Elevated d-dimer cut-off values for computed tomography pulmonary angiography—d-dimer correlates with location of embolism
Introduction
Acute venous thromboembolism is a common disorder with an incidence in the general population of 1–2 cases per 1,000 persons per year with a sharp increase with increasing age (1-4). Acute pulmonary embolism (APE) is a potentially fatal presentation of this condition with an incidence of 0.6–0.7 per 1,000 persons per year (1,3). The mortality rate of APE varies from 1% for small embolism to up to 30% for massive embolism (1).
The clinical presentation of APE is equally diverse ranging from asymptomatic to right ventricular failure and cardiac arrest. Symptoms such as chest pain, dyspnoea, tachycardia, tachypnoea and cough are common but unspecific. Accurate and timely diagnosis is crucial in the emergency room setting. Because of increasing population age, especially in Western countries, diagnosis and treatment of APE has become a major challenge (1). Risk stratification with clinical scoring systems such as Wells score or revised Geneva score is of value, but the sensitivity of 64–79% and 44–74%, respectively; limit their use in ruling out the diagnosis (5). D-dimer, a fibrin degradation product, has a reported sensitivity of 95% and specificity of 36%, and is used to exclude APE in low and medium risk patients (6,7). Some guidelines recommend a d-dimer cut-off value of 0.5 mg/L for performing diagnostic imaging, however there is increasing evidence that cut-off values should be age adjusted (8).
With a sensitivity of 83% and specificity of 96%, computed tomography pulmonary angiography (CTPA) plays a central role in the workup of patients with suspected APE (9). Access, scan time and spatial resolution have all improved, and CT is now the modality of choice for investigation of APE (10,11). This is a contributing factor to the increasing load on emergency room CT scanners which tripled in the last decade (12). As the demand for CTPA increases, so does the necessity of improving patient selection.
The purpose of this study was to estimate a cut-off value of d-dimer, which would reduce the demand for CTPA while maintaining a high sensitivity and negative predictive value (NPV). We also investigated a possible correlation between d-dimer and APE location.
Methods
Our department serves a public hospital with a catchment population of approximately 500,000 in a suburban area. We retrospectively included all patients with suspected APE referred to our department for CTPA between 1st of January and 31st of December 2012. Inconclusive CTPA examinations were excluded. Approval for the study and waiver of informed consent was obtained from the Regional Ethics Committee and the Data Protection Office at our hospital.
CTPA technique
CTPA were performed on four different multidetector CT scanners (Phillips Brilliance 64 row, Phillips Ingenuity 128 Core, and two Phillips iCT 256 rows, Royal Philips, Amsterdam, The Netherlands). In accordance with our standard CTPA protocol patients received an age adapted 60–90 mL intravenous bolus of iomeron 350, iomeprol 350 mg Iodine per mL (Bracco Imaging, Courcouronnes, France) followed by a 35 mL chasing bolus of saline. Pregnant patients and patients with impaired kidney function were examined with a low dose protocol (80 kV) with a reduced age adapted contrast bolus of 35–45 mL followed by 35 mL of saline. We used bolus tracking to assure synchronization of image acquisition with the arrival of contrast media in the pulmonary trunk.
Diagnosis and location of embolism
All radiology reports were reviewed. Presence and location of pulmonary embolism were recorded. Consultants checked examinations read by residents. In cases where the report did not state clearly either the absence or presence or the location of pulmonary embolism, two radiology consultants reviewed the examination to ascertain this. Patients were categorized according to the CTPA result into four categories: no pulmonary embolism (category 0), peripheral pulmonary embolism (category I), pulmonary embolism in lobar arteries (category II) and central embolisms in the pulmonary trunk or pulmonary arteries (category III). Categories according to location of embolism are shown in Figure 1 and refer to the most proximal part of the embolism.
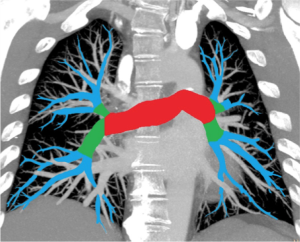
Isolated subsegmental pulmonary embolisms, constituted a subcategory of category I, and were defined as embolisms detected in one or more subsegmental arteries, but not in more proximal vessels (13).
D-dimer assay
D-dimer levels were determined with HemosIL d-dimer HS (Instrumentation Laboratory, Lexington, MA, USA), a rapid automated quantitative latex-based immune-agglutination assay, and obtained from the electronic patient records. We defined concomitant d-dimer analysis as within 48 hours prior to or after the CTPA examination. Earlier or later d-dimer analyses were not taken into consideration. In cases of multiple d-dimer analyses, the one closest in time to the CTPA examination was chosen. The minimal and maximal levels quantified by our lab are 0.3 and 20.0 mg/L, respectively. Lower levels, reported as <0.3 mg/L were recorded as 0, and higher levels, reported as >20.0 mg/L were recorded as 20.0 mg/L.
Statistical analysis
D-dimer values were compared to the current cut-off of 0.5 mg/L and to the suggested age-adjusted cut-off (age/100 mg/L). The diagnostic performance of d-dimer was also analyzed using receiver operating characteristic (ROC) curves, with CTPA result as the gold standard. New suggested cut-off levels were estimated by identifying the highest d-dimer level with a sensitivity and NPV ≥95%. Correlation between presence and location of embolism and levels of d-dimer were tested with Spearman correlation. Statistical analysis was performed using Statistical Package for Social Sciences, version 21 (IBM corp, Somers, NY, USA). All P values were two-sided and a significance level of 0.05 was applied.
Results
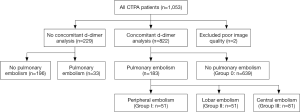
A total of 1,053 patients were referred to CT with a suspicion of APE during the study period. The distribution of these cases is shown in Figure 2. Fifty-three percent were females, mean age 64 years (range, 16–99 years). Two CTPA were inconclusive due to poor image quality, and were excluded from the analysis. We included 1,051 examinations; from which 216 (21%) were positive for pulmonary embolism. Two hundred and twenty-nine CTPA examinations had been conducted without concomitant d-dimer analysis (no d-dimer group) and 33 (14%) of these were positive for pulmonary embolism. In 822 CTPA examinations a concomitant d-dimer analysis had been carried out (d-dimer group), and these were used in the analysis of the diagnostic performance of d-dimer.
CTPA was carried out in 76 patients with d-dimer values below 0.5 mg/L, and from which one (1.3%) was positive for a peripheral pulmonary embolism, affecting segmental arteries. With this cut-off level d-dimer has a sensitivity and NPV of 99%. CTPA was carried out in 143 patients with d-dimer values below the age-adjusted cut-off, from which 3 (2%) were positive for pulmonary embolism. All three were peripheral embolisms, with two affecting segmental arteries and one being isolated subsegmental. The age-adjusted cut-off achieved a sensitivity and NPV of 98%. Table 1 shows the distribution of the 822 cases in the “d-dimer group” in four categories based on presence and location of pulmonary embolism.

Full table
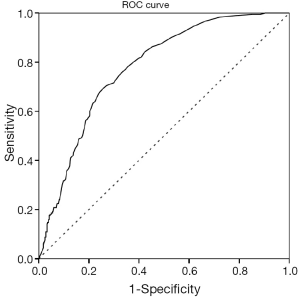
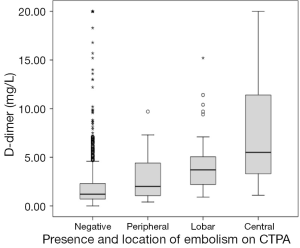
Figure 3 shows the ROC curve for d-dimer with an area under the curve of 0.78 for all APEs. In this population an elevated d-dimer cut-off of 0.9 mg/L achieved a NPV of 97% and sensitivity of 97% for all APE categories. Adopting this standard could have reduced the number of CTPA by 230 (27%) of the 822 at the cost of six missed peripheral pulmonary embolisms (three segmental and three isolated subsegmental). D-dimer values showed significant correlation with the location of the pulmonary embolism, Spearman’s rho: 0.43, P<0.01 (Figure 4).
Discussion
We found that an elevated d-dimer cut-off value of 0.9 mg/L achieved a sensitivity and NPV of 97% and could reduce the number of CTPAs in our department by 27%. In our study population 76 CTPA examinations were conducted on patients with d-dimer values below 0.5 mg/L, detecting a single case of peripheral pulmonary embolism. This indicates that conducting CTPA in this group was not the result of clinical risk stratification, and might advocate stricter requirements for documenting risk scores in the referral. Another 229 CTPAs were performed in the absence of a concomitant d-dimer analysis. This approach is appropriate for high-risk patients in which a negative d-dimer is not sufficient to rule out the condition, or when d-dimer is expected to be elevated due to comorbidities. In the “no d-dimer group” the rate of positive CTPAs (14%) was lower than in the “d-dimer group” (22%).
In our study population, adopting the suggested 0.9 mg/L cut-off for d-dimer would have resulted in pulmonary embolisms being missed in six patients. Interestingly, all six cases had peripheral embolisms, three of which were isolated subsegmental embolisms. We found a significant correlation between d-dimer value and the location of APE. This is consistent with a previous study, and may indicate that larger embolisms release more fibrin degradation products (14). Other studies report correlations between d-dimer levels and thrombotic burden/perfusion defects, recurrence and outcome (15-18). Hence, d-dimer analysis might contribute to a pre CTPA differentiation between peripheral and more central APE.
This possibility should not be ignored in light of studies suggesting that the introduction of CTPA has led to overdiagnosis of pulmonary embolism (19,20). Furthermore the accuracy of CTPA for peripheral pulmonary embolisms is not excellent. The a radiological finding of peripheral pulmonary embolism has a moderate interobserver agreement whit a kappa of 0.47, and the positive predictive value of apparent isolated subsegmental pulmonary embolisms is reported to be as low as 25% (9,21). Coupled with an increased complication rate from anticoagulation therapy this gives cause for concern (19). Patients with isolated subsegmental pulmonary embolisms are reported to have a higher risk of major haemorrhage from anticoagulation therapy than recurrence of embolisms, and both the Fleischner Society and the European Society of Cardiology recognize that these patients might not benefit from anticoagulation therapy (22-24). Considering the correlation between d-dimer values and location of embolism, and the finding that all embolisms detected by CTPA in patients with low d-dimer values were peripheral, these factors support the suggestion of elevated the d-dimer cut-off.
CTPA is especially useful to clinicians because it provides a wide range of diagnostic information other than the presence or absence of pulmonary embolism (11,25). This is a double-edged sword however, revealing an alternate cause of symptoms such as pneumonia or pleural effusion in one third of cases, while revealing incidental findings requiring diagnostic follow-up in one fourth (26). Our study was limited to a retrospective assessment of d-dimer values and CTPA findings. We did not consider clinical presentation, risk stratification or the delay between onsets of symptoms to sampling of blood. Patients with more central embolisms may be investigated in an earlier phase due to a more acute presentation, which may account for their higher d-dimer values. The half-life of d-dimer, which is 13 to 16 hours, is consistent with this interpretation, although these estimates were made in the absence of a thromboembolism (27,28). Our findings would have to be confirmed in a prospective study to ascertain their value.
Conclusions
In conclusion the elevated d-dimer cut-off of 0.9 mg/L achieved a high sensitivity and NPV, while reducing the number of CTPA by 27%. The correlation between d-dimer values and location of embolisms supports the suggestion of an elevated d-dimer value.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval for the study and waiver of informed consent was obtained from the Regional Ethics Committee and the Data Protection Office at our hospital.
References
- Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129-38. [PubMed]
- Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. [PubMed]
- Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d'Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost 2000;83:657-60. [PubMed]
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722-7. [Crossref] [PubMed]
- Shen JH, Chen HL, Chen JR, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis 2016;41:482-92. [Crossref] [PubMed]
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416-20. [PubMed]
- Flores J, García-Avello A, Ruíz A, et al. Can the tandem measurement of age adjusted D-dimer and tissue plasminagen activator improve the clinical utility of a conventional D-dimer in the pulmonary embolism diagnosis? Int Angiol 2016;35:62-70. [PubMed]
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014;311:1117-24. [Crossref] [PubMed]
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317-27. [Crossref] [PubMed]
- Hunsaker AR, Lu MT, Goldhaber SZ, et al. Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardiovasc Imaging 2010;3:491-500. [Crossref] [PubMed]
- Weiss CR, Scatarige JC, Diette GB, et al. CT pulmonary angiography is the first-line imaging test for acute pulmonary embolism: a survey of US clinicians. Acad Radiol 2006;13:434-46. [Crossref] [PubMed]
- Health, United States, 2012: With Special Feature on Emergency Care. Report No. National Center for Health Statistics (NCHS), 2013. Available online: http://www.cdc.gov/nchs/data/hus/hus12.pdf
- Stein PD, Goodman LR, Hull RD, et al. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb Hemost 2012;18:20-6. [Crossref] [PubMed]
- De Monyé W, Sanson BJ, Mac Gillavry MR, et al. Embolus location affects the sensitivity of a rapid quantitative D-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 2002;165:345-8. [Crossref] [PubMed]
- Bauer RW, Frellesen C, Renker M, et al. Dual energy CT pulmonary blood volume assessment in acute pulmonary embolism - correlation with D-dimer level, right heart strain and clinical outcome. Eur Radiol 2011;21:1914-21. [Crossref] [PubMed]
- Goldin Y, Berliner S, Rogowski O, et al. Correlated expression of D-dimer concentrations with thrombotic burden in acute pulmonary embolism. Blood Coagul Fibrinolysis 2008;19:153-8. [Crossref] [PubMed]
- Grau E, Tenías JM, Soto MJ, et al. D-dimer levels correlate with mortality in patients with acute pulmonary embolism: Findings from the RIETE registry. Crit Care Med 2007;35:1937-41. [Crossref] [PubMed]
- Eichinger S, Minar E, Bialonczyk C, et al. D-dimer levels and risk of recurrent venous thromboembolism. JAMA 2003;290:1071-4. [Crossref] [PubMed]
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011;171:831-7. [PubMed]
- Burge AJ, Freeman KD, Klapper PJ, et al. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol 2008;63:381-6. [Crossref] [PubMed]
- Chartrand-Lefebvre C, Howarth N, Lucidarme O, et al. Contrast-enhanced helical CT for pulmonary embolism detection: inter- and intraobserver agreement among radiologists with variable experience. AJR Am J Roentgenol 1999;172:107-12. [Crossref] [PubMed]
- Donato AA, Khoche S, Santora J, et al. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res 2010;126:e266-70. [Crossref] [PubMed]
- Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 2007;245:315-29. [Crossref] [PubMed]
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [Crossref] [PubMed]
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000;217:447-55. [Crossref] [PubMed]
- Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961-5. [Crossref] [PubMed]
- Rühl H, Berens C, Winterhagen A, et al. Label-Free Kinetic Studies of Hemostasis-Related Biomarkers Including D-Dimer Using Autologous Serum Transfusion. PLoS One 2015;10:e0145012. [Crossref] [PubMed]
- Ardaillou N, Yvart J, Le Bras P, et al. Catabolism of human fibrinogen fragment D in normal subjects and patients with liver cirrhosis. Thromb Haemost 1980;44:146-9. [PubMed]