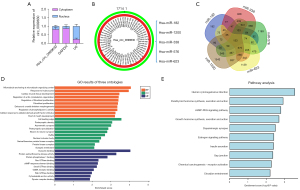
We also performed GO and pathway analyses of these target genes. As the GO analysis showed, the enriched GO-biological process terms mainly included the response to hydroxyurea, and fibroblast proliferation; the enriched GO-cellular component terms mainly included the cell leading edge, and postsynaptic density; the enriched GO-molecular function terms mainly included dynamin binding, protein kinase activity, and protein phosphatase I binding (see Figure 5D). The KEGG analysis results showed that the enriched pathways mainly included human cytomegalovirus infection, the cGMP-PKG signaling pathway, and the estrogen signaling pathway (see Figure 5E). Thus, we examined the vast miRNA/mRNA axis of hsa_circ_0099630, and preliminarily identified the possible function of the target genes.
Discussion
Chronic inflammation can affect the repair and regeneration of bone tissue, probably because inflammation can alter the cellular microenvironment (24). Periodontitis, as an infectious disease, is a major cause of loose and missing teeth in adults (25). It has been reported that periodontitis is mainly caused by G-bacterial infections (26). Among them, Pg, a melanin-producing anaerobic bacillus, is recognized as the main causative agent of chronic periodontitis (27). LPS, as the primary component of the G-bacterial cell wall, is a key factor for Pg (28). Research has shown that LPS participates in the destruction of periodontal tissues (29). LPS not only acts directly on periodontal tissue causing tissue destruction, but also acts as a potential cell activator to stimulate the activation and differentiation of osteoclasts (30). Pg-LPS, as a key inflammatory mediator, can activate the host immune system (31). Pg-LPS can also enter the blood circulation and produce a certain inflammatory response to the blood vessel wall (32). Studies have shown that Pg-LPS can act on mononuclear macrophages, induce the production of inflammatory cytokines, and participate in the development of periodontitis (33,34). Pg- LPSs, as cellular inflammation inducers, can be applied to construct inflammatory cell models, as reported in previous studies (35,36). In our study, we also used Pg-LPS to stimulate HPLFs to construct inflammatory model cells. Our data showed that Pg-LPS treatment suppressed the proliferation and induced the apoptosis of HPLFs.
The regeneration of periodontal tissue has been the focus of periodontal disease research (37). HPLFs have been reported to have a multidirectional differentiation ability and to participate in tissue repair and regeneration (38). Thus, the study of molecular regulatory mechanisms in the osteogenic differentiation of HPLFs is crucial for periodontal therapy, and it has extremely broad clinical application prospects. The identification of osteogenic markers is the basis for studying the osteogenic differentiation of HPLFs. ALP is one of the early marker enzymes of osteoblast differentiation and a key enzyme in the mineralization process (39). To some extent, ALP activity reflects the osteogenic differentiation capacity of the cells (40). RUNX2, as a bone differentiation-specific transcription factor, is involved in activating and initiating osteoblast differentiation and maturation (41). RUNX2 also promotes the synthesis and expression of Collagen I. Collagen I is the main component of the organic matter of bone tissue (42). Osteocalcin, an inducer of matrix mineralization, plays a key role in adaptive responses that alter energy homeostasis (43). According to one report, circ_0076906 can induce the osteogenic differentiation of bone marrow mesenchymal stem cells (BM-MSCs) and reduce osteoporosis by binding miR-1305 to regulate osteocalcin (44). Osterix (OSX) is a transcription factor that determines the differentiation of osteoblasts (45). It was found that changes in OSX gene expression affect the osteogenic differentiation of stem cells, such as dental pulp stem cells, periodontal cells, and adipose stem cells (46,47). In our study, we found that Pg-LPS stimulation notably weakened ALP activity, and downregulated RUNX2, Collagen I, osteocalcin, and Osterix in the HPLFs. Thus, we observed that osteogenic differentiation ability was reduced in Pg-LPS-induced HPLFs.
Currently, most studies on circRNAs are directed at cancer (48). However, some studies have shown that circRNAs affect tissue regeneration and stem cell differentiation processes; for example, studies have shown that circRNAs participate in liver regeneration in rats and promote the osteogenic differentiation of maxillary sinus membrane stem cells (49,50). Further, research has shown that BMP2 may induce osteoblast differentiation by targeting the miRNA/mRNA axis through circRNAs (51). Several circRNAs, including circ_0062491, circCDK8, circ_0095812, circ_0081572, and circCDR1as, were found aberrant expression in periodontal ligament (PDL) or gingival tissues from sites with periodontitis. The circRNA-miRNA interactions play important roles during osteogenic differentiation with an inhibitory mechanism, where circRNAs suppresses miRNAs expression, as shown by the gain-of-function or loss-of-function experiments. This suggests that circRNAs are able to modulate several critical biological pathways in the periodontium (49). In our study, we first found that hsa_circ_0099630 was upregulated in patients with periodontitis, indicating that hsa_circ_0099630 has an inductive role on the progression of periodontitis. Our data also revealed that hsa_circ_0099630 overexpression, like Pg-LPS, led to the inhibition of proliferation and osteogenic differentiation and the induction of apoptosis in HPLFs; the knockdown of hsa_circ_0099630 also accelerated proliferation and osteogenic differentiation prevented apoptosis in the Pg-LPS-induced HPLFs. Overall, the silencing of hsa_circ_0099630 may be a target for periodontal therapy. Nevertheless, the problems and challenges in the clinical application of circRNA containing two sides. On the one hand, the main challenge of circRNA targeting is the off-target effect of circRNA knockdown technology. At least half of the sequences of siRNA targeting circRNA are paired with the parent gene mRNA, which may have miRNA-like non-specific effects. This effect may affect cell function to varying degrees and produce non-specific effects. On the other hand, the challenge in circRNA therapy is to improve the translation efficiency of circRNA. We will focus on the problems and challenges to improve the clinical application of circRNA.
Many circRNAs have been reported to have rich and stable potential functions as competing endogenous RNAs (48). CircRNAs have potential functions in the regulation of gene expression (52). A recent study has shown that endogenous circRNAs act as miRNA sponges to attenuate the function of miRNAs (53). A study has also confirmed that the decrease in osteogenic potential in chronic inflammatory environments is associated with miRNA dysregulation (54). In the current study, vast miRNAs, especially miR-182, miR-1200, miR-338, miR-576, miR-623, were identified as possibly interacting with hsa_circ_0099630 based on a bioinformatics analysis. Additionally, we predicted that 153 mRNAs might interact with these 5 miRNAs.
Conclusions
In summary, our findings suggested that hsa_circ_0099630 regulates the proliferation, osteogenic differentiation, and apoptosis of Pg-LPS-induced HPLFs. We also preliminarily screened out a large number of miRNA/mRNA axis related to hsa_circ_0099630, which is also the main direction of our future research. Furthermore, how to judge the prognostic characteristics of periodontitis based on the expression of hsa_circ_0099630 and provide hsa_circ_0099630 as a reliable target for the treatment of periodontitis need to be further investigated.
Acknowledgments
Funding: This research was supported by grants from the National Natural Science Foundation of China (No. 81800954).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4209/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4209/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of the Hospital of Stomatology, Sun Yat-sen University (No. 2020-07-162). All the participants signed a written informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. Int Dent J 2021;71:462-76. [Crossref] [PubMed]
- White DJ. Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur J Oral Sci 1997;105:508-22. [Crossref] [PubMed]
- Manresa C, Sanz-Miralles EC, Twigg J, et al. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst Rev 2018;1:CD009376. [Crossref] [PubMed]
- Nuñez J, Vignoletti F, Caffesse RG, et al. Cellular therapy in periodontal regeneration. Periodontol 2000 2019;79:107-16. [Crossref] [PubMed]
- Tamashunas AC, Katiyar A, Zhang Q, et al. Osteoprotegerin is sensitive to actomyosin tension in human periodontal ligament fibroblasts. J Cell Physiol 2021;236:5715-24. [Crossref] [PubMed]
- Shi J, Li J, Su W, et al. Loss of periodontal ligament fibroblasts by RIPK3-MLKL-mediated necroptosis in the progress of chronic periodontitis. Sci Rep 2019;9:2902. [Crossref] [PubMed]
- Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019;20:675-91. [Crossref] [PubMed]
- He AT, Liu J, Li F, et al. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther 2021;6:185. [Crossref] [PubMed]
- Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022;185:2016-34. [Crossref] [PubMed]
- Yang Q, Li F, He AT, et al. Circular RNAs: Expression, localization, and therapeutic potentials. Mol Ther 2021;29:1683-702. [Crossref] [PubMed]
- Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol 2020;21:475-90. [Crossref] [PubMed]
- Li D, Yang Y, Li ZQ, et al. Circular RNAs: from biogenesis and function to diseases. Chin Med J (Engl) 2019;132:2457-64. [Crossref] [PubMed]
- Li Z, Li X, Xu D, et al. An update on the roles of circular RNAs in osteosarcoma. Cell Prolif 2021;54:e12936. [Crossref] [PubMed]
- Chen X, Yang T, Wang W, et al. Circular RNAs in immune responses and immune diseases. Theranostics 2019;9:588-607. [Crossref] [PubMed]
- Lin Z, Tang X, Wan J, et al. Functions and mechanisms of circular RNAs in regulating stem cell differentiation. RNA Biol 2021;18:2136-49. [Crossref] [PubMed]
- Sun Q, Liu Z, Xu X, et al. Identification of a circRNA/miRNA/mRNA ceRNA Network as a Cell Cycle-Related Regulator for Chronic Sinusitis with Nasal Polyps. J Inflamm Res 2022;15:2601-15. [Crossref] [PubMed]
- Li J, Xie R. Circular RNA expression profile in gingival tissues identifies circ_0062491 and circ_0095812 as potential treatment targets. J Cell Biochem 2019;120:14867-74. [Crossref] [PubMed]
- Wang J, Wang Z, Huang M, et al. Circ_0099630 Participates in SPRY1-Mediated Repression in Periodontitis. Int Dent J 2022;S0020-6539(22)00169-1. Epub ahead of print. [Crossref] [PubMed]
- Zhao C, Chen Q, Yu S, et al. Effect of interleukin-22 on osteogenic differentiation and the osteoclastogenic response of human periodontal ligament fibroblasts in vitro. J Periodontol 2020; Epub ahead of print. [Crossref] [PubMed]
- Wu X, Zhang G, Feng X, et al. Transcriptome analysis of human periodontal ligament fibroblasts exposed to Porphyromonas gingivalis LPS. Arch Oral Biol 2020;110:104632. [Crossref] [PubMed]
- The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res 2017;45:D331-8. [Crossref] [PubMed]
- Li CB, Wang HF, Feng ZK, et al. Identification of immune-related genes for Hepatocellular Carcinoma: a study based on TCGA data. J Mens Health 2021;17:101-13. [Crossref]
- Kanehisa M, Furumichi M, Tanabe M, et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 2017;45:D353-61. [Crossref] [PubMed]
- Newman H, Shih YV, Varghese S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021;277:121114. [Crossref] [PubMed]
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [Crossref] [PubMed]
- Kim WJ, Soh Y, Heo SM. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol Ther (Seoul) 2021;29:263-7. [Crossref] [PubMed]
- Xu W, Zhou W, Wang H, et al. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol 2020;120:45-84. [Crossref] [PubMed]
- Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer's disease. J Oral Microbiol 2019;11:1563405. [Crossref] [PubMed]
- Aquino-Martinez R, Rowsey JL, Fraser DG, et al. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 2020;132:115220. [Crossref] [PubMed]
- AlQranei MS, Chellaiah MA. Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms. J Oral Biosci 2020;62:123-30. [Crossref] [PubMed]
- Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000 2010;54:53-70. [Crossref] [PubMed]
- Kunnen A, van Pampus MG, Aarnoudse JG, et al. The effect of Porphyromonas gingivalis lipopolysaccharide on pregnancy in the rat. Oral Dis 2014;20:591-601. [Crossref] [PubMed]
- Akkaoui J, Yamada C, Duarte C, et al. Contribution of Porphyromonas gingivalis lipopolysaccharide to experimental periodontitis in relation to aging. Geroscience 2021;43:367-76. [Crossref] [PubMed]
- Zhao J, Geng W, Wan K, et al. Lipoxin A4 promotes autophagy and inhibits overactivation of macrophage inflammasome activity induced by Pg LPS. J Int Med Res 2021;49:300060520981259. [Crossref] [PubMed]
- Bozkurt SB, Tuncer Gokdag I, Hakki SS. Porphyromonas gingivalis-Lipopolysaccharide induces cytokines and enzymes of the mouse cementoblasts. Cytokine 2021;138:155380. [Crossref] [PubMed]
- Yao S, Jiang C, Zhang H, et al. Visfatin regulates Pg LPS-induced proinflammatory/prodegradative effects in healthy and inflammatory periodontal cells partially via NF-κB pathway. Biochim Biophys Acta Mol Cell Res 2021;1868:119042. [Crossref] [PubMed]
- Kocher T, König J, Borgnakke WS, et al. Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontol 2000 2018;78:59-97. [Crossref] [PubMed]
- Sokos D, Everts V, de Vries TJ. Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. J Periodontal Res 2015;50:152-9. [Crossref] [PubMed]
- Kim YH, Jang WG, Oh SH, et al. Fenofibrate induces PPARα and BMP2 expression to stimulate osteoblast differentiation. Biochem Biophys Res Commun 2019;520:459-65. [Crossref] [PubMed]
- Lin Z, He H, Wang M, et al. MicroRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Prolif 2019;52:e12688. [Crossref] [PubMed]
- Hou Z, Wang Z, Tao Y, et al. KLF2 regulates osteoblast differentiation by targeting of Runx2. Lab Invest 2019;99:271-80. [Crossref] [PubMed]
- Akhir HM, Teoh PL. Collagen type I promotes osteogenic differentiation of amniotic membrane-derived mesenchymal stromal cells in basal and induction media. Biosci Rep 2020;40:BSR20201325. [Crossref] [PubMed]
- Tsao YT, Huang YJ, Wu HH, et al. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int J Mol Sci 2017;18:159. [Crossref] [PubMed]
- Wen J, Guan Z, Yu B, et al. Circular RNA hsa_circ_0076906 competes with OGN for miR-1305 biding site to alleviate the progression of osteoporosis. Int J Biochem Cell Biol 2020;122:105719. [Crossref] [PubMed]
- Xu Y, Li D, Zhu Z, et al. miR-27a-3p negatively regulates osteogenic differentiation of MC3T3-E1 preosteoblasts by targeting osterix. Mol Med Rep 2020;22:1717-26. [Crossref] [PubMed]
- Wu L, Wu Y, Lin Y, et al. Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol Cell Biochem 2007;301:83-92. [Crossref] [PubMed]
- Kurata H, Guillot PV, Chan J, et al. Osterix induces osteogenic gene expression but not differentiation in primary human fetal mesenchymal stem cells. Tissue Eng 2007;13:1513-23. [Crossref] [PubMed]
- Cao YZ, Sun JY, Chen YX, et al. The roles of circRNAs in cancers: Perspectives from molecular functions. Gene 2021;767:145182. [Crossref] [PubMed]
- Jiao K, Walsh LJ, Ivanovski S, et al. The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. Int J Mol Sci 2021;22:4636. [Crossref] [PubMed]
- Iwamoto N, Kawakami A. Recent findings regarding the effects of microRNAs on fibroblast-like synovial cells in rheumatoid arthritis. Immunol Med 2019;42:156-61. [Crossref] [PubMed]
- Qian DY, Yan GB, Bai B, et al. Differential circRNA expression profiles during the BMP2-induced osteogenic differentiation of MC3T3-E1 cells. Biomed Pharmacother 2017;90:492-9. [Crossref] [PubMed]
- Li J, Sun D, Pu W, et al. Circular RNAs in Cancer: Biogenesis, Function, and Clinical Significance. Trends Cancer 2020;6:319-36. [Crossref] [PubMed]
- Yang Y, Yujiao W, Fang W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res 2020;53:40. [Crossref] [PubMed]
- Bravo Vázquez LA, Moreno Becerril MY, Mora Hernández EO, et al. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules 2021;27:211. [Crossref] [PubMed]


