Is afatinib a treatment option for brain metastases in patients with EGFR mutation-positive non-small cell lung cancer?
Introduction
Treatment for advanced non-small cell lung cancer (NSCLC) depends on the molecular characteristics of the tumor. Mutations of the gene for the epidermal growth factor receptor (EGFR) are present in ~32% of Asians and ~7% of individuals of other ethnic groups with NSCLC, with deletions in exon 19 and an L858R point mutation in exon 21 accounting for ~90% of such genetic alterations detected at diagnosis (1). NSCLC tumors that harbor EGFR mutations are oncogene addicted and therefore usually sensitive to treatment with EGFR tyrosine kinase inhibitors (TKIs).
Three EGFR-TKIs—gefitinib, erlotinib, and afatinib—are widely available in the clinic. Gefitinib was the first such drug to be approved for patients with NSCLC positive for EGFR mutations. The IPASS study assessed gefitinib in comparison with carboplatin-paclitaxel as a first-line treatment for patients with advanced NSCLC in East Asia (2). A subset analysis of this study found that gefitinib significantly improved progression-free survival (PFS) compared with the standard chemotherapy in patients with EGFR mutation-positive NSCLC [9.5 vs. 6.3 months; hazard ratio (HR) of 0.48 with a 95% confidence interval (CI) of 0.36–0.64; P<0.001]. Overall survival (OS) was not increased by gefitinib, however, in this subset of patients (21.6 vs. 21.9 months; HR of 1.00; P=0.990) (3). Another two phase III trials performed in Japan reported similar outcomes (4,5).
Erlotinib was also found to be beneficial in first-line treatment of EGFR mutation-positive NSCLC. The EURTAC trial compared erlotinib with platinum-doublet chemotherapy in European patients, finding that the median PFS for erlotinib was 9.7 months compared with only 5.2 months for chemotherapy (HR of 0.37 with a 95% CI of 0.25–0.54; P<0.0001) (6).
In contrast to gefitinib and erlotinib, both of which are reversible inhibitors, afatinib is a highly selective, irreversible EGFR-TKI, often being referred to as a second-generation EGFR-TKI. In a phase III trial (LUX-Lung 3) performed with EGFR mutation-positive NSCLC patients, afatinib improved PFS compared with cisplatin-pemetrexed in the first-line setting (11.1 vs. 6.9 months; HR of 0.58 with a 95% CI of 0.43–0.78; P=0.001) (7). Similar results were obtained in the LUX-Lung 6 trial, which compared gefitinib with cisplatin-gemcitabine in patients in East Asia (PFS of 11.0 vs. 5.6 months; HR of 0.28 with a 95% CI of 0.20–0.39; P<0.0001) (8). The LUX-Lung 7 trial further showed that afatinib was superior to gefitinib in terms of OS in the first-line setting (9).
Brain metastases (BM) in non-small cell lung cancer (NSCLC)
BM are manifest in 16% to 20% of NSCLC patients at diagnosis (10,11). The introduction of magnetic resonance imaging and improvement in OS of such patients likely account for a recent apparent increase in the incidence of BM. BM can cause neurological symptoms and thereby reduce quality of life in NSCLC patients.
A review of 1,127 NSCLC patients found that those with EGFR mutations were more likely to develop BM than those without such mutations (12). The frequency of BM was thus 31.4% for the mutation-positive patients but only 19.7% for the negative ones [odds ratio of 1.86, with a 95% CI of 1.39–2.49; P<0.001). Of note, BM were smaller (P=0.031) and the frequency of leptomeningeal dissemination was higher (30.8% vs. 12.7%; odds ratio of 3.04 with a 95% CI of 1.64–5.78; P<0.001) in the EGFR mutation-positive patients than in those wild type for EGFR. Median OS after diagnosis of BM was also significantly longer in patients with EGFR mutation-positive tumors (HR of 2.23 with a 95% CI of 1.62–3.10; P<0.001). Another study showed that NSCLC patients with a deletion in exon 19 of EGFR had more and smaller metastases with a reduced extent of peritumoral brain edema compared with patients with wild-type EGFR alleles, whereas the characteristics of BM in patients with the L858R point mutation of EGFR were similar to those of the metastases in wild-type patients (13).
The standard management for BM to date has been irradiation [including whole-brain radiation therapy (WBRT) and stereotactic radiosurgery] and surgical resection. Traditional cytotoxic agents usually do not penetrate the blood-brain barrier. However, the possibility of systemic EGFR-TKI treatment for BM in patients with EGFR mutation-positive NSCLC is receiving increasing attention.
EGFR-TKIs for treatment of brain metastases (BM)
A phase II study evaluated gefitinib alone (without irradiation) for the treatment of BM in 41 patients with EGFR mutation-positive NSCLC (14). The response rate (RR) for BM, median PFS, and median OS were 87.8%, 14.5 months (95% CI of 10.2–18.3 months), and 21.9 months (95% CI of 18.5–30.3 months), respectively (Table 1). This favorable outcome suggested that EGFR-TKIs might delay the need for irradiation and the associated risk of neurocognitive decline in such patients. Erlotinib achieves a higher cerebrospinal fluid concentration than gefitinib (18), but the clinical efficacy of erlotinib alone for BM has not been well assessed in a prospective study. A retrospective study of erlotinib treatment in 17 patients with EGFR mutation-positive NSCLC and BM found that the RR for BM, median time to progression (TTP) in the brain, and median OS were 82.4%, 11.7 months (95% CI of 7.9–15.5 months), and 12.9 months (95% CI of 6.2–19.7 months), respectively (15) (Table 1). Nine of these 17 patients had a history of WBRT. Another retrospective study compared erlotinib, WBRT, and stereotactic radiosurgery for 110 EGFR-TKI-naïve NSCLC patients with BM (n=63, 32, and 15, respectively) (16) (Table 1). Although no significant difference in median OS was apparent between the WBRT and erlotinib groups (35 vs. 26 months, respectively; P=0.62), median intracranial TTP was significantly longer in the WBRT group than in the erlotinib group (24 vs. 16 months; P=0.04). Among patients in the WBRT group who received erlotinib within 2 months of completing irradiation (n=21), the median TTP for BM during erlotinib treatment was 25 months, which was significantly longer than that in the erlotinib group by univariate analysis (P=0.01) but not significantly longer by multivariate analysis (P=0.20). Thus, although erlotinib appears to prolong TTP in the brain, its effectiveness for treatment of BM in patients with EGFR mutation-positive NSCLC might be enhanced by prior WBRT.
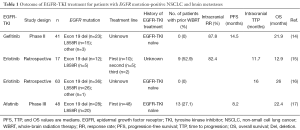
Full table
Afatinib has been even less well validated for treatment of BM than has gefitinib or erlotinib. A subset analysis for LUX-Lung 2, a phase II study of afatinib for patients with EGFR mutation-positive NSCLC, found that the overall RR did not differ significantly between patients with or without BM (65% vs. 60%, respectively; HR of 1.20 with a 95% CI of 0.52–2.78) (19).
A recent study reported a subset analysis for patients with common EGFR mutations (exon 19 deletion or L858R), and BM in the LUX-Lung 3 and LUX-Lung 6 trials (17) (Table 1). Whereas LUX-Lung 3 compared afatinib with cisplatin-pemetrexed in 345 treatment-naïve patients with EGFR mutation-positive NSCLC (7), LUX-Lung 6 compared afatinib with cisplatin-gemcitabine in 364 such patients of Asian ethnicity (8). The two trials included 42 (12.2%) and 49 (13.5%) patients with clinically asymptomatic and controlled BM, respectively, most of whom had common EGFR mutations [n=81 (89%)]. Among these patients with BM, there was a trend toward improved PFS on treatment with afatinib compared with standard chemotherapy in both LUX-Lung 3 (11.1 vs. 5.4 months; HR of 0.54 with a 95% CI of 0.23–1.25; P=0.1378) and LUX-Lung 6 (8.2 vs. 4.7 months; HR of 0.47 with a 95% CI of 0.18–1.21; P=0.1060). Combined analysis of both trials revealed a significant improvement in PFS for the afatinib group compared with the chemotherapy group (8.2 vs. 5.4 months; HR of 0.50 with a 95% CI of 0.27–0.95; P=0.0297). Of note, the PFS benefit of afatinib compared with chemotherapy was enhanced by prior WBRT treatment, with median PFS values of 13.8 vs. 4.7 months (HR of 0.37 with a 95% CI of 0.12–1.17; P=0.0767) for patients with prior WBRT (n=24) and of 6.9 vs. 5.4 months (HR of 0.62 with a 95% CI of 0.28–1.36; P=0.2222) for those without prior WBRT (n=57). One possible explanation for this finding is that WBRT followed by afatinib can confer longer intracranial and extracranial PFS, respectively. Alternatively, WBRT might have disrupted the blood-brain barrier and thereby facilitated the entry of afatinib into the brain (20). Rates of central nervous system (CNS) progression in patients with BM at baseline were similar for afatinib treatment [n=9 (45.0%) in LUX-Lung 3 and n=6 (21.4%) in LUX-Lung 6] and chemotherapy [n=5 (33.3%) in LUX-Lung 3 and n=5 (27.8%) in LUX-Lung 6]. Similar rates of CNS progression were observed in the two trials for all patients without BM at baseline [n=3 (3.7%) in LUX-Lung 3 and n=4 (4.7%) in LUX-Lung 6]. Median OS in patients with BM did not differ significantly between afatinib and chemotherapy for LUX-Lung 3 (19.8 vs. 33.2 months, respectively; HR of 1.15 with a 95% CI of 0.49–2.67; P=0.7517), for LUX-Lung 6 (22.4 vs. 24.7 months; HR of 1.13 with a 95% CI of 0.56–2.26; P=0.7315), or for the combined data set (22.4 vs. 25.0 months; HR of 1.14 with a 95% CI of 0.66–1.94; P=0.6412). An OS benefit for afatinib over chemotherapy was apparent for total patients with a deletion in exon 19 of EGFR, whereas no significant difference was observed between afatinib and chemotherapy for patients with an exon 19 deletion and BM (22.4 vs. 20.6 months, respectively; HR of 0.78 with a 95% CI of 0.37–1.66; P=0.5229) (21). This difference might be due to an effect of subsequent therapy or to the small number of patients with BM included in the analysis. In conclusion, this study demonstrated superiority of afatinib over chemotherapy in patients with EGFR mutation-positive NSCLC and BM.
Reported OS times for the various studies of EGFR mutation-positive NSCLC patients with BM treated with EGFR-TKIs are similar (Table 1). Given that there have been no head-to-head comparisons among gefitinib, erlotinib, and afatinib for such patients, the best EGFR-TKI for their treatment is not yet known. In addition, prospective data are currently limited, with most of the published studies of EGFR-TKI efficacy in this patient population having been retrospective in nature. The combined subset analysis of the LUX-Lung 3 and LUX-Lung 6 trials is the first such report from a phase III study. Given that the data suggest that afatinib is superior to chemotherapy in terms of PFS for patients with EGFR mutation-positive NSCLC and BM, this drug is a potential treatment option for such patients.
Whether WBRT or an EGFR-TKI should be selected for patients with symptomatic BM is unclear. Patients with symptomatic or unstable BM have been excluded from most clinical trials of EGFR-TKIs, with traditional WBRT thus still being preferred for such cases. In patients with asymptomatic and stable BM, however, EGFR-TKIs have the potential to prolong the time to the onset of intracranial radiation therapy and consequent side effects. EGFR-TKIs without irradiation might be appropriate for patients for whom treatment-related neurocognitive decline is a particular concern. The combined analysis of the LUX-Lung 3 and LUX-Lung 6 trials suggested that prior WBRT prolonged PFS in patients with BM treated with afatinib (17). A retrospective study of erlotinib treatment also suggested that prior WBRT prolongs TTP in the brain (16). Whether an EGFR-TKI alone or together with prior WBRT should be selected for EGFR mutation-positive patients with symptomatic BM should thus be addressed carefully on a case-by-case basis, with further studies exploring the effects of EGFR-TKIs in such patients being warranted.
What about treatment for patients with BM and NSCLC positive for a secondary T790M mutation of EGFR, which confers resistance to gefitinib, erlotinib, and afatinib? The efficacy of osimertinib, a third-generation EGFR-TKI that is effective against the T790M mutant form of EGFR, for such patients is unclear. Furthermore, a recent study found that the CNS metastases including leptomeningeal metastases of 10 of 12 patients whose extracranial tumor was positive for T790M were negative for this mutation (22). If the CNS metastases of most patients with T790M-positive extracranial tumors are indeed T790M negative, then the metastatic lesions may be susceptible to control by first- or second-generation EGFR-TKIs. AZD3759 is an investigational EGFR-TKI that shows high penetrance into the CNS in vivo and is currently under evaluation in a phase I clinical trial (23). This agent may thus hold promise for the treatment of patients with EGFR mutation-positive NSCLC and BM.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Chunwei Xu, MD, PhD (Pathology and Biobank Department, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China).
Conflicts of Interest: H Hayashi has received lecture fees from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Bristol Myers Squibb, and Taiho Pharmaceutical Co. Ltd.; research funding from Ono Pharmaceutical Co. Ltd.; as well as advisory fees from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., and Eli Lilly Japan K.K. K Nakagawa has received lecture fees and advisory fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., and Nippon Boehringer Ingelheim Co., Ltd. S Watanabe has no conflicts of interest to declare.
References
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98:1817-24. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer 2012;77:64-9. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [Crossref] [PubMed]
- Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys 2014;89:322-9. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012;13:539-48. [Crossref] [PubMed]
- Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 2015;6:8366-76. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
- Zeng Q, Wang J, Cheng Z, et al. Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. J Med Chem 2015;58:8200-15. [Crossref] [PubMed]


