
Newly found variations of the right posterior portal vein identified radiologically in 1,003 Chinese patients: a cross-sectional study
Highlight box
Key findings
• Variation of the RPPV without a common trunk was found in 216 (21.54%) of 1,003 patients in this series, and further categorized into three subtypes.
What is known and what is new?
• In our routine radiological studies and liver surgical practices, we have encountered not a few abnormal branching patterns and variations of the RPPV.
• We provided data of the incidence of the RPPV without a common trunk in large group of Chinese persons.
What is the implication, and what should change now?
• Variations of the RPPV without a common trunk were not rare in Chinese population. An awareness of this newly proved anatomy of the RPPV is greatly important for hepatic and transplant surgeons and interventional radiologists.
Introduction
The portal vein (PV) anatomy is the foundation for Couinaud’s liver segmentation (1). Awareness of the branching patterns and variations of the PV is important for accurately interpreting preoperative imaging findings and planning liver surgery or interventional radiological procedures (2-8). In our routine radiological studies and liver surgical practices, we have encountered not a few abnormal branching patterns and variations of the right posterior PV (RPPV). Therefore, we investigated the incidence of variations of the RPPV without a common trunk in Chinese people, and discussed its clinical significances. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4837/rc).
Methods
Patients
We retrospectively reviewed the imaging data of 1,933 patients who were suspected of various abdominal diseases and underwent abdominal triphasic multidetector computed tomography (MDCT) examinations in hospital between September 28, 2018 through May 23, 2019. A total of 1,144 patients who met all of the following criteria were screened for the inclusion criteria: (I) age ≥14 years; (II) no history of liver resection; (III) no history of major upper abdominal surgery; (IV) no apparent liver cirrhosis; and (V) liver with small tumors (<2 cm in diameter and located in the perihepatic region). Patients with main PV (MPV) variations were excluded, including MPV trifurcation in 41 patients, the RPPV as the first branch of the MPV in 98 patients, total ramification in 1 patient, and separate origin of P7 from the MPV in 1 patient. After excluding 930 patients, 1,003 patients comprised the research group [(396 (39.48%) female, 607 (60.52%) male; median age, 50 years (range 14–86 years)]. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of the Second Xiangya Hospital of Central South University (No. 2020-374) and individual consent for this retrospective analysis was waived.
MDCT examination protocol
All abdominal MDCT scans were performed with a dual-source dual-energy CT scanner (Somatom Force, Siemens Healthcare, Germany) while the patient was in a supine position. Before scanning, 1.5–2.0 mL/kg of body weight of nonionic iodinated contrast material (Ultravist 370, 370 mg I/mL, Bayer Schering Pharma Limited Company, Berlin, Germany) were injected through a dorsal vein of the hand using an Ulrich power injector (Ulrich Medical, Germany) at a rate of 3.0–4.0 mL/s. Scans were acquired in the hepatic artery, PV and hepatic vein phases, and were triggered using the bolus tracking technique and a threshold set at 100 HU. The MDCT contrast-enhanced scanning parameters were: individual detector collimation 0.6 mm; pitch 1.2; tube rotation speed 0.5 s/r; tube potential and effective tube current-time product set at 100 kVp and 180 Quality Reference mAs for tube A, and Sn 150 kVp and 90 Quality Reference mAs for tube B; automatic tube current modulation (Care Dose 4D, Siemens Healthcare, Germany) switched on; reconstruction layer thickness of 0.75 mm and reconstruction interval of 0.55 mm.
Image interpretation
The MDCT imaging data were post-processed using imaging software (Syngo Via, VB 10 version, Siemens Healthcare, Germany) equipped with two dedicated software applications (liver analysis software and vascular processing software, Siemens Healthcare, Germany). The processing procedure of the imaging software was as follows: (I) 100 kVp thin layer reconstruction imaging data uploaded to the liver analysis software and (II) segmentation of intrahepatic vessels automatically processed and then manually corrected for three-dimensional reconstruction images, maximum-intensity projection (MIP) and volume-rendered (VR) images of the PV generated from the PV phase data; and (III) branches of the PV and corresponding supplying liver areas clearly shown in the reconstructed images. Five radiologists with over 8 years of experience independently interpreted all images for branching patterns of the PV, and reached a consensus on all the PV variations. ‘Pn’ was defined as the branch of the PV supplying Couninaud’s segment n (9). Variations of the RPPV without a common trunk were categorized according to the classifications introduced by Koç et al. (10) and Sureka et al. (11).
Statistical analysis
Categorical variables are presented as frequencies and proportion. Continuous variables are expressed as median and range. The statistical analyses were performed with SPSS software (Version 25.0, IBM, USA).
Results
Variation of the RPPV without a common trunk
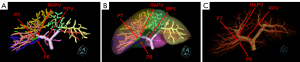
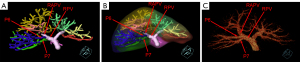
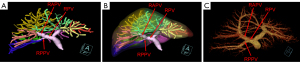
Variation of the RPPV without a common trunk was found in 216 (21.54%) of 1,003 patients in the present study (Table 1), and further categorized into three subtypes. Subtype 1 was characterized as the first separate origin of P6 and then P7 from the right PV (RPV), and there were 15 patients (1.50%) with this subtype (Figure 1). Subtype 2 was characterized by the first separate origin of P7 and then P6 from the RPV, and there were 66 patients (6.58%) with this subtype (Figure 2). Subtype 3 was characterized by simultaneous separate origins of P6 and P7 from the common site of the RPV, and there were 135 patients (13.46%) with this subtype (Figure 3). Lastly, among the 1,003 patients, 787 patients (78.46%) showed common RPPV bifurcation in which the RPPV was normally divided into P6 and P7 branches (Table 1, Figure 4).
Table 1
| Description | n | % |
| Variation of the RPPV without a common trunk | 216 | 21.54 |
| 1. First separate origin of the Segment VI PV branch and then the Segment VII PV branch from the RPV | 15 | 1.50 |
| 2. First separate origin of the Segment VII PV branch and then the Segment VI PV branch from the RPV | 66 | 6.58 |
| 3. Simultaneous separate origin of the Segments VI and VII PV branches from the common site of the RPV | 135 | 13.46 |
| RPPV normally dividing into Segments VI and VII PV branches | 787 | 78.46 |
| Total | 1,003 | 100.00 |
RPPV, right posterior portal vein; PV, portal vein; RPV, right portal vein.

Discussion
In the English literature, Koç et al. from Turkey in 2007 reported that the RPPV did not have a common trunk in only 35 (3.22%) of 1,087 cases (10). Sureka et al. reported from India in 2015 that the RPPV without a common trunk was seen in few (4.34%, 42/967) patients (11). Both included large sample sizes [>1,000 (including) patients] and both studies carefully and completely explored the intrahepatic PV variations on triphasic abdomen MDCT. Given that the studies focusing on the variations of the RPPV are rare, conclusions drawn from these two studies may not apply to other racial groups. Moreover, to our best knowledge, no study has investigated variations of the RPPV without a common trunk in a single tertiary referral center in China, and none has focused on racial differences in the RPPV without a common trunk. In the present study, we demonstrated a high incidence (21.54%) of variation of the RPPV without a common trunk in a Chinese population, higher than in the Turkish (3.22%, 35/1,087) and Indian (4.34%, 42/967) populations (10,11). These results may suggest a great racial difference in the RPPV without a common trunk between the Chinese population and patients from Turkey and India. The smallest sample size of the three studies was 967 patients, which greatly enhances the credibility of the findings and provides sound evidence of racial differences in the anatomy of the RPPV among different races. This inference needs to be furtherly investigated by more studies including large groups of population. The possible reason for this difference may be different genetic backgrounds of the racial groups. Currently, it is unclear which genes control the genesis and development of the PV. More comprehensive studies are needed to fully explore the reasons for racial differences in the anatomy of the RPPV.
Liver transplantation is an effective treatment for patients with end-stage hepatic diseases. While ensuring the safety of the donor in living donor liver transplantation (LDLT), sufficient graft volume must be provided for the recipient. It is generally believed that suitable donors should have a graft-to-recipient weight ratio (GRWR) >0.8%, and a residual liver volume of 35% (for young donors without steatosis) or 40% (for older donors or donors suffering from steatosis) of the total liver volume (TLV) (12-14). The right hemiliver and left hemiliver grafts are the most commonly used in LDLT. However, we often encounter GRWR mismatch in donors in clinical practice. In the case of difficult graft selection, the right posterior sector (RPS) graft is an alternative for liver graft to ensure the safety of the donor and overcome these problems (15-17). Currently, RPS transplantation is mainly carried out in Asian countries, especially Japan and South Korea. In the present study, variation of the RPPV without a common trunk was found in 216 (21.54%) of 1,003 cases. In case of RPS as the donor liver graft in such patients, P6 and P7 anastomoses have to be separately performed in the recipient, which dramatically increases the complexity of the surgical procedure. In addition, the inherent surgical difficulties and limited experience in LDLT using the RPS graft means the RPS cannot be considered as a single liver sector and cannot be selected as a donor liver graft in liver donor candidates with this anatomic variation. In fact, the RPS graft forms only 1.5% of the LDLT carried out in Japan (18). A thorough and precise preoperative evaluation of intrahepatic PV distribution and variation is necessary to perform a perfect and successful LDLT.
Segment-based hepatic resection is defined as complete removal of a single or multiple segment(s) of the liver (19-21). The pedicles can be isolated, looped, divided and suture-ligated as one of the bundles. In theory, any anatomic liver resection may be performed using this technique (22). However, the RPPV without a common trunk identified in this study does not completely support that theory. It is very difficult and dangerous to perform anatomic right posterior sectionectomy by the Glissonean pedicle approach in those patients with the variation of the RPPV without a common trunk. As we all know, the pedicle of the RPS is the most deeply placed of the right hepatic pedicles. In patients with the variation of the RPPV without a common trunk, the pedicle of the RPS does not exist and it is necessary to separately isolate the pedicle of Segment VI or VII by dissecting along the right hepatic pedicle. This surgical procedure is time-consuming and dangerous because the pedicles of Segments VI and VII are located deep within the hepatic parenchyma and are closely adjacent to major vasculature. In such a situation, we can use intraoperative ultrasound (IOUS) to identify and stain the pedicles of Segments VI and VII, and then perform a combined or single anatomic sectionectomy VI and/or VII. Moreover, in order to avoid injury to the separate pedicle of Segment VI or VII, a safety margin >1 cm distal to its origin must be kept while dividing them (23). Variation of the RPPV without a common trunk encountered in anatomic hepatic resection should also be paid attention to in associated liver partition and PV ligation for staged hepatectomy (ALPPS). As a result, careful preoperative imaging is the key to successfully performing anatomical right posterior sectionectomy.
PV embolization (PVE) involves the PV main trunk of the tumor-bearing hemiliver or single tumor-bearing sector of the liver and will be generally carried out about 4 weeks prior to surgery (24). A sufficient future liver residual (FLR) is a necessary prerequisite for performing successfully hepatectomy. Without an adequate FLR, postresection liver failure will inevitably occur after hepatectomy. Most liver surgeons agree that a FLR volume of at least 25% is appropriate in patients with normal liver function, and 40% is accepted in patients with impaired liver function. When preoperative assessment of FLR is insufficient, PVE is carried out in an attempt to increase the volume of FLR. The technique can be performed through the contralateral or ipsilateral approach, and a variety of embolic materials can be used (25). However, anatomic variations of the intrahepatic PV, such the RPPV without a common trunk, increase the technical difficulties of PVE (26). In this situation, the PV branches of Segments VI and VII need to be embolized separately to achieve embolization of the entire RPS and thus the risk of embolic migration will increase. Embolic migration may cause ischemic damage to the right anterior sector (RAS) and even result in failure of the PVE. Accurate knowledge of the PV anatomy is of great importance when atypical PVE is performed.
Transjugular intrahepatic portosystemic shunt (TIPS) is one of the treatments for portal hypertension, and has partly replaced the surgical shunt operation (27-29). A successful TIPS often is built up between the right hepatic vein (RHV) and the RPV. TIPS is widely used in clinical practice, but transhepatic PV puncture is technically demanding and requires precise understanding of the anatomy and spatial location of the branches of the RPV. Diversified techniques have been used to improve the rate of success of image-guided PV puncture, such as intravascular or percutaneous ultrasound guidance, CT-guided or MR-guided portography, and transhepatic catheterization of the PV (30-32). However, it is difficult to place a stent between the RHV and separate P6 or P7, and the effect of a portosystemic shunt may be poor in those patients with the RPPV without a common trunk (33). The number of punctures, operation time, total amount of radiation exposure for patients, and the use of contrast media may increase in this situation. In addition, the incidence of the PV post-puncture-related complications, such as intraperitoneal hemorrhage, bile leakage, hepatic artery pseudoaneurysm and extracapsular puncture, will also be increased. Therefore, precise pre-procedural imaging is advocated to evaluate the intrahepatic PV distribution and its variation. A complete awareness of the intrahepatic PV distribution and variation is crucial for performing successful TIPS.
Conclusions
In conclusion, the present study suggested that variations of the RPPV without a common trunk were not rare in Chinese population. Awareness of this newly proved knowledge may be extremely important for hepatic and transplant surgeons and interventional radiologists.
Acknowledgments
Funding: This study was supported by the National Key R&D Program of China (No. 2019YFE0190500, to Jiang-Sheng Huang) and the Key Research and Development Program of Hunan Province, China (No. 2018SK2090, to Xian-Ling Liu).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4837/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4837/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4837/coif). JSH reports funding from the National Key R&D Program of China (No. 2019YFE0190500). XLL reports funding from the Key Research and Development Program of Hunan Province, China (No. 2018SK2090). The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iqbal S, Iqbal R, Iqbal F. Surgical Implications of Portal Vein Variations and Liver Segmentations: A Recent Update. J Clin Diagn Res 2017;11:AE01-5. [Crossref] [PubMed]
- Hu J, Pi Z, Yu MY, et al. Obstructive jaundice caused by tumor emboli from hepatocellular carcinoma. Am Surg 1999;65:406-10. [PubMed]
- Xiao E, Li D, Shen S, et al. Effect of preoperative transcatheter arterial chemoembolization on apoptosis of hepatocellular carcinoma cells. Chin Med J (Engl) 2003;116:203-7. [PubMed]
- Hu JX, Dai WD, Miao XY, et al. Anatomic resection of segment VIII of liver for hepatocellular carcinoma in cirrhotic patients based on an intrahepatic Glissonian approach. Surgery 2009;146:854-60. [Crossref] [PubMed]
- Miao XY, Hu JX, Dai WD, et al. Anatomic mesohepatectomy with extrahepatic control of hepatic veins. Hepatogastroenterology 2009;56:1730-4. [PubMed]
- Bian DJ, Xiao EH, Hu DX, et al. Magnetic resonance spectroscopy on hepatocellular carcinoma after transcatheter arterial chemoembolization. Chin J Cancer 2010;29:198-201. [Crossref] [PubMed]
- Dai WD, Huang JS, Hu JX. Isolated caudate lobe resection for huge hepatocellular carcinoma (10 cm or greater in diameter). Am Surg 2014;80:159-65. [Crossref] [PubMed]
- Choe JW, Lee HY, Rim CH. Will the collaboration of surgery and external radiotherapy open new avenues for hepatocellular carcinoma with portal vein thrombosis? World J Gastroenterol 2022;28:704-14. [Crossref] [PubMed]
- Mise Y, Satou S, Shindoh J, et al. Three-dimensional volumetry in 107 normal livers reveals clinically relevant inter-segment variation in size. HPB (Oxford) 2014;16:439-47. [Crossref] [PubMed]
- Koç Z, Oğuzkurt L, Ulusan S. Portal vein variations: clinical implications and frequencies in routine abdominal multidetector CT. Diagn Interv Radiol 2007;13:75-80. [PubMed]
- Sureka B, Patidar Y, Bansal K, et al. Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol 2015;88:20150326. [Crossref] [PubMed]
- Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl 2003;9:S29-35. [Crossref] [PubMed]
- Ito T, Kiuchi T, Egawa H, et al. Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation 2003;76:158-63. [Crossref] [PubMed]
- Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7. [Crossref] [PubMed]
- Sugawara Y, Makuuchi M, Takayama T, et al. Right lateral sector graft in adult living-related liver transplantation. Transplantation 2002;73:111-4. [Crossref] [PubMed]
- Hori T, Kirino I, Uemoto S. Right posterior segment graft in living donor liver transplantation. Hepatol Res 2015;45:1076-82. [Crossref] [PubMed]
- Qu X, Wan P, Feng M, et al. Pediatric living-donor liver transplantation using right posterior segment grafts. BMC Gastroenterol 2021;21:249. [Crossref] [PubMed]
- Takagi K, Domagala P, Polak WG, et al. Right posterior segment graft for living donor liver transplantation: A systematic review. Transplant Rev (Orlando) 2020;34:100510. [Crossref] [PubMed]
- Takasaki K, Kobayashi S, Tanaka S, et al. Highly anatomically systematized hepatic resection with Glissonean sheath code transection at the hepatic hilus. Int Surg 1990;75:73-7. [PubMed]
- Yamamoto M, Katagiri S, Ariizumi S, et al. Glissonean pedicle transection method for liver surgery (with video). J Hepatobiliary Pancreat Sci 2012;19:3-8. [Crossref] [PubMed]
- Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17-23. [Crossref] [PubMed]
- Cristino H, Hashimoto T, Takamoto T, et al. Advanced concept of anatomic resection of the liver: preservation of subsegment during right paramedian sectoriectomy. J Am Coll Surg 2012;214:e5-7. [Crossref] [PubMed]
- Figueroa R, Laurenzi A, Laurent A, et al. Perihilar Glissonian Approach for Anatomical Parenchymal Sparing Liver Resections: Technical Aspects: The Taping Game. Ann Surg 2018;267:537-43. [Crossref] [PubMed]
- de Baere T, Roche A, Vavasseur D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology 1993;188:73-7. [Crossref] [PubMed]
- Schmidt S, Demartines N, Soler L, et al. Portal vein normal anatomy and variants: implication for liver surgery and portal vein embolization. Semin Intervent Radiol 2008;25:86-91. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215-25. [Crossref] [PubMed]
- Rössle M. TIPS: 25 years later. J Hepatol 2013;59:1081-93. [Crossref] [PubMed]
- Perry BC, Kwan SW. Portosystemic Shunts: Stable Utilization and Improved Outcomes, Two Decades After the Transjugular Intrahepatic Portosystemic Shunt. J Am Coll Radiol 2015;12:1427-33. [Crossref] [PubMed]
- Lee HL, Lee SW. The role of transjugular intrahepatic portosystemic shunt in patients with portal hypertension: Advantages and pitfalls. Clin Mol Hepatol 2022;28:121-34. [Crossref] [PubMed]
- Kew J, Davies RP. Intravascular ultrasound guidance for transjugular intrahepatic portosystemic shunt procedure in a swine model. Cardiovasc Intervent Radiol 2004;27:38-41. [Crossref] [PubMed]
- Bloch R, Fontaine A, Borsa J, et al. CT-guided transfemoral portocaval shunt creation. Cardiovasc Intervent Radiol 2001;24:106-10. [Crossref] [PubMed]
- Kee ST, Rhee JS, Butts K, et al. 1999 Gary J. Becker Young Investigator Award. MR-guided transjugular portosystemic shunt placement in a swine model. J Vasc Interv Radiol 1999;10:529-35. [Crossref] [PubMed]
- Raza SA, Walser E, Hernandez A, et al. Transhepatic puncture of portal and hepatic veins for TIPS using a single-needle pass under sonographic guidance. AJR Am J Roentgenol 2006;187:W87-91. [Crossref] [PubMed]


