
Effect of Roxadustat versus erythropoietin (EPO) for treating anemia in patients with diabetic kidney disease: a retrospective cohort study
Highlight box
Key findings
• In this retrospective cohort study, we found Roxadustat effectively improves anemia in patients with diabetic kidney disease (DKD).
What is known and what is new?
• Renal anemia in DKD has higher incidence rate, earlier onset and higher severity than other chronic kidney disease (CKD). Roxadustat, a novel oral hypoxia-inducible factor-prolyl hydroxylase inhibitor, improves CKD anemia.
• In this retrospective cohort study, we evaluated if Roxadustat could treat the inflammation related DKD anemia effectively.
What is the implication, and what should change now?
• Roxadustat is a novel and effective choice for DKD patients with anemia.
Introduction
Diabetic kidney disease (DKD), a major microvascular complication of diabetes mellitus (DM), has become the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (1). Anemia as a common complication among CKD patients not only accelerates the decline of kidney function (2,3), but is also an independent risk factor for the development of cardiovascular disease (CVD) and all-cause mortality (3-5).
Compared to other CKDs, renal anemia of DKD is rather special with higher incidence rate, earlier onset and higher severity (6,7). The incidence of renal anemia is significantly higher in DKD patients (68.0% in DKD versus 56.6% in hypertensive nephropathy, and 46.1% in chronic glomerulonephritis) as reported in a muti-center cross-sectional study (6). A nested case-control study also demonstrated a high incidence rate of anemia in DKD patients (7). Additionally, anemia and erythropoietin (EPO) deficiency can occur in the early stage of DKD, but not in other renal diseases with similar levels of renal dysfunction (8). DKD patients usually develop more severe anemia than patients with non-DKD (9). Study enrolled 106 DKD and 100 non-DKD participants showed that in stage 3 and 4 CKD, Hb levels were lower in DKD (10).
Traditionally, patients suffering from renal anemia are managed with erythropoiesis stimulating agents (ESAs), iron supplements, and blood transfusion in severe cases. However, ESA treatment is associated with an increased cardiovascular risk, especially when used at high doses in inflamed and hyporesponsive patients. Additionally, resistance to ESA also creates challenges in treating anemia (11), A post hoc analysis of PROMPT study demonstrated that 90.2% CKD patients with DM could achieved Hb level larger than 110 g/L when treated with 10,000 U epoetin alfa once a week, lower than the 96.5% of CKD patients without DM (12).
In the continuous search for treatment, a new class of drugs, [i.e., HIF—prolyl hydroxylase inhibitors (HIF-PHIs)] has gradually been used in clinical practice. Inhibition of the prolyl hydroxylase domain protein prevents the degradation of the HIF-α subunit, which simulates a mild hypoxia condition, and thus induces the translocation of HIF-α to the cell nucleus and the combination with HIF-β. The heterodimeric HIF-α/HIF-β complex then promote the expression of genes related to EPO synthesis and iron metabolism (11).
Roxadustat, a HIF-PHI, can elevate hemoglobin (Hb) levels in both non-dialysis dependent and dialysis dependent CKD (including DKD) patients according to previous phase-Ⅱ and phase-Ⅲ clinical trials (13-32). Post hoc analysis of the 1517-CL-0310 study showed that Roxadustat elevated and maintain Hb level of renal anemia patients with or without DM to a similar extent (33). Clinical trials showed that the safety of Roxadustat was comparable to positive controls though adverse events occurred more frequently in the Roxadustat group than in the placebo group. Common treatment-emergent adverse events include hyperkalemia, metabolic acidosis, hypertension, peripheral edema, pneumonia, urinary tract infection, upper respiratory tract infection, nausea, arteriovenous fistula thrombosis, headache, diarrhea, etc. (14,17,18,25,26).
In addition, Roxadustat can improved iron metabolism related indexes (14,17,24-26). The Roxadustat dose required for effective anti-anemia effect may be less affected by ESA hyporesponsive related factors such as lack of iron and inflammation (34,35), which is common in DKD renal anemia patients. Moreover, basic research discovered that Roxadustat could ameliorate high glucose induced glomerular endothelial cells injury (36). In 2020, we reported a case in which Roxadustat effectively treated the refractory renal anemia in a DKD patient with significantly elevated ferritin (37).
In this retrospective study, we investigated the efficacy of Roxadustat in terms of anemia improvement [e.g., Hb and hematocrit (Hct) levels] in DKD patients. Our results provide new evidence for Roxadustat’s application in DKD patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4344/rc).
Methods
Study design and subjects
This study is a retrospective cohort study. Patients with DKD who presented to The Second Xiangya Hospital between December 31, 2018 and December 31, 2021 and the Third Xiangya Hospital between December 31, 2018 and January 31, 2022 were enrolled in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The Second Xiangya Hospital of Central South University (No. LYF2022171). The Third Xiangya Hospital was informed and agreed the study. Individual consent for this retrospective analysis was waived. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have been diagnosed with DKD; (II) meet the diagnostic criteria for anemia (i.e., a Hb level: male <130 g/L, female <120 g/L); and (III) have received standard Roxadustat or EPO treatment. DKD was diagnosed based on a clear DM history, a urinary albumin (ALB)-to-creatinine ratio ≥30 mg/g and/or an estimate glomerular filtration rate (eGFR) <60 mL/min·1.73 m2. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had been diagnosed with a CKD in addition to DKD; (II) had anemia unrelated to kidney disease; (III) failed to finish the follow-up; (IV) had experienced Roxadustat or EPO withdrawal and discontinuance; (V) had received a combination of Roxadustat and EPO in the observation period; and/or (VI) had undergone kidney transplantation.
A total 61 of DKD patients with anemia who received Roxadustat treatment were ultimately enrolled in this retrospective study. Anemia DKD patients treated with recombinant human EPO (rHuEPO) were matched to patients in the Roxadustat group at a 1:1 ratio (n=61) based on age, gender and approximately baseline Hb level. Baseline data, including age, sex, dialysis condition, height, body weight, Hb, Hct, serum ALB, serum creatinine (Scr), eGFR, C-reactive protein (CRP), and intact parathyroid hormone (iPTH) levels were recorded. After 3 months of Roxadustat or EPO treatment, the patients’ Hb and Hct levels were examined again.
The efficacy endpoint was anemia alleviation. The primary outcome was change of Hb (ΔHb) and Hct (ΔHct). The secondary outcomes were Hb response rate and Hb qualified rate. Hb response rate was the proportion of participants who achieved ΔHb larger than 10 g/L after the 3-month treatment. Hb qualified rate was the proportion of participants whose Hb level reached 110 g/L at the end of the 3-month treatment. The safety endpoint was evaluated by the incidence and severity of adverse events according to medical record.
Sample size estimation
Based on previous study (28) and the unpublished preliminary data, we supposed that the Hb response rate of Roxadustat and EPO group would be approximately 80% and 50%, respectively. Sample size estimation was performed with α level of 0.05 (two-sided) and power of 90%. About 96 participants (48 in each group) were needed. If the loss rate was approximately 10%, at least 108 subjects were needed.
Statistical analysis
All the statistical analyses were conducted using SPSS and R studio. When examining the baseline comparability of the two groups, the variation analysis for the continuous variables with a normal distribution was performed using the Student’s t test for the 2-group comparisons, while one-way ANOVA was used for the multiple-group comparisons. Continuous variables with a normal distribution are expressed as mean ± standard deviations, or otherwise as the median and inter-quartile range. The Wilcoxon rank-sum test was used for the non-normally distributed continuous variables. The categorical variables are expressed in terms of the number of people and percentages, and Chi-square tests or Fisher’s exact tests were used for the variation analysis. Non-parametric Wilcoxon rank-sum tests were used for comparisons between ordinal categorical variables. A P value <0.05 was considered statistically significant.
In order to adjust for incomparable baseline variables, an analysis of covariance (ANCOVA) model was used to calculate the least square mean, i.e., the adjusted mean with baseline value as covariates, of Hb, Hct, ΔHb and ΔHct. The effect size of the anemia treatment was evaluated by Cohen’s d for ΔHb and ΔHct.
Logistic regression analysis was used to calculate the odds ratio (OR) of the Hb qualified rate and Hb response rate. If a categorical confounder was found when exploring the correlation between 2 dichotomous variables, the Cochran-Mantel-Haenszel test was used.
Sensitivity analyses of the primary and secondary outcomes were performed. Analysis was done before and after missing value handling. As for a missing value, if it was <5%, we substituted it with the mean value. Additionally, for indicators that do not change much in the short term, the last observation carried forward and the next observation carried backward methods were used. However, if a missing value was >5%, we examined whether there was a significant difference between the 2 groups first. If the data of the Roxadustat group differed significantly to that of the EPO group, we enrolled the missing value as another factor when performing the logistic regression.
Results
Characteristics of the study participants
61 patients met all the inclusion criteria and treated with Roxadustat for 3 months at The Second Xiangya Hospital between December 31, 2018 and December 31, 2021, or The Third Xiangya Hospital between December 31, 2018 and January 31, 2022 were enrolled in this study. The anemia DKD patients treated with rHuEPO were matched to patients in the Roxadustat group at a 1:1 ratio (n=61). The baseline data of the patients are presented in Table 1. No significant differences were observed in terms of age, gender, body mass index (BMI), Scr, eGFR, Hb, Hct, CRP, and dialysis therapy between the 2 groups. However, the ALB, iPTH level, and the stage of DKD differed significantly between the 2 groups (see Table 1). In this study, 19 participants lacked iPTH data, and 60 participants lacked CRP data.
Table 1
| Characteristic | Overall (N=122) | Roxadustat group (N=61) | EPO group (N=61) | P value |
|---|---|---|---|---|
| Age-year, | 60.37±12.52 | 60.52±12.50 | 60.21±12.66 | 0.891 |
| Sex-male, n (%) | 86 (70.5) | 46 (75.4) | 40 (65.6) | 0.234 |
| BMI (kg/m2), | 24.13±3.48 | 24.04±3.56 | 24.21±3.43 | 0.795 |
| ALB (g/L), | 32.03±5.73 | 32.98± 6.20 | 31.08±5.10 | 0.067 |
| ALB category, n (%) | 0.025 | |||
| <38 g/L | 103 (84.40) | 47 (77.00) | 56 (91.80) | |
| ≥38 g/L | 19 (15.60) | 14 (23.00) | 5 (8.20) | |
| Scr (μmol/L), M (Q1, Q3) | 446.90 (307.15, 605.45) | 426.00 (252.50, 638.95) | 447.80 (336.00, 583.95) | 0.504 |
| eGFR (1.73/min/m2), M (Q1, Q3) | 11.64 (8.01, 18.15) | 12.50 (7.50, 19.97) | 11.06 (8.39, 15.99) | 0.214 |
| DKD stage, n (%) | 0.012 | |||
| 3 | 7 (5.70) | 7 (11.50) | 0 (0.00) | |
| 4 | 36 (29.50) | 19 (31.10) | 17 (27.90) | |
| 5 | 79 (64.80) | 35 (57.40) | 44 (72.10) | |
| Dialysis therapy, n (%) | 56 (45.90) | 29 (47.50) | 27 (44.30) | 0.716 |
| Type, n (%) | 0.051 | |||
| Hemodialysis | 41 (73.2) | 18 (62.1) | 23 (85.2) | |
| Peritoneal dialysis | 15 (26.8) | 11 (37.9) | 4 (14.8) | |
| iPTH (pg/mL), M (Q1, Q3) | 38.05 (15.54, 135.65) | 91.80 (26.25, 197.78) | 25.46 (12.67, 104.28) | 0.008 |
| iPTH category, n (%) | 0.015 | |||
| ≤88 pg/mL | 64 (62.1) | 22 (48.9) | 42 (72.4) | |
| >88 pg/mL | 39 (37.9) | 23 (51.1) | 16 (27.6) | |
| CRP (mg/L), M (Q1, Q3) | 5.11 (2.61, 12.30) | 5.48 (2.94, 12.34) | 4.97 (1.86, 13.75) | 0.412 |
| CRP category, n (%) | 0.701 | |||
| ≤6 mg/L | 34 (54.8) | 19 (52.8) | 15 (57.7) | |
| >6 mg/L | 28 (45.2) | 17 (47.2) | 11 (42.3) | |
| Hb (g/L), | 80.90±11.49 | 81.05±12.42 | 80.75±10.57 | 0.888 |
| Hct (%), | 25.07±3.65 | 24.93±4.08 | 25.22±3.18 | 0.666 |
Missing values: iPTH data were missing in 19 cases (15.57%); CRP data were missing in 60 cases (49.18%). DKD, diabetic kidney disease; EPO, erythropoietin; BMI, body mass index; , mean; s, standard deviation; ALB, serum albumin; Scr, serum creatinine; M, median; Q1, lower quartile; Q3, upper quartile; eGFR, estimated glomerular filtration rate; iPTH, intact parathyroid hormone; CRP, C-reactive protein; Hb, hemoglobin; Hct, hematocrit.
The effect of Roxadustat on Hb elevation in DKD patients was similar to that of EPO
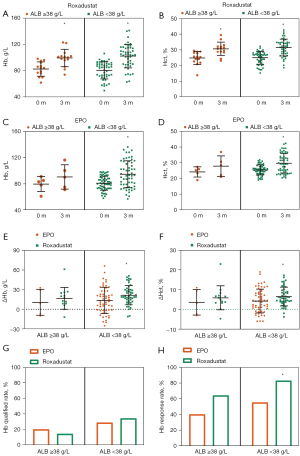
After 3 months of Roxadustat and EPO treatment, the Hb levels increased significantly in both groups (see Figure 1A). The change of Hb (ΔHb) of the Roxadustat group was 20.49±15.14 g/L, larger than that of the EPO group (ΔHb =13.38±19.94 g/L, P=0.0283, Cohen’s d =0.4; see Figure 1B and Table 2). After adjusting for confounding factors without missing value handling, the least-squares means (LSM) of Hb and ΔHb in the two groups were calculated (see Table 2). However, the difference in LSM changes (95% CI) was 8.7 (–1.5, 18.8), while the Cohen’s d of ΔHb was 0.23. Additionally, after confounders adjustment and missing value manipulation, the difference in LSM changes was 4.9 (–2.4, 12.1), while the Cohen’s d of ΔHb was 0.18, which showed that Roxadustat’s ability to boost Hb within a 3-month period was similar to that of EPO (see Table 2).
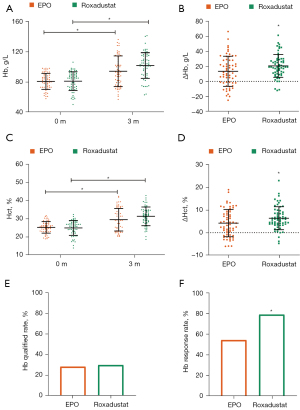
Table 2
| Variable | Roxadustat | EPO | Difference in LSM changes (95% CI) | Cohen’s d for ΔHb | |||
|---|---|---|---|---|---|---|---|
| Hb | ΔHb | Hb | ΔHb | ||||
| Mean ± SD | 101.5±16.8 | 20.5±15.1 | 94.1±20.2 | 13.4±19.9 | – | 0.40 | |
| Least-squares mean ± SE* | 103.4±4.2 | 22.4±4.2 | 94.7±5.4 | 13.8±5.4 | 8.7 (–1.5, 18.8) | 0.23 | |
| Least-squares mean ± SE* (after handling missing value) | 99.7±2.9 | 18.8±2.9 | 94.8±3.9 | 13.9±3.9 | 4.9 (–2.4, 12.1) | 0.18 | |
*, least-square mean adjusted for age, gender, baseline Hb, baseline Hct, BMI, DKD stage (G3–4 or G5), dialysis therapy (non-dialysis dependent or dialysis dependent), iPTH (iPTH >88 pg/mL, PTH ≤88 pg/mL or missing), CRP (CRP ≤6 mg/L, CRP >6 mg/L or missing) and ALB (ALB <38 g/L or ALB ≥38 g/L). Hb, hemoglobin; ΔHb, change of Hb; DKD, diabetic kidney disease; EPO, erythropoietin; LSM, least-squares mean; CI, confidence interval; SD, standard deviation; SE, standard error; Hct, hematocrit; BMI, body mass index; iPTH, intact parathyroid hormone; PTH, parathyroid hormone; CRP, C-reactive protein; ALB, albumin.
Similar to the Hb level, a 3-month treatment with Roxadustat or EPO significantly elevated the participants’ Hct level (see Figure 1C,1D). However, after confounders adjustment and missing value manipulation, the difference in LSM changes was 1.2 (–1.1, 3.4), while the Cohen’s d of ΔHb was 0.14 (see Table 3).
Table 3
| Variable | Roxadustat | EPO | Difference in LSM changes (95% CI) | Cohen’s d for ΔHct | |||
|---|---|---|---|---|---|---|---|
| Hct | ΔHct | Hct | ΔHct | ||||
| Mean ± SD | 31.3±5.1 | 6.4±5.1 | 29.4±6.2 | 4.2±6.0 | – | 0.40 | |
| Least-squares mean ± SE* | 32.2±1.4 | 7.1±1.4 | 30.0±1.7 | 4.8±1.7 | 2.3 (–1.1, 5.6) | 0.19 | |
| Least-squares mean ± SE* (after handling missing value) | 31.0±0.9 | 6.0±0.9 | 29.9±1.2 | 4.8±1.2 | 1.2 (–1.1, 3.4) | 0.14 | |
*, least-square mean adjusted for age, gender, baseline Hb, baseline Hct, BMI, DKD stage (G3–4 or G5), dialysis therapy (non-dialysis dependent or dialysis dependent), iPTH (iPTH >88 pg/mL, PTH ≤88 pg/mL or missing), CRP (CRP ≤6 mg/L, CRP >6 mg/L or missing) and ALB (ALB <38 g/L or ALB ≥38 g/L). Hct, hematocrit; ΔHct, change of Hct; DKD, diabetic kidney disease; EPO, erythropoietin; LSM, least-squares mean; CI, confidence interval; SD, standard deviation; SE, standard error; Hb, hemoglobin; BMI, body mass index; iPTH, intact parathyroid hormone; PTH, parathyroid hormone; CRP, C-reactive protein; ALB, albumin.
Roxadustat showed higher Hb response rate in DKD patients than EPO
No significant difference was observed in the Hb qualified rate between the 2 groups (see Figure 1E). However, the Hb response rate of the Roxadustat group (78.7%) was significantly higher than that of the EPO group (54.1%; see Figure 1F). When performing logistic regression analysis without adjusting for any confounders and without missing value handling, we found the Hb response rate of Roxadustat was 9.57 (1.67, 91.14) times higher than that of EPO (P=0.0023). After missing value handling and adjusting for all the confounders (i.e., age, gender, baseline Hb, baseline Hct, stage of DKD, BMI, dialysis therapy, iPTH, CRP, and ALB), the Hb response rate of the Roxadustat group was 3.30 (1.20, 9.94) times higher than that of the EPO group (P= 0.0251; see Table 4).
Table 4
| Index | Group | OR (95% CI), P value | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Hb response rate | EPO as reference | 1 | 1 | 1 | 1 |
| Roxadustat | 9.57 (1.67, 91.14) 0.0023 | 3.13 (1.44, 7.10) 0.0048 | 3.01 (1.36, 6.89) 0.0074 | 3.30 (1.20, 9.94) 0.0251 | |
| Hb qualified rate | EPO as reference | 1 | 1 | 1 | 1 |
| Roxadustat | 0.65 (0.09, 3.96) 0.6440 | 1.08 (0.49, 2.39) 0.8414 | 1.04 (0.47, 2.30) 0.9270 | 0.80 (0.26, 2.33) 0.6829 | |
Model 1: analysis without adjustment and missing value handling; Model 2: analysis without adjustment; model 3: analysis adjusted for age and sex; model 4: analysis adjusted for age, gender, baseline Hb, baseline Hct, BMI, DKD stage (G3–4 or G5), dialysis therapy (non-dialysis dependent or dialysis dependent), iPTH (iPTH >88 pg/mL, PTH ≤88 pg/mL or missing), CRP (CRP ≤6 mg/L, CRP >6 mg/L or missing) and ALB (ALB <38 g/L or ALB ≥38 g/L). Hb, hemoglobin; DKD, diabetic kidney disease; OR, odds ratio; CI, confidence interval; EPO, erythropoietin; Hct, hematocrit; BMI, body mass index; iPTH, intact parathyroid hormone; PTH, parathyroid hormone; CRP, C-reactive protein; ALB, albumin.
Subgroup analysis of Roxadustat in the treatment of DKD renal anemia
In this study, data of 60 participants lacked baseline CRP. Data missing was mainly discovered in subjects who did not receive dialysis therapy (P=0.006) and showed relatively higher level of iPTH (P=0.001, see Table 5). Therefore, we considered CRP data missed was missing not at random (MNAR). For further analysis, we divided the participants into normal CRP subgroup (CRP ≤6 mg/L) and high CRP subgroup (CRP >6 mg/L). In the normal CRP subgroup (CRP ≤6 mg/L), both Roxadustat and EPO increased the Hb level and Hct (see Figure 2A-2D). Conversely, in the high CRP subgroup (CRP >6 mg/L), Roxadustat significantly boosted the Hb level and Hct (see Figure 2A-2D). For ΔHb and ΔHct, no significant difference was observed when comparing the two groups in the participants with normal CRP (CRP ≤6 mg/L) (see Figure 2E,2F). In participants with high CRP (CRP >6 mg/L), ΔHb and ΔHct induced by Roxadustat was 23.35±16.94 g/L and 7.51%±5.68%, greater than that induced by EPO (7.36±21.55 g/L, P<0.05 and 2.68%±6.64%, P<0.05; see Figure 2E,2F).
Table 5
| Characteristic | Not missing (n=62, 50.82%) | Missing (n=60, 49.18%) | P value |
|---|---|---|---|
| Roxadustat, n (%) | 0.07 | ||
| Yes | 36 (59.0) | 25 (41.0) | |
| No | 26 (42.6) | 35 (57.4) | |
| Age-year, | 61.89±12.80 | 58.80±12.14 | 0.175 |
| Sex-male, n (%) | 48 (55.8) | 28 (44.2) | 0.088 |
| BMI (kg/m2), | 24.35±3.84 | 23.89±3.08 | 0.467 |
| ALB (g/L), | 31.60±5.47 | 32.48±6.01 | 0.402 |
| ALB category, n (%) | 0.408 | ||
| <38 g/L | 54 (52.4) | 49 (47.6) | |
| ≥38 g/L | 8 (42.1) | 11 (57.9) | |
| Scr (μmol/L), M (Q1, Q3) | 469.75 (310.60, 632.58) | 427.75 (269.35, 557.83) | 0.537 |
| eGFR (1.73/min/m2), M (Q1, Q3) | 11.56 (8.87, 17.78) | 11.72 (7.38, 19.71) | 0.804 |
| DKD stage, n (%) | 0.912 | ||
| 3 | 3 (42.9) | 4 (57.1) | |
| 4 | 19 (52.8) | 17 (47.2) | |
| 5 | 40 (50.6) | 39 (49.4) | |
| Dialysis therapy, n (%) | 0.006 | ||
| Yes | 36 (64.3) | 20 (35.7) | |
| No | 26 (39.4) | 40 (60.6) | |
| Dialysis type, n (%) | 0.393 | ||
| Hemodialysis | 25 (61.0) | 16 (39.0) | |
| Peritoneal dialysis | 11 (73.3) | 4 (26.7) | |
| iPTH (pg/mL), M (Q1, Q3) | 22.92 (12.10, 85.60) | 95.45 (26.68, 213.84) | 0.001 |
| iPTH category, n (%) | 0.004 | ||
| ≤88 pg/mL | 40 (62.5) | 24 (37.5) | |
| >88 pg/mL | 13 (33.3) | 26 (66.7) | |
| Baseline Hb (g/L), | 81.11±11.00 | 80.68±12.06 | 0.837 |
| Baseline Hct (%), | 25.18±3.76 | 24.96±3.55 | 0.734 |
Missing values: iPTH data were missing in 19 cases (15.57%) among the total 122 cases. CRP, C-reactive protein; , mean; s, standard deviation; BMI, body mass index; ALB, serum albumin; Scr, serum creatinine; M, median; Q1, lower quartile; Q3, upper quartile; eGFR, estimated glomerular filtration rate; DKD, diabetic kidney disease; iPTH, intact parathyroid hormone; Hb, hemoglobin; Hct, hematocrit.
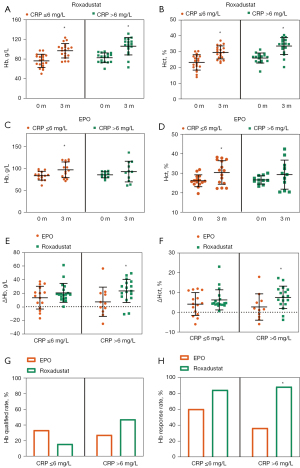
Additionally, under different CRP levels, the Hb qualified rate did not differ significantly between the two groups (see Figure 2G). In relation to the Hb response rate comparison, the results remained non-significant in the normal CRP subgroup, but the Hb response rate was significantly higher in the Roxadustat treated group (88.2% versus 36.4%; see Figure 2H).
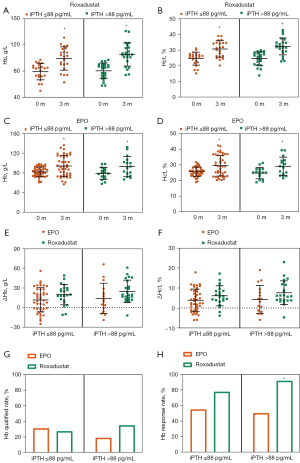
Additionally, 19 participants lacked baseline iPTH data. Participants who received Roxadustat treatment, had higher baseline ALB levels and eGFRs, lower Scr levels, and were diagnosed as G3 DKD had missing iPTH data more frequently (see Table 6). We formed the view that the missing iPTH data was also missing not at random (MNAR), and classified iPTH ≤88 pg/mL as normal iPTH, and iPTH >88 pg/mL as high iPTH. Under different iPTH levels, both Roxadustat and EPO improved Hb and Hct levels, and the level of efficacy in terms of the ΔHb and ΔHct was similar (see Figure 3A-3G). However, in the high iPTH subgroup, the Hb response rate of Roxadustat was 91.3%, higher than the 50% Hb response rate of EPO (see Figure 3H).
Table 6
| Characteristics | Not missing (n=103, 84.43%) | Missing (n=19, 15.57%) | P value |
|---|---|---|---|
| Roxadustat, n (%) | 0.001 | ||
| Yes | 45 (73.8) | 16 (26.2) | |
| No | 58 (95.1) | 3 (4.9) | |
| Age-year, | 59.24±11.50 | 66.47±16.08 | 0.075 |
| Sex-male, n (%) | 73 (84.9) | 13 (15.1) | 0.829 |
| BMI (kg/m2), | 24.18±3.55 | 23.82±3.13 | 0.673 |
| ALB (g/L), | 31.56±5.50 | 34.58±6.42 | 0.034 |
| ALB category, n (%) | 0.005 | ||
| <38 g/L | 91 (88.3) | 12 (11.7) | |
| ≥38 g/L | 12 (63.2) | 7 (36.8) | |
| Scr (μmol/L), M (Q1, Q3) | 480.00 (334.00, 646.60) | 232.00 (173.00, 375.00) | <0.001 |
| eGFR (1.73/min/m2), M (Q1, Q3) | 11.06 (7.65, 16.73) | 19.14 (12.15, 29.85) | 0.002 |
| DKD stage, n (%) | 0.003 | ||
| 3 | 3 (42.9) | 4 (57.1) | |
| 4 | 28 (77.8) | 8 (22.2) | |
| 5 | 72 (91.1) | 7 (8.9) | |
| Dialysis therapy, n (%) | 0.018 | ||
| Yes | 52(92.9) | 4 (7.1) | |
| No | 51 (77.3) | 15 (22.7) | |
| Dialysis type | 0.565 | ||
| Hemodialysis | 37 (90.2) | 4 (9.8) | |
| Peritoneal dialysis | 15 (100.0) | 0 (0) | |
| CRP (mg/L), M (Q1, Q3) | 5.09 (2.48, 10.88) | 6.39 (4.26, 17.05) | 0.363 |
| CRP category, n (%) | 0.650 | ||
| ≤6 mg/L | 32 (94.1) | 2 (5.9) | |
| >6 mg/L | 25 (89.3) | 3 (10.7) | |
| Baseline Hb (g/L), | 80.62±11.14 | 82.42±13.43 | 0.532 |
| Baseline Hct (%), | 25.05±3.64 | 25.22±3.75 | 0.853 |
Missing values: CRP data were missing in 60 cases (49.18%) among the total 122 cases. iPTH, intact parathyroid hormone; , mean; s, standard deviation; BMI, body mass index; ALB, serum albumin; Scr, serum creatinine; M, median; Q1, lower quartile; Q3, upper quartile; eGFR, estimated glomerular filtration rate; DKD, diabetic kidney disease; CRP, C-reactive protein; Hb, hemoglobin; Hct, hematocrit.
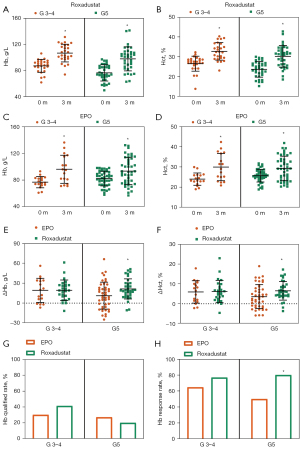
In addition to the missing values, when analyzing the baseline characteristic of the participants, we also found that DKD stage and ALB level differed significantly between the Roxadustat and EPO treatment groups (see Table 1). In this case, we divided the participants into stage G3–4 DKD and stage G5 DKD subgroups for the analysis. Patients suffering from stage G3–5 DKD with renal anemia could all benefit from 3 months of Roxadustat or EPO treatment (in terms of Hb and Hct level). However, in the stage G3–4 DKD subgroup, the ΔHb and ΔHct of the Roxadustat group were similar to those of the EPO group (see Figure 4A-4F), while the ΔHb (21.31±15.11 g/L) and the ΔHct (6.51%±4.68%) of the Roxadustat group were significantly higher than those of the EPO group (ΔHb =11.11±20.33 g/L, ΔHct =3.54%±6.06%) in patients with stage G5 DKD (see Figure 4E,4F). Moreover, in patients with stage G3–4 DKD, there was no obvious change of the Hb qualified rate and response rate when comparing the Roxadustat and EPO groups (see Figure 4G,4H). Among patients with G5 DKD, the Hb response rate induced by Roxadustat was 80%, higher than the 50% response rate induced by EPO (see Figure 4H). However, Hb qualified rates were similar between the 2 groups (see Figure 4G).
Based on the ALB levels, the participants were divided into the normal ALB group (ALB ≥38 g/L) and the low ALB group (ALB <38 g/L). In the low ALB subgroup, ΔHb of Roxadustat was 21.60±14.65 g/L, higher than that of EPO (13.61±20.11 g/L). ΔHct was also higher in the Roxadustat group than the EPO group (6.51%±4.76% versus 4.26%±6.00%). The Hb response rate was 83% in the Roxadustat group, significantly higher than the 55.4% response rate of the EPO group (see Figure 5).
Discussion
This retrospective study evaluated the efficacy of Roxadustat in the treatment of DKD patients with renal anemia over a 3-month period compared to that of EPO. Roxadustat significantly elevated the Hb and Hct levels of the patients from their baseline levels. Based on the analysis of the ΔHb, ΔHct, and the Hb response rate of both therapies, Roxadustat was not different from EPO in anti-anemia effect on DKD patients, but showed high Hb response rate. Further, according to the subgroup analysis, Roxadustat might have better efficacy in treating DKD patients with an advanced stage, or high CRP, high iPTH, and low ALB levels. The effect of Roxadustat is consistent with the findings from previous phase Ⅱ and Ⅲ clinical trials of CKD patients (13-32). Moreover, previous case have reported that DKD patient with refractory anemia could benefit from the application of Roxadustat (37).
We allocated the participants to normal CRP and high CRP groups and performed a subgroup analysis, as the CRP levels differed significantly between the Roxadustat and EPO groups. Notably, there were missing baseline CRP data (see Table 1). The missing CRP data were more common in patients undergoing dialysis and patients with high iPTH levels (see Table 5). This may be because the non-dialysis–dependent DKD patients had not received invasive procedures, such as peritoneal dialysis, hemodialysis catheterization, and arteriovenous fistulas. Thus, their CRP levels were not tested routinely, as the risk of secondary infection was relatively low.
Roxadustat was better at improving renal anemia in DKD patients with higher CRP levels; however, the 2 therapies had similar efficacy among patients with normal CRP levels. These findings support those of previous studies (13,17,24,30,31,34,35), which suggests that the effect of Roxadustat is less affected by inflammation. Inflammation directly increases the production and secretion of hepcidin, contributes to an impaired response to hypoxia, and suppresses the proliferation of erythroid progenitors (38). Thus, the inflammation state is one of the key causes of EPO resistance. However, Roxadustat mimics a mild hypoxia condition and improves the endogenous production of EPO and iron homeostasis. Notably, further studies should be conducted to explore the use of Roxadustat in inflamed state.
Additionally, iPTH level was also verified to be MNAR according to our analysis (see Table 6). This may be because iPTH screening is not commonly conducted in non-dialysis-dependent CKD patients with relatively fair nutritional status and renal function, given the low probability of the onset of hyperparathyroidism. In patients with iPTH >88 pg/mL, the Hb response rate of Roxadustat was higher than that of EPO. Previous research has shown that CKD patients with high iPTH had a lower Hb level and required a higher dose of ESA than those with normal iPTH (39). Together with our findings, this suggests that Roxadustat may be a choice for ameliorating anemia in DKD patients suffering from calcium and phosphorus metabolism disorders, which still needs further studies to confirm.
In addition to inflammation and secondary hyperparathyroidism, disease severity and malnutrition may also contribute to the development of EPO hyporesponsiveness, albeit to a lesser extent (38). This was also consistent with our subgroup analysis findings based on disease stage and ALB level (see Figures 4,5).
This study had a number of limitations. First, the data collection was incomplete. In addition to the missing values for CRP and iPTH mentioned above, we failed to obtain important data related to iron metabolism (e.g., hepcidin) and serum lipid (e.g., cholesterol, low density lipoprotein, and high-density lipoprotein). As the data were collected from the electronic medical record system, it should be noted that more attention should be paid to the iron metabolism of patients with renal anemia. Second, the sample size of this study was rather small. Thus, inconformity in the baseline characteristics between the 2 groups was unavoidable. We used the least-square method for ΔHb and ΔHct and logistic regression analysis for Hb response rate to adjust for the confounders, but the imbalance may still have affected the comparisons between the effects of Roxadustat and EPO. Third, it was also difficult to perform the subgroup analyses, especially the analyses based on dialysis dependency and dialysis modalities. Fourth, the 3-month observational period was not long enough to collect data on any adverse events induced by the drugs. Recall bias makes it difficult to confirm the causality between drugs and events. In this case, we did not provide any adverse drug reaction profiles. Further prospective studies should be conducted in the future. The study was conducted at 2 hospitals in Changsha, the results of a larger-scale multi-center study would have better generalizability.
To further investigate the effect of Roxadustat in DKD patients, we are now recruiting participants for a single-arm, open-label, prospective study focusing on the drug’s efficacy, safety, and the possible long-term benefits, such as the protection of kidney function and the prevention of CVD.
Conclusions
In conclusion, Roxadustat effectively improves DKD renal anemia. The effect of Roxadustat is similar to that of the commonly used EPO.
Acknowledgments
We thank Dr. Jingzheng Shi (Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China) for statistical support.
Funding: This study was supported by grants from the National Natural Science Foundation of China (Nos. NSFC82170744 and NSFC82270733) and the Hunan Province Traditional Chinese Medicine Research Program (No. C2022002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4344/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4344/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4344/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The Second Xiangya Hospital of Central South University (No. LYF2022171). The Third Xiangya Hospital was informed and agreed the study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ruiz-Ortega M, Rodrigues-Diez RR, Lavoz C, et al. Special Issue "Diabetic Nephropathy: Diagnosis, Prevention and Treatment". J Clin Med 2020;9:813. [Crossref] [PubMed]
- Silverberg DS, Wexler D, Iaina A, et al. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol 2006;38:295-310. [Crossref] [PubMed]
- Babitt JL, Eisenga MF, Haase VH, et al. Controversies in optimal anemia management: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 2021;99:1280-95. [Crossref] [PubMed]
- Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol 2005;16:3403-10. [Crossref] [PubMed]
- Kim-Mitsuyama S, Soejima H, Yasuda O, et al. Anemia is an independent risk factor for cardiovascular and renal events in hypertensive outpatients with well-controlled blood pressure: a subgroup analysis of the ATTEMPT-CVD randomized trial. Hypertens Res 2019;42:883-91. [Crossref] [PubMed]
- Li Y, Shi H, Wang WM, et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Medicine (Baltimore) 2016;95:e3872. [Crossref] [PubMed]
- Loutradis C, Skodra A, Georgianos P, et al. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: A nested case-control study. World J Nephrol 2016;5:358-66. [Crossref] [PubMed]
- Bosman DR, Winkler AS, Marsden JT, et al. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care 2001;24:495-9. [Crossref] [PubMed]
- Ito K, Yokota S, Watanabe M, et al. Anemia in Diabetic Patients Reflects Severe Tubulointerstitial Injury and Aids in Clinically Predicting a Diagnosis of Diabetic Nephropathy. Intern Med 2021;60:1349-57. [Crossref] [PubMed]
- Li Vecchi M, Fuiano G, Francesco M, et al. Prevalence and severity of anaemia in patients with type 2 diabetic nephropathy and different degrees of chronic renal insufficiency. Nephron Clin Pract 2007;105:c62-7. [Crossref] [PubMed]
- Del Vecchio L, Minutolo R. ESA, Iron Therapy and New Drugs: Are There New Perspectives in the Treatment of Anaemia? J Clin Med 2021;10:839. [Crossref] [PubMed]
- Provenzano R, Singh AK. Hemoglobin maintenance with use of extended dosing of epoetin alfa in patients with diabetes and anemia of chronic kidney disease. Endocr Pract 2007;13:251-9. [Crossref] [PubMed]
- Hou YP, Mao XY, Wang C, et al. Roxadustat treatment for anemia in peritoneal dialysis patients: A randomized controlled trial. J Formos Med Assoc 2022;121:529-38. [Crossref] [PubMed]
- Fishbane S, Pollock CA, El-Shahawy M, et al. Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study. J Am Soc Nephrol 2022;33:850-66. [Crossref] [PubMed]
- Shutov E, Sułowicz W, Esposito C, et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a Phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol Dial Transplant 2021;36:1629-39. [Crossref] [PubMed]
- Provenzano R, Shutov E, Eremeeva L, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant 2021;36:1717-30. [Crossref] [PubMed]
- Fishbane S, El-Shahawy MA, Pecoits-Filho R, et al. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J Am Soc Nephrol 2021;32:737-55. [Crossref] [PubMed]
- Csiky B, Schömig M, Esposito C, et al. Roxadustat for the Maintenance Treatment of Anemia in Patients with End-Stage Kidney Disease on Stable Dialysis: A European Phase 3, Randomized, Open-Label, Active-Controlled Study (PYRENEES). Adv Ther 2021;38:5361-80. [Crossref] [PubMed]
- Barratt J, Sulowicz W, Schömig M, et al. Efficacy and Cardiovascular Safety of Roxadustat in Dialysis-Dependent Chronic Kidney Disease: Pooled Analysis of Four Phase 3 Studies. Adv Ther 2021;38:5345-60. [Crossref] [PubMed]
- Barratt J, Andric B, Tataradze A, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a Phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant 2021;36:1616-28. [Crossref] [PubMed]
- Akizawa T, Yamaguchi Y, Otsuka T, et al. A Phase 3, Multicenter, Randomized, Two-Arm, Open-Label Study of Intermittent Oral Dosing of Roxadustat for the Treatment of Anemia in Japanese Erythropoiesis-Stimulating Agent-Naïve Chronic Kidney Disease Patients Not on Dialysis. Nephron 2020;144:372-82. [Crossref] [PubMed]
- Akizawa T, Ueno M, Shiga T, et al. Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies. Ther Apher Dial 2020;24:628-41. [Crossref] [PubMed]
- Akizawa T, Otsuka T, Reusch M, et al. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial 2020;24:115-25. [Crossref] [PubMed]
- Akizawa T, Iwasaki M, Yamaguchi Y, et al. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol 2020;31:1628-39. [Crossref] [PubMed]
- Chen N, Hao C, Peng X, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 2019;381:1001-10. [Crossref] [PubMed]
- Chen N, Hao C, Liu BC, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med 2019;381:1011-22. [Crossref] [PubMed]
- Akizawa T, Iwasaki M, Otsuka T, et al. Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther 2019;36:1438-54. [Crossref] [PubMed]
- Chen N, Qian J, Chen J, et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 2017;32:1373-86. [Crossref] [PubMed]
- Provenzano R, Besarab A, Wright S, et al. Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study. Am J Kidney Dis 2016;67:912-24. [Crossref] [PubMed]
- Provenzano R, Besarab A, Sun CH, et al. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin J Am Soc Nephrol 2016;11:982-91. [Crossref] [PubMed]
- Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol 2016;27:1225-33. [Crossref] [PubMed]
- Besarab A, Provenzano R, Hertel J, et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 2015;30:1665-73. [Crossref] [PubMed]
- Akizawa T, Tanaka-Amino K, Otsuka T, et al. Clinical parameters among patients in Japan with anemia and non-dialysis-dependent chronic kidney disease with and without diabetes mellitus who received roxadustat. Clin Exp Nephrol 2022;26:843-50. [Crossref] [PubMed]
- Akizawa T, Yamaguchi Y, Majikawa Y, et al. Factors affecting the doses of roxadustat vs darbepoetin alfa for anemia treatment in hemodialysis patients. Ther Apher Dial 2021;25:575-85. [Crossref] [PubMed]
- Akizawa T, Tanaka-Amino K, Otsuka T, et al. Factors Affecting Doses of Roxadustat Versus Darbepoetin Alfa for Anemia in Nondialysis Patients. Am J Nephrol 2021;52:702-13. [Crossref] [PubMed]
- Xie RY, Fang XL, Zheng XB, et al. Salidroside and FG-4592 ameliorate high glucose-induced glomerular endothelial cells injury via HIF upregulation. Biomed Pharmacother 2019;118:109175. [Crossref] [PubMed]
- Zhang H, Huang Z, He L, et al. Successful treatment of anti-EPO antibody associated refractory anemia with hypoxia-inducible factor prolyl hydroxylase inhibitor. Ren Fail 2020;42:860-4. [Crossref] [PubMed]
- Weir MR. Managing Anemia across the Stages of Kidney Disease in Those Hyporesponsive to Erythropoiesis-Stimulating Agents. Am J Nephrol 2021;52:450-66. [Crossref] [PubMed]
- Amnuay K, Srisawat N, Wudhikarn K, et al. Factors associated with erythropoiesis-stimulating agent hyporesponsiveness anemia in chronic kidney disease patients. Hematol Rep 2019;11:8183. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


