Management and postoperative outcome in primary lung cancer and heart disease co-morbidity: a systematic review and meta-analysis
Introduction
Co-morbidity of primary lung cancer (LC) and heart disease (HD) characterizes a high-risk group of patients necessitating prompt diagnosis and treatment. By extensively reviewing the relevant literature of the last 36 years, from January 1 1980 to present, a number of studies, being cited below, have focused on how to manage these patients.
Issues investigated deal with the impact of: (I) age (1-4); (II) safety of choice for a combined or staged surgical procedure (5-19); (III) on- or off-pump technique on postoperative complications occurrence (12,16,20-27); (IV) an extended surgery, due to LC infiltration in the left atrium and/or great vessels, on postoperative outcome (24,28-30); (V) video-assisted thoracoscopic surgery (VATS) method on primary LC patients (31-36); (VI) neo-adjuvant or adjuvant treatment (37-40); and (VII) factors, other than the aforementioned ones, influencing postoperatively patients operated for primary LC and HD. These factors were: myocardial ischemia (41), arrhythmias (41,42), type of grafts selected (20), high preoperative serum level of lactate dehydrogenase (LDH) (43), low preoperative forced expiratory volume in one second (FEV1) (% of predicted) (L) (2), low postoperative FEV1 (% of predicted) (L) (43), no immediate extubation (43), tumor’s staging (2,22), postoperative bleeding (22,44), histologic type of LC (2,45,46), completeness of lymph node dissection (44,46), timing of cardiac compared to lung surgery (47), early administration of anti-coagulant therapy after LC surgery to prevent arrhythmias (48) and finally completion pneumonectomy (49).
The aim of this article is the review of available evidence guiding management decisions in patients with primary LC and cardiac disease co-morbidity, both requiring surgical treatment. This is answered in a three pronged arm by:
- Performing a systematic review and meta-analysis of studies published since 1985 to present, examining patients with primary LC and HD co-morbidity, focusing on parameters such as mortality, postoperative complications and survival rate;
- Making an overall medical interpretation of the factors influencing postoperative outcome;
- Elucidating whether combined or staged surgery is preferable for prolongation of life in these patients.
Methods
Study design
Two literature searches were conducted, each one followed by the respective meta-analysis. Particularly, the first search was about the impact of perioperative management on postoperative outcome of patients surgically treated for primary LC while the second was about the impact of combined or staged surgical procedures on postoperative outcome of patients operated for both primary LC and HD co-morbidity. In each meta-analysis the same parameters were studied such as thirty-day postoperative mortality, postoperative complications, three- and five-year survival probabilities. Also, both searches covered the period from January 1 1990 to present (April 05 2016). The reason for choosing this time period was to obtain newer information published in the relevant literature during the last 26 years. Concerning the second literature search the start date extended back to January 1 1980 because articles reporting information for the postoperative outcome of patients undergoing combined primary LC and HD surgery first appeared in literature during the eighties. The PubMed (National Library of Medicine/National Institute of Health, United States) database was used.
Both searches were conducted by the Hellenic National Documentation Centre. The key words and research items to locate the relevant articles as well as the selection of the articles included in meta-analyses were determined by the primary investigator (George D. Bablekos).
The information provided from the selected articles focused on: (I) 30-day postoperative mortality; (II) type of postoperative complications; (III) histologic type of LC; (IV) p-T status; (V) type of the operated co-existing HD; (VI) use of extra-corporeal circulation (ECC); and (VII) 3- and 5-year survival rate. There was no overlap between searches, while the methodology concerning the approach for each literature search is as follows:
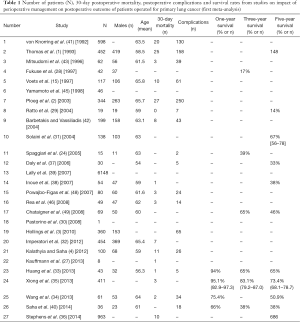
- The first literature search, based on the impact of perioperative management on the postoperative outcome of patients undergoing surgical operation for primary LC, intended to select data for the first meta-analysis. For LC the international terms were: (i) lung neoplasms [Mesh]/surgery [Mesh] AND (surgical procedures) [Mesh], (operative) [Mesh] OR (pulmonary surgical procedures) [Mesh]. The terms AND, OR are internationally characterized as Boolean operators. For HD the international term was: (ii) heart diseases [Mesh]. These two terms were combined with and (1 AND 2) and the result was combined with the following subtitles: (i) (survival rate) OR (quality of life) [Mesh]; (ii) (perioperative period) [Mesh] OR (perioperative care) [Mesh] OR (preoperative period) [Mesh]; (iii) (intra-operative complications) [Mesh]; and (iv) (combined modality therapy) [Mesh]. Combined modality therapy included also terms such as: neo-adjuvant therapy [Mesh], chemo-radiotherapy [Mesh], adjuvant [Mesh] and radiotherapy [Mesh]. The allocated results were combined with OR. A total of 122 studies were relevant to the subject under investigation. Five further studies (keywords: lobectomy, pneumonectomy, surgery, thoracoscopic, video-assisted, non-small cell lung cancer) from other sources (Scopus data base) and published in the relevant literature from January 1 2004 to May 31 2014, were also selected for the present work. Twenty-seven of the above 127 studies were assessed for eligibility, based on their title and abstract, to extract information for the first meta-analysis (Table 1);
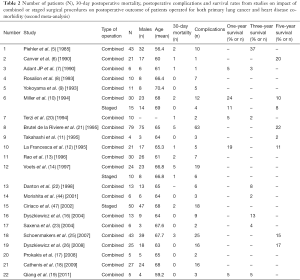
- The second literature search, focusing on the impact of combined or staged surgical procedures on postoperative outcome of patients with co-morbidity of primary LC and HD both requiring surgical treatment, was designed for the collection of data for the second meta-analysis. The terms used for LC and HD were: (i) lung neoplasms/surgery [Mesh] OR (lung neoplasms) [Mesh] AND (surgical procedures, operative) [Mesh] OR (pulmonary surgical procedures) [Mesh]; (ii) heart diseases [Mesh]. One and 2 were combined between with and (1 AND 2) and 1,038 articles resulted. These articles were successively combined by using and with the following combinations of terms: (i) staged surgical [title/abstract]; (ii) staged surgery [title/abstract]; (iii) staged operation [title/abstract]; and (iv) combined [title], concomitant [title]. The final results were combined with or and 77 studies were identified. Eighteen of the 77 articles were selected based on title and abstract to extract information for the second meta-analysis. Moreover, ten articles (keywords: combined, cardiac, pulmonary, concomitant, surgery, lung cancer) from other sources (Scopus data base) were further selected for the second meta-analysis. Information resulted from the above ten articles covered period from January 1 1990 to December 31 2007.
Full table
Twenty-two of the 28 articles were assessed for eligibility based on their title and abstract to extract information for the second meta-analysis (Table 2).
Full table
Forty-nine studies were included in qualitative synthesis for both meta-analyses among which thirty-seven were finally used in quantitative synthesis. Twelve articles were excluded due to lack of sufficient information (Figure 1).
Moreover, to determine the quality of the literature reviewed in our study, we determined the number of citations of these articles since January 1 1985 and the total impact factor (IF) of their corresponding journals. For the number of citations the base of Science Citation Index Expanded (SCI-Expanded) Thomson Reuters was used. Besides, for the total IF the base of Journal Citation Reports (JCR) version 2013 Thomson Reuters was used. The number of citations for articles included in both meta-analyses was found to be 837 in 552 overall hits on April 05 2016. Also, the total IF was calculated as 44.222. Both citations and impact factors were provided by the Hellenic National Documentation Center.
Statistical analysis
For the meta-analysis of the 30-day mortality and complications proportions, both fixed and random effects approaches were used. An overall proportion with exact binomial confidence intervals (CIs) was calculated in the fixed effects approach. In the presence of significant heterogeneity, pooled proportions and relevant 95% CIs were estimated by summarizing the Freeman-Tukey transformed proportions, using random effects meta-analysis (DerSimonian and Laird’s method) (50). Heterogeneity was assessed using the I2 index (51). Meta- regression was used to explore significant heterogeneity.
A similar approach was used for survival probability, since in several studies relevant to the clinical outcome the number of patients that survived at various time points (one to five years) was reported.
Analysis was performed using Stata v10.
Results
Sixteen and twenty-one studies, with information on 30-day postoperative mortality and postoperative complications, were qualified for the first and the second meta-analysis, respectively. The majority of the studies reported the survival rate but not in a consistent way: most studies relevant to first meta-analysis reported a survival rate (%) at various time points but not a measure of variability (CIs or standard errors) while studies relevant to second meta-analysis reported the number of patients that survived at various time points (Tables 1,2).
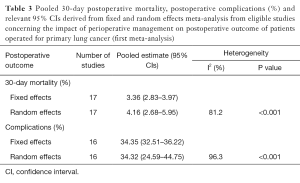
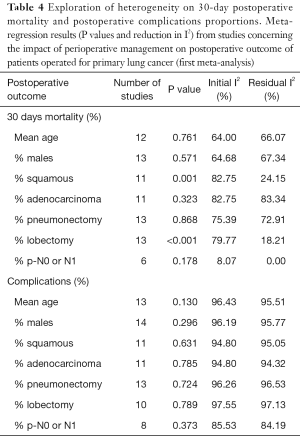
The reported results for 30-day postoperative mortality from studies of the first meta-analysis (impact of perioperative management on postoperative outcome of patients operated for primary LC) appear to be very heterogeneous (I2=81.2%, P value <0.001) (Table 3, Figure 2A). The random effects pooled percentage (%) was 4.16% (95% CI: 2.68–5.95). We used available information to explain heterogeneity. Particularly, we used age (mean age), gender (% males), type of tumor (% squamous, % adenocarcinoma), type of operation (% pneumonectomy, % lobectomy) and severity (% p-N0 or p-N1). From the above variables, (%) squamous tumor and (%) lobectomy were found to substantially reduce heterogeneity in a subset of the 16 studies; this reduction was statistically significant (Table 4). Specifically, higher (%) squamous tumors were associated with higher 30-day postoperative mortality, while higher (%) lobectomy with lower. An indication for publication bias was found (Egger’s test P value =0.074).
Full table

Full table
Heterogeneity was also evident when postoperative complications were considered (I2=96.3%, P value <0.001) (Table 3, Figure 2B). The random effects pooled percentage was 34.32% (95% CI: 24.59–44.75). None of the aforementioned variables were found to reduce heterogeneity (Table 4). There was no evidence of publication bias regarding postoperative complications (Egger’s test P value =0.799).
Twenty-one studies on the second meta-analysis (impact of combined or staged surgical procedures on postoperative outcome of patients operated for both primary LC and HD co-morbidity) reported 30-day postoperative mortality figures. Eighteen of them used a combined procedure, two used both combined and staged and one used only staged. The overall 30-day postoperative mortality proportion was 5.26% (95% CI: 3.47–7.62) (Table 5, Figure 3A). No heterogeneity between studies was found (I2=0.0%, P value =0.753). Furthermore, no evidence for publication bias was found (Egger’s test P value =0.815).
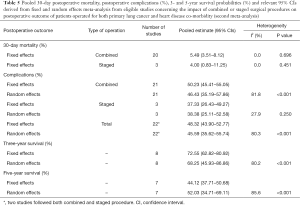
Full table

On the other hand, reported proportions of postoperative complications were highly heterogeneous (I2=80.3%, P value <0.001), reflecting the heterogeneity between studies following a combined procedure. The random effects overall pooled percentage was 45.59% (95% CI: 35.62–55.74) (Table 5, Figure 3B). Similarly with 30-day postoperative mortality, postoperative complications proportions were higher for the combined procedure but due to the small number of studies following a staged procedure, comparisons were avoided.
Meta-regression identified (%) squamous histology, (%) lobectomy and (%) p-T1 or p-T2 as statistically significant parameters that reduce heterogeneity. Studies with higher (%) squamous histology (P=0.001), (%) lobectomy (P=0.002) and (%) p-T1 or p-T2 (P=0.034) were associated with higher proportion of postoperative complications (Table 6). No evidence for publication bias was found (Egger’s test P value =0.372).

Full table
Only 3- and 5-year pooled survival probabilities (Figure 4A,B) from studies of the second meta-analysis were estimated, based on data availability, both displaying significant heterogeneity between studies (Table 5). The random effects pooled 3- and 5-year survival probabilities were 68.25% (95% CI: 45.93–86.86) and 52.03% (95% CI: 34.71–69.11), respectively. Higher mean age (P=0.046) and (%) lobectomy (P=0.009) were associated with decreased 5-year survival while for the 3-year survival none of the available parameters were found to significantly reduce heterogeneity (Table 7). Egger’s test for publication bias was not statistically significant (P values =0.301 and 0.153 for 3- and 5-year survival, respectively).

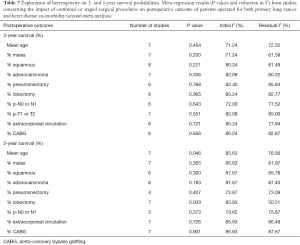
Full table
Moreover, concerning both meta-analyses, no statistically significant differences were found postoperatively between arrhythmias versus respiratory complications as well as total cardiac versus respiratory complications (Tables 8,9).
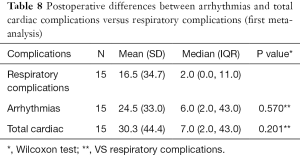
Full table
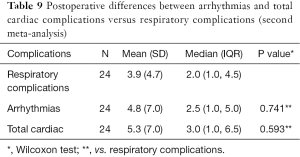
Full table
In addition, focusing on the types of LC surgery performed, the frequency of lobectomies was significantly increased compared to pneumonectomies, both in studies of the first [median (IQR): 68.0 (22.0, 186.5) vs. 21.5 (9.5, 154) respectively, P=0.004, 12 studies] and in studies of the second meta-analysis [median (IQR): 8.5 (5.0, 22.0) vs. 3.0 (2.0, 5.0) respectively, P<0.001, 18 studies]. Lobectomies were also significantly increased compared to wedge resections [median (IQR): 7.5 (4.0, 15.5) vs. 3.0 (2.0, 7.0) respectively, P=0.043] in 16 studies of the second meta-analysis. The number of pneumonectomies and wedge resections was comparable in 12 studies of the second meta-analysis [median (IQR): 3.0 (1.5, 4.0) vs. 4.0 (2.0, 7.0) respectively, P=0.345].
Regarding the types of cardiac surgery in the studies of the second meta-analysis, the frequency of the aorto-coronary bypass grafting (CABG) [median (IQR): 14.5 (9.0, 23.0)] was significantly higher compared to both aortic valve replacement (AVR) [median (IQR): 1.0 (1.0, 3.0), P=0.001, 14 studies] and mitral valve replacement (MVR) [median (IQR): 1.0 (1.0, 2.0), P=0.012, 8 studies]. AVR and MVR frequency was similar [median (IQR): 1.0 (1.0, 4.0) vs. 1.5 (1.0, 2.0) respectively, P=0.899, 6 studies].
Discussion
This study is based on available evidence concerning decision making in management of patients with primary LC and HD both requiring surgical treatment. A concise description of the main results is firstly presented. Then, an overall medical interpretation of the factors potentially influencing the postoperative outcome of these patients is attempted followed by the comments on our findings.
The basic meta-analytic findings are:
- Higher percentages (%) of squamous LC histology and lobectomy were associated with a significant increase (P=0.001) and decrease (P<0.001) respectively, in 30-day postoperative mortality (first meta-analysis) (Table 4);
- Combined surgical operations were significantly (P<0.001) correlated with higher postoperative complications (second meta-analysis) (Table 5);
- Higher percentages for squamous LC histology, lobectomy and p-T1 or p-T2 were accompanied by significantly higher (P=0.001, P=0.002 and P=0.034, respectively) postoperative proportions of complications (second meta-analysis) (Table 6). Also, the percentage of p-N0 or p-N1 was found to exert a marginal significance (P=0.064) in postoperative proportions of complications (second meta-analysis) (Table 6);
- Use of ECC does not significantly affect the postoperative proportions of complications (second meta-analysis) (P=0.580, Table 6) or the three- and five- year survival (second meta-analysis) (P=0.121 and P=0.726 respectively, Table 7);
- Both higher percentages (%) of lobectomy and mean age were factors significantly decreasing the five-year survival (second meta-analysis) (P=0.009 and P=0.046 respectively, Table 7);
- No statistically significant difference was found between postoperative cardiac and respiratory complications, including cardiac arrhythmias, in patients undergoing surgery for LC and HD co-morbidity (Tables 8,9).
Parameters that seem to be related to postoperative outcome of patients with LC and HD, both requiring surgical treatment, are: (I) patient’s age to tolerate impending postoperative morbidity; (II) priority of performing cardiac before LC surgery; (III) graft suitability for CABG; (IV) use of on- or off-pump technique in cardiac surgery; (V) type of adopted surgical incision for optimal surgical plan; and (VI) application of neo-adjuvant and/or adjuvant therapy to improve postoperative surgical results.
Concerning the age, elderly patients undergoing surgery for bronchogenic carcinoma presented high mortality rates of about 20% (52), given that age is a strong risk factor for atrial fibrillation (AF) following pulmonary lobectomy (3). Also, the in-hospital mortality was significantly higher in patients >70 years compared to younger ones (1). For patients >70 years, less extensive oncologic operations based on standard lung resections contribute to lower mortality (1,53-58), while a thorough preoperative assessment concerning pulmonary and cardiac functional status should be performed (1).
The priority of cardiac compared to LC surgery deals particularly with myocardial revascularization. Critical stenosis in coronary arteries should be restored before LC resection to prevent myocardial ischemia, myocardial infarction (MI) and postoperative arrhythmias [supraventricular tachydysrhythmias (SVTs)] resulting in reduction of survival (32,59,60). Patient’s age, duration of the oncologic operation, hypoxemia and hypercapnia, further contribute to the emergence of cardiac complications after LC surgery (61).
Regarding the grafts suitability for coronary artery revascularization, the use of the internal mammary artery (IMA) should be avoided if CABG is planned simultaneously with lobectomy (20). If IMA grafting is inevitable, then LC and HD operations should be carried out in staged surgical procedures with cardiac surgery first (20). The IMA should be fixed behind the sternum so that its stability is not influenced by the vacuum created by the lobectomy planned four or six weeks after cardiac surgery (20). Saphenous vein grafts with sufficient blood flow are the method of choice for simultaneous LC and HD surgery particularly for patients with poor LC differentiation and five-year survival expectancy lower than 50% (8,62).
For the use of the on- versus off-pump technique, our search focuses mainly on patients undergoing CABG rather than all heart surgery like valve or aortic surgery. This is due to the fact that, to the best of our knowledge, the majority of patients with primary LC and HD co-morbidity both requiring surgical treatment, apart from anatomical LC resection, were, simultaneously or in a staged surgical procedure, operated for CABG. Cardiopulmonary bypass (CPB) brings about hemorrhagic predisposition and/or hemorrhage because of heparine administration and disorders in coagulation mechanisms (8,10). The exaggerated dose of heparine being incompletely neutralized by protamine sulfate (22), the rebound phenomenon (22) and disturbances in thrombocytes function (resulting from use of CPB) (9,22,63), all contribute to the emergence of hemorrhages. However, for combined LC and HD operations the priority should be given to cardiac surgery while the timing of subsequent LC resection depends on hemodynamic stability after the cardiac procedure (13). The left lower lobe LC surgical removal should be performed during CPB due to better mobilization of the left lung (9,20,64). This improves the surgical plan while arrhythmias or bradycardia associated with hypotension (65-67) and hemodynamic instability (5,9,20) are avoided. Also, combined LC [T4 locally advanced non-small cell lung cancer (NSCLC)] and cardiac surgery under CPB presents tolerable postoperative long-term survival reaching 21.6 months (27). This is in accordance with another study (68) showing 5-year survival for 37% of patients undergoing LC surgery under circulatory bypass. If combined LC and HD surgery, performed under CPB, concerns valvular repair or replacement, then after cardiac operation but before LC resection, the pericardium should be surgically closed to avoid dissemination either of neoplastic cells or infectious micro-organisms of respiratory system origin (13). The disadvantage of the off-pump technique is the incomplete revascularization when the coronary obstruction is anatomically positioned in circumflex ramifications (69,70), necessitating a surgical approach using left thoracotomy (69,71). The prerequisites for success of CABG with the off-pump method are: (I) sufficient graft patency; (II) sufficient venous return; (III) surgical skills for quick anastomosis completion; (IV) twine sutures for stability; (V) placement of anastomosis as distally as possible; and (VI) sufficient surgical plan (69).
Moreover, for combined LC and HD surgery median sternotomy and lateral open thoracotomy are the more prevalent approaches (64), with median sternotomy being preferable for the following reasons: (I) lung volumes, flow rates and compliance are postoperatively less affected (72,73); (II) postoperative pain requires less administration of analgesia (72); (III) surgical plan is sufficient for right lung tumors (74); (IV) feasibility of safe intra-operative examination, before establishment of CPB, for detection of possible lymph nodes metastases not preoperatively diagnosed (5); and (V) the possibility of performing mediastinal lymphadenectomy except for subcarinal nodes (74).
Concerning the neo-adjuvant and/or adjuvant therapy, the postoperative outcome of patients with locally advanced LC depends on a radical surgical excision and responsiveness to neo-adjuvant treatment (37,75-78), both contributing to an increase of the five-year postoperative survival rate in 38% (37,78) and 46.2% (79) of patients. The more dangerous complications after pneumonectomy in LC patients who received neo-adjuvant treatment are pulmonary edema and broncho-pleural fistula (BPF) (37). Pulmonary edema is attributed to the adverse effects of preoperative radiation in healthy lungs producing sclerosis in mediastinal lymph vessels being further enhanced by mediastinal lymphadenectomy (37). The appearance of BPF depends on vascularization sufficiency of the flaps of pericardial and intercostal or serratus anterior muscle origin, used to stabilize the cut in the main bronchus after pneumonectomy (37). Also, postoperative radiotherapy in LC patients with N2 lymph nodes produced a survival increase related to either the death of neoplastic cells or a decrease of the toxicity level (80). Adverse effects of postoperative radiotherapy in LC patients with HD co-morbidity are: (I) pacemaker dysfunction (81-85); (II) local disturbances in myocardial perfusion (86); (III) MI (87); and (IV) coronary arterial spasm followed by cardiac arrest (88). Cardiotoxicity, mainly dependent on the tumor’s location in lung (39), should be also taken into account following radiation after LC surgery except for right upper lobe tumors (39).
Moreover, implementation of adjuvant treatment does not significantly affect disease free survival (DFS) in early-stage LC patients (89). However, the benefits of neo-adjuvant and/or adjuvant treatment in five-year survival after pneumonectomy for LC stage III patients are also reported in literature (40). Individualization of the adjuvant radiotherapy dose along with improvement of technological equipment both significantly reduced cardiotoxicity (39).
In the context of LC therapy, although stereotactic ablative radiotherapy treatment (SABR) is not the method of choice for patients with LC and HD co-morbidity, it’s worth mentioning some information concerning this newer treatment modality. Particularly, during the last 10 years SABR has been considered the main treatment for early-stage primary LC or for LC patients with oligometastatic disease (90-92). Ideal candidates for SABR are T1N0 or T2N0 NSCLC patients (93). SABR can substitute lobectomy or wedge resection for local control of early-stage NSCLC (93,94). Complications attributed to SABR are radiation pneumonitis (RP) and radiation-induced lung injury (RILI) (95). Although RILI is more frequently associated with SABR treatment (95), patients with large tumors or interstitial lung disease (ILD) present a higher risk for RP after SABR (95). Besides, it is important to distinguish between RILI and local recurrence of LC in order to protect patients from inappropriate medical decisions (95). Also, FEV1, forced vital capacity (FVC) and carbon monoxide diffusion capacity (DLCO) are slightly affected, without statistical significance, by SABR treatment (96-98). Nevertheless, chest wall pain, rib fractures, skin toxicity, nausea and injuries to great vessels, trachea and bronchial tree, are the adverse effects of SABR if lung tumors are positioned within 2 cm of the mediastinal structures (95). Recurrences usually appear within three years of SABR (99). Although for NSCLC stage II or III (N0, N1 or T3 tumors) surgery seems to be the treatment of choice (100), local control of the disease is considered feasible for inoperable NSCLC stage II or III by improved imaging techniques and radiotherapy including dose escalation in the use of SABR (100).
The main important issue to be elucidated concerns the choice between combined or staged operation, as preferable surgical treatment, in patients with primary LC and HD co-morbidity. To the best of our knowledge, we didn’t find what operations are actually occurring regarding the surgical trend for these patients, while provided information (10,14,47) was not sufficient for statistical comparison between combined and staged surgical procedures. However, it was previously reported that staged procedures, with HD being first surgically repaired, are preferable due to less perioperative mortality and better long-term survival (5,6,10,14).
Nevertheless, combined surgical operation for both primary LC and HD co-morbidity seems to be feasible if: (I) LC histology is of squamous type with stage IA to IIIA without N2 nodules infiltration (16); (II) there is no medical history of recent myocardial cancerous infiltration (16); and (III) the presence of unstable angina with no possibility for coronary angioplasty or stenting (16). Contra-indications for a combined LC and HD surgery are: (I) co-existence of cancer in the esophagus, pancreas and other organs (11); (II) heart failure requiring high dose of catecholamines (11); (III) application of mechanical circulatory assistance (11); (IV) a tendency for serious postoperative bleeding specifically after CABG surgery (11); (V) N2 LC disease (16); and (VI) when lung tumors infiltrate the diaphragm and/or parietal pleura or being adjacent to the oesophagus or descending aorta (16).
For primary LC patients stage I or II with HD both requiring surgical repair, the combined surgical procedure is preferred (5,6,9,10) to prevent metastasis, thus avoiding a second surgical stress condition. This seems to be in accordance with other studies (5-8,10,11,13,19,44) reporting that combined LC and HD surgery can be safe with good results and prognosis in carefully selected patients including the ones unable to tolerate a second operation. It was further supported that simultaneous surgical treatment for cardiac and operable oncologic disease could prevent rapid growth of tumor (11). Also, candidates for a combined procedure should undergo preoperative mediastinoscopy even if no lymph node enlargement is identified in a preoperative chest CT-scan (16). Furthermore, when staged procedure is decided, HD has to be treated-first and lung resection should follow four or six weeks later (12,20,47,101). However, if coronary angioplasty took place instead of conventional cardiac surgery, then lung resection should be performed one or two weeks later (14,47,102). In case of prior coronary arterial stenting, LC resection is recommended one year after stent’s placement because of significant risk for mortality attributed to perioperative major adverse cardiac events (103,104) such as stent thrombosis (103) and MI necessitating revascularization (103). Nevertheless, if coronary stenting occurs and LC surgery must be performed, the following options exist: (I) delay of the oncologic operation; (II) lung resection under cessation of dual anti-platelet therapy or under anti-coagulative treatment by inducing increased risk for perioperative bleeding; and (III) application of stereotactic radiotherapy (105-107).
As combined LC and HD operation entails greater perioperative risks (14), the decision between combined or staged surgical procedure can be facilitated by careful and detailed preoperative assessment of factors potentially influencing the postoperative outcome of these patients. These factors, related to LC surgery, were divided in preoperative and postoperative predictors (108). Preoperative predictors were male sex, age, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), renal disease, any prior tumor diagnosed during the last five years before LC surgery and clinical stage (108). Postoperative predictors were the type of LC resection and the pathological stage, both being the most accurate to estimate the postoperative long-term survival (108). Also, according to another study, risk factors related with morbidity after LC surgery were independently divided for the younger (≤70 years) and for the elderly (≥70 years) patients (109). Concerning the younger patients (≤70 years) postoperative morbidity was significantly related to the histological cell type, the extent of lung resection and FEV1% (109). For the elderly patients (≥70 years), hypertension, serum creatinine level, (%) DLCO and smoking status had significant contribution to the increase of postoperative morbidity (109). Thoracotomy (110), pneumonectomy (111), co-morbidities (112) and induction of neo-adjuvant therapy (113) were also found to increase morbidity after LC surgery.
As for the in-hospital mortality for candidates for LC surgery this can be estimated by using independent variables such as age, sex, American Society of Anaesthesiologists score (ASA), performance status, side, lobectomy, pneumonectomy, extended resection, stage III and stage IV disease, forced expiratory volumes (FEV), body mass index (BMI) and the number of co-morbidities per patient (114). Although the possibility for in-hospital death was estimated to be three times higher for the right-sided pneumonectomy compared to lobectomy or limited lung resection, the risk of death following pneumonectomy or extended resection was not correlated to FEV which was not the case for lobectomy (114). Also, the positive cardiologic history is not a risk factor for increased postoperative morbidity and mortality for the elderly, on the assumption that lung resections will be limited to lobar, segmental or wedge resections (104,106,115,116). However, surgery for both middle and lower lobectomy (bilobectomy) was associated with significant cardio-respiratory postoperative complications compared to lower lobectomy (117). Moreover, the risk of perioperative cardiac complications and the 5-year survival in candidates for early stage LC surgery can be evaluated by using the thoracic revised cardiac risk index (ThRCRI) which is an independent prognostic factor (118).
After having presented and medically interpreted issues potentially influencing the postoperative outcome of patients requiring surgical treatment for LC and HD co-morbidity, a commentary of our meta-analytic findings is as follows.
It is well known that tumor’s histology influences outcome after LC surgery. Our finding from the first meta-analysis that higher percentages of squamous LC were correlated to significant increase in 30-day postoperative mortality, is enhanced by a previous study reporting that squamous LC is included among risk factors contributing to death or major complications during the first thirty postoperative days (119). Also, according to Takamochi et al. squamous histologic type was more frequent in the elderly compared to younger patients with LC (109). However, in our study, and for both meta-analyses, squamous LC was found to be frequently detected in younger patients (≤70 years, Tables 1,2). Moreover, our finding, from the first meta-analysis, that higher percentages of lobectomy were accompanied by significant decrease in 30-day postoperative mortality seems to be in accordance with Myrdal et al. reporting that lobectomy was associated with lower early postoperative mortality compared to more extensive surgery such as pneumonectomy (119). This can be attributed to the type of surgical procedure per se which maintains more pulmonary reserves compared to pneumonectomy or more extended lung resection (114). Other studies further supported that pneumonectomies were associated with higher morbidity and postoperative mortality (119-121). Also, the early mortality after LC surgery depended on occurrence of major postoperative complications, particularly pulmonary complications, including respiratory failure (119,122). This was significantly (P=0.001) frequent for pneumonectomy compared to lobectomy (119). The importance of FEV1 to predict the remaining pulmonary capacity along with the possibility of respiratory complications after LC surgery is underlined in the relevant literature (119,123). Besides, the contribution of FEV1 in determining morbidity and in-hospital mortality following LC resection is further reported by other studies (109,114).
As for our finding of the second meta-analysis that combined LC and HD surgical procedures were significantly associated with higher postoperative proportions of complications, this appears to be in accordance with previous studies supporting that higher perioperative risks and overall perioperative mortality are compatible rather to simultaneous than staged cardiac and LC surgery (5,6,9,14,15,20,21). A possible explanation could be that combination of LC and cardiac surgery constitutes by itself a factor predisposing to a more severe early postoperative clinical condition attributed to increased surgical stress.
Furthermore, our findings of the second meta-analysis, that higher percentages for squamous LC histology, lobectomy and p-T1 or p-T2 were associated with significantly higher postoperative proportions of complications can be explained as follows. Squamous LC histology usually presents a centrally located tumor (124,125). This can necessitate a more extended pulmonary resection, affecting postoperative survival (66,126,127) which coincides with the suggestion that LC histology is included among risk factors influencing outcome after surgery (119). Also, our finding that higher percentages for lobectomy were related to significantly higher postoperative complications for combined LC and HD surgery, can be further attributed to the fact that lobectomy affects postoperative FEV (114) which have an important role in the outcome after LC surgery (109,114,119,123). Additional cardiac co-morbidity should be also taken into account regarding postoperative complications. The negative impact of co-morbidity in postoperative outcome of LC patients is reported in literature (128), which might be in favor for the choice of a simultaneous surgical approach for LC and HD co-morbidity. Concerning our finding between percentages of pT1 or pT2 and postoperative complications, the majority of patients, according to our literature search, treated for combined LC and cardiac surgery, were of pT1 or pT2 status (numerically suitable for statistical analysis). These patients underwent rather lobectomy than other types of lung surgery. Postoperative pulmonary capacity can deteriorate by this type of LC surgery (114), particularly in patients with an impaired cardiac function, thus contributing to postoperative complications. We suggest that the possibility of performing a specific program of cardio-pulmonary rehabilitation before combined LC and HD surgery can improve preoperative value of peak VO2 (maximal oxygen consumption), which globally reflects patient’s functional status including muscular oxygenation (129,130). This might result in reduction of postoperative complications. The usefulness of a similar preoperative program was reported in literature for LC patients with COPD co-morbidity who underwent lobectomy (131).
As for the systematic side effects attributed to the ECC, these have been extensively reported in literature (132-140). Immunologic disturbances induced by ECC, are considered to contribute to neoplastic disease spreading (101,141). However, for combined LC and cardiac surgery performed under ECC, resection of lung parenchyma is safer to be performed rather before than during CPB (13,20,21), with the exception of the left lower lobe (20), or after CPB (9,20). The potential increase of neoplastic metastasis resulting from ECC use was supported by a number of studies (9,20,21), while the adverse effects of the ECC on pulmonary function were reported as well (142). The possibility to avoid the use of the ECC in combined LC and cardiac disease surgery, particularly for myocardial ischemia, was also reported (44,143). Other studies did not confirm the fact that the ECC either worsens postoperative survival in patients with malignancy (13,19,25) or to make for spreading of lung tumor (14). These works (13,14,19,25) are in accordance with our finding of the second meta-analysis that the postoperative outcome of patients simultaneously operated for both LC and HD under ECC is not significantly affected, something which enhances the hypothesis of reversibility of adverse effects by ECC.
A finding from the second meta-analysis which should also to be commented on is that the five-year survival was significantly affected by lobectomy, which represents a less extensive procedure. In a previous study the five-year survival after lobectomy was of 67% to 83% and of 43% to 78% for T1N0/stage IA and T2N0/stage IB patients, respectively (144). This is in agreement with the Lung Cancer Study Group reporting a 5-year DFS of 65% for T1N0M0 NSCLC patients undergoing surgery (145), while the 5-year survival after combined procedure for pT1N0M0 NSCLC patients was of 87.5% (6). Other studies, for combined LC and cardiac surgery, reported a 5-year survival of 88% (6), 52.4% (12) and 42% (21), while long-term survival in patients who underwent simultaneous LC and CABG surgery was significantly affected only by recurrence of the neoplastic disease (26). The negative impact of combined LC and HD surgery by itself on long-term survival of these patients is also reported in literature (10,20). The fact that the second meta-analysis displayed significant decrease in 5-year survival for higher percentages of lobectomy can have the following possible explanation. Particularly: (I) the usually central location in lung parenchyma of the tumor as the majority, according to our literature search, of patients were of squamous histology which can involve a more extended surgical procedure; (II) the impact of lobectomy in pulmonary capacity affecting the outcome after LC surgery (109,114,119,123); and (III) the issue of co-morbidity by itself in patients suffering from LC (129). Moreover, for both meta-analyses, no statistically significant differences were found for the type of postoperative complications, i.e., respiratory or cardiac complications including arrhythmias. Additionally, in second meta-analysis, the five-year survival is significantly decreased by higher mean age although the advanced age is not a contradiction for LC surgery (2). Nevertheless, in LC surgery, age strongly increases in-hospital mortality (146) while it is included among risk factors for life-threatening morbidity (147) by also affecting the long-term survival (108). These studies (108,147) seem to support our finding of a decreased five-year survival. Besides, co-morbidities should be taken into account concerning their impact on postoperative outcome of patients undergoing LC surgery (112,114).
Conclusions
Lobectomy, where possible, was accompanied by lower postoperative mortality. However, lobectomy (as selected type of operation) and age were both associated with a reduced five-year survival rate. Patients undergoing a combined surgical procedure such as CABG and lobectomy for squamous pT1 or pT2 display a higher risk of postoperative complications not attributable either to the ECC or the type of cardiac operation, given that in the reviewed literature the majority of cardiac operations were CABG compared to other types of cardiac surgery. Male patients showed a tendency for increased postoperative complications. Nevertheless, the medical decision between combined and staged surgical procedure for LC and HD disease co-morbidity should be individualized for each patient. Although combined operation seems to spare patient from both spreading of cancerous cells and a second surgical stress condition, we think that the final decision should depend on parameters such as adequacy of myocardial arterial flow, age, patient’s preoperative performance status (taking into account the number and the type of co-morbidities per patient), staging of tumor and the extent of lung resection.
Acknowledgements
We thank Mr. Antonios Kardasis (Information Specialist, Hellenic National Documentation Centre, EKT/NHRF, 48 Vas. Constantinou Ave GR-11635 Athens, Greece, email: akarda@ekt.gr) for his valuable contribution to the literature search and for locating the articles citations and impact-factors of the corresponding journals and Mrs Nektaria Vathi [Librarian, Library of Technological Educational Institute (T.E.I.) of Athens, Agiou Spyridonos 12243, Egaleo, Athens, Greece, email: nvathi@teiath.gr] for her valuable contribution to the literature search and for locating the articles, as well as to the final format of this manuscript according to the journal’s instructions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thomas P, Sielezneff I, Ragni J, et al. Is lung cancer resection justified in patients aged over 70 years? Eur J Cardiothorac Surg 1993;7:246-50; discussion 250-1. [Crossref] [PubMed]
- Ploeg AJ, Kappetein AP, van Tongeren RB, et al. Factors associated with perioperative complications and long-term results after pulmonary resection for primary carcinoma of the lung. Eur J Cardiothorac Surg 2003;23:26-9. [Crossref] [PubMed]
- Hollings DD, Higgins RSD, Faber Penfield L, et al. Age is a strong risk factor for atrial fibrillation after pulmonary lobectomy. Am J Surg 2010;199:558-61. [Crossref] [PubMed]
- Kalathiya RJ, Saha SP. Pneumonectomy for non-small cell lung cancer:outcomes analysis. South Med J 2012;105:350-4. [Crossref] [PubMed]
- Piehler JM, Trastek VF, Pairolero PC, et al. Concomitant cardiac and pulmonary operations. J Thorac Cardiovasc Surg 1985;90:662-7. [PubMed]
- Canver CC, Bhayana JN, Lajos TZ, et al. Pulmonary resection combined with cardiac operations. Ann Thorac Surg 1990;50:796-9. [Crossref] [PubMed]
- Adant JP, Defraigne JO, Limet R. Combined one stage cardiac and pulmonary surgery by median sternotomy. Acta Chir Belg 1990;90:197-202. [PubMed]
- Rosalion A, Woodford NW, Clarke CP, et al. Concomitant coronary revascularization and resection of lung cancer. Aust N Z J Surg 1993;63:336-40. [Crossref] [PubMed]
- Yokoyama T, Derrick MJ, Lee AW. Cardiac operation with associated pulmonary resection. J Thorac Cardiovasc Surg 1993;105:912-6; discussion 916-7. [PubMed]
- Miller DL, Orszulak TA, Pairolero PC, et al. Combined operation for lung cancer and cardiac disease. Ann Thorac Surg 1994;58:989-93; discussion 993-4. [Crossref] [PubMed]
- Takahashi T, Nakano S, Shimazaki Y, et al. Concomitant coronary bypass grafting and curative surgery for cancer Surg Today 1995;25:131-5. [Crossref] [PubMed]
- La Francesca S, Frazier OH, Radovancevic B, et al. Concomitant cardiac and pulmonary operations for lung cancer. Tex Heart Inst J 1995;22:296-300. [PubMed]
- Rao V, Todd TR, Weisel RD, et al. Results of combined pulmonary resection and cardiac operation. Ann Thorac Surg 1996;62:342-6; discussion 346-7. [Crossref] [PubMed]
- Voets AJ, Joesoef KS, van Teeffelen ME. Synchroneously occurring lung cancer (stages I-II) and coronary artery disease: concomitant versus staged surgical approach. Eur J Cardiothorac Surg 1997;12:713-7. [Crossref] [PubMed]
- Voets AJ, Joesoef KS, van Teeffelen ME. The influence of open-heart surgery on survival of patients with co-existent surgically amenable lung cancer (stages I and II). Eur J Cardiothorac Surg 1997;12:898-902. [Crossref] [PubMed]
- Dyszkiewicz W, Jemielity MM, Piwkowski CT, et al. Simultaneous lung resection for cancer and myocardial revascularization without cardiopulmonary bypass (off-pump coronary artery bypass grafting). Ann Thorac Surg 2004;77:1023-7. [Crossref] [PubMed]
- Prokakis C, Koletsis E, Apostolakis E, et al. Combined heart surgery and lung tumor resection. Med Sci Monit 2008;14:CS17-CS21. [PubMed]
- Cathenis K, Hamerlijnck R, Vermassen F, et al. Concomitant cardiac surgery and pulmonary resection. Acta Chir Belg 2009;109:306-11. [Crossref] [PubMed]
- Fu Q, Li QZ, Liang DG, et al. Early and long-term results of combined cardiac surgery and neoplastic resection in patients with concomitant severe heart disease and neoplasms. Chin Med J (Engl) 2011;124:1939-42. [PubMed]
- Terzi A, Furlan G, Magnanelli G, et al. Lung resections concomitant to coronary artery bypass grafting. Eur J Cardiothorac Surg 1994;8:580-4. [Crossref] [PubMed]
- Brutel de la Rivière A, Knaepen P, Van Swieten H, et al. Concomitant open heart surgery and pulmonary resection for lung cancer. Eur J Cardiothorac Surg 1995;9:310-3; discussion 313-4. [Crossref] [PubMed]
- Danton MH, Anikin VA, McManus KG, et al. Simultaneous cardiac surgery with pulmonary resection: presentation of series and review of literature. Eur J Cardiothorac Surg 1998;13:667-72. [Crossref] [PubMed]
- Saxena P, Tam RK. Combined off-pump coronary artery bypass surgery and pulmonary resection. Ann Thorac Surg 2004;78:498-501. [Crossref] [PubMed]
- Spaggiari L, D’ Aiuto M, Veronesi G, et al. Extended pneumonectomy with partial resection of the left atrium, without cardiopulmonary bypass, for lung cancer. Ann Thorac Surg 2005;79:234-40. [Crossref] [PubMed]
- Schoenmakers MC, van Boven WJ, van den Bosch J, et al. Comparison of on-pump or off-pump coronary artery revascularization with lung resection. Ann Thorac Surg 2007;84:504-9. [Crossref] [PubMed]
- Dyszkiewicz W, Jemielity M, Piwkowski C, et al. The early and late results of combined off-pump coronary artery bypass grafting and pulmonary resection in patients with concomitant lung cancer and unstable coronary heart disease. Eur J Cardiothorac Surg 2008;34:531-5. [Crossref] [PubMed]
- Kauffmann M, Kruger T, Aebert H. Surgery on Extracorporeal circulation in early and advanced non-small cell lung cancer. Thorac Cardiovasc Surg 2013;61:103-8. [Crossref] [PubMed]
- Fukuse T, Wada H, Hitomi S. Extended operation for non-small cell lung cancer invading great vessels and left atrium. Eur J Cardiothorac Surg 1997;11:664-9. [Crossref] [PubMed]
- Ratto GB, Costa R, Vassalo G, et al. Twelve-year experience with left atrial resection in the treatment of non-small cell lung cancer. Ann Thorac Surg 2004;78:234-7. [Crossref] [PubMed]
- Pastorino U, Yang XN, Massimo F, et al. Long-term survival after salvage surgery for invasive thymoma with intracardiac extension. Tumori 2008;94:772-6. [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Long-term results of video-assisted thoracic surgery lobectomy for stage I non-small cell lung cancer: a single-centre study of 104 cases. Interact Cardiovasc Thorac Surg 2004;3:57-62. [Crossref] [PubMed]
- Imperatori A, Mariscalo G, Riganti G, et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg 2012;7:4. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Wang W, Xu Z, Xiong X, et al. Video-assisted thoracoscopic lobectomy for non-small cell lung cancer in patients with severe chronic obstructive pulmonary disease. J Thorac Dis 2013;5:S253-9. [PubMed]
- Xiong X, Shao W, Yin W, et al. Video-assisted thoracoscopic surgery for stage I non-small cell lung cancer: long-term survival and prognostic factors. Tumour Biol 2013;34:3389-96. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Daly BD, Fernando HC, Ketchedjian A, et al. Pneumonectomy after high-dose radiation and concurrent chemotherapy for nonsmall cell lung cancer. Ann Thorac Surg 2006;82:227-31. [Crossref] [PubMed]
- Inoue M, Okumura M, Minami M, et al. Cardiopulmonary co-morbidity: a critical negative prognostic predictor for pulmonary resection following preoperative chemotherapy and / or radiation therapy in lung cancer patients. Gen Thorac Cardiovasc Surg 2007;55:315-21. [Crossref] [PubMed]
- Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007;110:911-7. [Crossref] [PubMed]
- Saha SP, Kalathiya RJ, Davenport DL, et al. Survival after pneumonectomy for stage III non-small cell lung cancer. Oman Med J 2014;29:24-7. [Crossref] [PubMed]
- Von Knorring J, Lepantalo M, Lindgren L, et al. Cardiac arrhythmias and myocardial ischemia after thoracotomy for lung cancer. Ann Thorac Surg 1992;53:642-7. [Crossref] [PubMed]
- Barbetakis N, Vassiliadis M. Is amiodarone a safe antiarrhythmic to use in supraventricular tachyarrhythmias after lung cancer surgery. BMC Surg 2004;4:7. [Crossref] [PubMed]
- Mitsudomi T, Mizoue T, Yoshimatsu T, et al. Postoperative complications after pneumonectomy for treatment of lung cancer: multivariate analysis. J Surg Oncol 1996;61:218-22. [Crossref] [PubMed]
- Morishita K, Kawaharada N, Watanabe T, et al. Simultaneous cardiac operations with pulmonary resection for lung carcinoma. Jpn J Thorac Cardiovasc Surg 2001;49:685-9. [Crossref] [PubMed]
- Yamamoto S, Uchiyama T, Yamaoka N, et al. Surgical results of T3 lung cancer with combined resection (Article in Japanese). Kyobu Geka 1998;51:935-8. [PubMed]
- Rea F, Marulli G, Schiavon M, et al. Tracheal sleeve pneumonectomy for non-small cell lung cancer (NSCLC): Short and long-term results in a single institution. Lung Cancer 2008;61:202-8. [Crossref] [PubMed]
- Ciriaco P, Carretta A, Calori G, et al. Lung resection for cancer in patients with coronary arterial disease: analysis of short-term results. Eur J Cardiothorac Surg 2002;22:35-40. [Crossref] [PubMed]
- Figas-Powajbo E, Gawor Z, Kozak J. Perioperative cardiac arrhythmias in patients undergoing surgical treatment for lung cancer. Pol Arch Med Wewn 2007;117:290-6. [PubMed]
- Chataigner O, Fadel E, Yildizeli B, et al. Factors affecting early and long-term outcomes after completion pneumonectomy. Eur J Cardiothorac Surg 2008;33:837-43. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Weiss W. Operative mortality and five-year survival rates in patients with bronchogenic carcinoma. Am J Surg 1974;128:799-804. [Crossref] [PubMed]
- Breyer RH, Zippe C, Pharr WF, et al. Thoracotomy in patients over age seventy years. Ten years’ experience. J Thorac Cardiovasc Surg 1981;81:187-93. [PubMed]
- Errett LE, Wilson J, Chiu RC, et al. Wedge resection as an alternative procedure for peripheral bronchogenic carcinoma in poor-risk patients. J Thorac Cardiovasc Surg 1985;90:656-61. [PubMed]
- Ginsberg RJ, Hill LD, Eagan RT. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983;86:654-8. [PubMed]
- Ishida T, Yokoyama H, Kaneko S, et al. Long-term results of operation for non-small cell lung cancer in the elderly. Ann Thorac Surg 1990;50:919-22. [Crossref] [PubMed]
- Kadri MA, Dussek JE. Survival and prognosis following resection of primary non small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 1991;5:132-6. [Crossref] [PubMed]
- Yellin A, Benfield JR. Surgery for bronchogenic carcinoma in the elderly. Am Rev Respir Dis 1985;131:197. [PubMed]
- Nataf P, Régnard JF, Nottin R, et al. Lung resection for cancer in coronary patients. Immediate and medium-term results. Retrospective study in a series of 51 patients. Presse Med 1991;20:789-93. [PubMed]
- Amar D, Burt M, Reinsel RA, et al. Relationship of early postoperative dysrhythmias and long-term outcome after resection of non-small cell lung cancer. Chest 1996;110:437-9. [Crossref] [PubMed]
- Liu YZ. Postoperative cardiac intensive care for patients with thoracic cancer: analysis of 430 cases. Zhonghua Wai Ke Za Zhi 1992;30:675-6, 700. [PubMed]
- Turley K. Thoracic wall, pleura, mediastinum, and lung. In: Way LW, editor. Current Surgical Diagnosis and Treatment, 9th edn. Connecticut: Appleton & Lange, 1991:Chapter 19.
- Moores DW, Miller SJ Jr, McKneally MF. Lung cancer: a surgeon's approach. Curr Probl Surg 1987;24:679-758. [Crossref] [PubMed]
- Girardet RE, Marsi ZA, Lansing AM. Pulmonary lesions in patients undergoing open heart surgery: approach and management. The Journal of the Kentucky Medical Association 1981;79:645-8. [PubMed]
- Asaph JW, Keppel JF. Midline sternotomy for the treatment of primary pulmonary neoplasms. Am J Surg 1984;147:589-92. [Crossref] [PubMed]
- Johnston MR. Median sternotomy for resection of pulmonary metastases. J Thorac Cardiovasc Surg 1983;85:516-22. [PubMed]
- Urschel HC Jr, Razzuk MA. Median sternotomy as a standard approach for pulmonary resection. Ann Thorac Surg 1986;41:130-4. [Crossref] [PubMed]
- Muralidaran A, Detterbeck FC, Boffa DJ, et al. Long-term survival after lung resection for non-small cell lung cancer with circulatory bypass: a systematic review. J Thorac Cardiovasc Surg 2011;142:1137-42. [Crossref] [PubMed]
- Kondo K, Minohara S, Sawada Y, et al. Indications and problems of coronary artery bypass grafting without cardiopulmonary bypass. Surg Today 1997;27:202-6. [Crossref] [PubMed]
- Benetti FJ, Naseli G, Wood M, et al. Direct myocardial revascularization without extracorporeal circulation. Experience in 700 patients. Chest 1991;100:312-6. [Crossref] [PubMed]
- Fanning WJ, Kakos GS, Williams TE. Preoperative coronary artery bypass grafting without cardiopulmonary bypass. Ann Thorac Surg 1993;55:486-9. [Crossref] [PubMed]
- Cooper JD, Nelems JM, Pearson FG. Extended indications for median sternotomy in patients requiring pulmonary resection. Ann Thorac Surg 1978;26:413-20. [Crossref] [PubMed]
- Mattila T, Laustela E, Tala P. On the effect of sternotomy and thoracotomy incision on pulmonary function after open heart operations. Ann Chir Gynaecol Fenn 1967;56:58-61. [PubMed]
- Apostolakis E, Prokakis C, Koletsis E, et al. Median sternotomy for combined coronary artery bypass grafting and lung tumor resection: is it valid or not? Eur J Cardiothorac Surg 2009;35:1117; author reply 1117-8. [Crossref] [PubMed]
- Albain KS, Rush VW, Crowley JJ, et al. Concurrent cisplatin / etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [PubMed]
- Doddoli C, Barlesi F, Trousse D, et al. One hundred consecutive pneumonectomies after induction therapy for stage IIIA (PN2) non-small cell lung cancer: An uncertain balance between risks and benefits. J Thorac Cardiovasc Surg 2005;130:416-25. [Crossref] [PubMed]
- Bueno R, Richards WG, Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 2000;70:1826-31. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Spencer SA, et al. Pulmonary resection after high-dose and low-dose chest irradiation. Ann Thorac Surg 2005;80:1224-30. [Crossref] [PubMed]
- Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg 2004;78:1200-5; discussion 1206. [Crossref] [PubMed]
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol 2006;24:2998-3006. [Crossref] [PubMed]
- Quertermous T, Megahy MS, Das Gupta DS, et al. Pacemaker failure resulting from radiation damage. Radiology 1983;148:257-8. [Crossref] [PubMed]
- Katzenberg CA, Marcus FI, Heusinkveld RS, et al. Pacemaker failure due to radiation therapy. Pacing Clin Electrophysiol 1982;5:156-9. [Crossref] [PubMed]
- Lewin AA, Serago CF, Schwade JG, et al. Radiation induced failures of complementary metal oxide semiconductor containing pacemakers: a potentially lethal complication. Int J Radiat Oncol Biol Phys 1984;10:1967-9. [Crossref] [PubMed]
- Souliman SK, Christie J. Pacemaker failure induced by radiotherapy. Pacing Clin Electrophysiol 1994;17:270-3. [Crossref] [PubMed]
- Venselaar JL, Van Kerkoerle HIJ, Vet AJ. Radiation damage to pacemakers from radiotherapy. Pacing Clin Electrophysiol 1987;10:538-42. [Crossref] [PubMed]
- Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery-distribution. Int J Radiat Oncol Biol Phys 2003;55:914-20. [Crossref] [PubMed]
- Joensuu H. Myocardial infarction after irradiation in Hodgkin’s disease: A review. Recent Results Cancer Res 1993;130:157-73. [Crossref] [PubMed]
- Martí V, García J, Augé JM, et al. Coronary arterial spasm and cardiac arrest following mediastinal radiation therapy for Hodgkin's disease. Chest 1991;100:1180-2. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Lewis S, Salama J, Nakamura N, et al. Stereotactic ablative radiotherapy (SABR) for extra-cranial oligometastatic disease: How do Canadian practice patterns compare to the rest of the world? [Abstract]. Canadian Association of Radiation Oncology Annual Meeting 2013.
- Pan H, Rose BS, Simpson DR, et al. Clinical practice patterns of lung stereotactic body radiation therapy in the United States: A secondary analysis. Am J Clin Oncol 2013;36:269-72. [Crossref] [PubMed]
- Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer 2011;117:4566-72. [Crossref] [PubMed]
- Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: Surgery or stereotactic ablative radiotherapy? Lancet Oncol 2013;14:e270-4. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Huang K, Palma DA. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015;10:412-9. [Crossref] [PubMed]
- Takeda A, Enomoto T, Sanuki N, et al. Reassessment of declines in pulmonary function > 1 year after stereotactic body radiotherapy. Chest 2013;143:130-7. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PTF) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early-stage peripheral non-small cell lung cancer: An analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- McGarry RC. Integrating stereotactic body radiation therapy in stage II / III non-small cell lung cancer: is local control important? Expert Rev Anticancer Ther 2014;14:1419-27. [Crossref] [PubMed]
- Peters RM, Swain JA. Management of the patient with emphysema, coronary artery disease and lung cancer. Am J Surg 1982;143:701-5. [Crossref] [PubMed]
- Licker M, de Perrot M, Hohn L, et al. Perioperative mortality and major cardiopulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg 1999;15:314-9. [Crossref] [PubMed]
- Fernandez FG, Crabtree TD, Liu J, et al. Incremental risk of prior coronary arterial stents for pulmonary resection. Ann Thorac Surg 2013;95:1212-8; discussion 1219-20. [Crossref] [PubMed]
- Hollis RH, Graham LA, Richman JS, et al. Adverse cardiac events in patients undergoing noncardiac surgery: a systematic review. Am J Surg 2012;204:494-501. [Crossref] [PubMed]
- Brilakis ES, Banerjee S, Berger PB. Perioperative management of patients with coronary stents. J Am Coll Cardiol 2007;49:2145-50. [Crossref] [PubMed]
- Cerfolio RJ, Minnich DJ, Bryant AS. General thoracic surgery is safe in patients taking clopidogrel (Plavix). J Thorac Cardiovasc Surg 2010;140:970-6. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Birim O, Kappetein AP, Waleboar M, et al. Long-term survival after non-small cell lung cancer surgery: Development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491-8. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Risk factors for morbidity after pulmonary resection for lung cancer in younger and elderly patients. Interact Cardiovasc Thorac Surg 2011;12:739-43. [Crossref] [PubMed]
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. [Crossref] [PubMed]
- Dyszkiewicz W, Pawlak K, Gasiorowski L. Early post-pneumonectomy complications in the elderly. Eur J Cardiothorac Surg 2000;17:246-50. [Crossref] [PubMed]
- Birim O, Zuydendorp HM, Maat AP, et al. Lung resection for non-small cell lung cancer in patients older than 70: mortality, morbidity, and late survival compared with the general population. Ann Thorac Surg 2003;76:1796-801. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Survival and outcomes of pulmonary resection for non-small cell lung cancer in the elderly: a nested case-control study. Ann Thorac Surg 2006;82:424-9; discussion 429-30. [Crossref] [PubMed]
- Bernard A, Rivera C, Pages Benoit P, et al. Risk model of in-hospital mortality after pulmonary resection for cancer: A national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J Thorac Cardiovasc Surg 2011;141:449-58. [Crossref] [PubMed]
- Pagni S, McKelvey A, Riordan C, et al. Pulmonary resection for malignancy in the elderly: is age still a risk factor? Eur J Cardiothorac Surg 1998;14:40-4; discussion 44-5. [Crossref] [PubMed]
- Senbaklavaci O, Taspinar H, Hartert M, et al. Lobar lung resection in elderly patients with non-small cell lung carcinoma: impact of cardiac comorbidity on surgical outcome. Swiss Med Wkly 2012;142:w13742. [PubMed]
- Gómez MT, Jiménez MF, Aranda JL, et al. The risk of bilobectomy compared with lobectomy: a retrospective analysis of a series of matched cases and controls. Eur J Cardiothorac Surg 2014;46:72-5. [Crossref] [PubMed]
- Brunelli A, Ferguson MK, Salati M, et al. Thoracic Revised Cardiac Risk Index Is Associated With Prognosis After Resection for Stage I Lung Cancer. Ann Thorac Surg 2015;100:195-200. [Crossref] [PubMed]
- Myrdal G, Gustafsson G, Lambe M, et al. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg 2001;20:694-9. [Crossref] [PubMed]
- Morandi U, Stefani A, Golinelli M, et al. Results of surgical resection in patients over the age of 70 years with non small-cell lung cancer. Eur J Cardiothorac Surg 1997;11:432-9. [Crossref] [PubMed]
- Pitz CC, Brutel de la Riviere A, Elbers HR, et al. Surgical treatment of 125 patients with non-small cell lung cancer and chest wall involvement. Thorax 1996;51:846-50. [Crossref] [PubMed]
- Duque JL, Ramos G, Castrodeza J, et al. Early complications in surgical treatment of lung cancer: A prospective multicenter study. Ann Thorac Surg 1997;63:944-50. [Crossref] [PubMed]
- Wang J, Olak J, Ultmann RE, et al. Assessment of pulmonary complications after lung resection. Ann Thorac Surg 1999;67:1444-7. [Crossref] [PubMed]
- Travis WD, Brabilla E, Burke AP, et al. WHO Classification of tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015:52.
- Tan D, Alrawi S. Usual Lung Cancers. In: Zander DS, Farver CF, editors. Pulmonary Pathology. Chapter 26. Edinburgh: Churchill Livingstone, Elsevier, 2008:552.
- Takita H, Edgerton F, Merrin C, et al. Management of multiple lung metastases. J Surg Oncol 1979;12:199-205. [Crossref] [PubMed]
- Vogt-Moykopf I, Meyer G, Merkle N, et al. Late results of surgical treatment of pulmonary metastasis. Thorac Cardiovasc Surg 1986;34:143-8. [Crossref] [PubMed]
- Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 1990;49:391-8; discussion 399-400. [Crossref] [PubMed]
- Jones LW, Eves ND, Mackey JR, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer 2007;55:225-32. [Crossref] [PubMed]
- Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol 2008;9:757-65. [Crossref] [PubMed]
- Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg 2013;44:e260-5. [Crossref] [PubMed]
- Christakis GT, Koch JP, Deemar KA, et al. A randomized study of the systemic effects of warm heart surgery. Ann Thorac Surg 1992;54:449-57; discussion 457-9. [Crossref] [PubMed]
- Cavarocchi NC, Pluth JR, Schaff HV, et al. Complement activation during cardiopulmonary bypass. J Thorac Cardiovasc Surg 1986;91:252-8. [PubMed]
- Moore FD, Warner KG, Assousa S, et al. The effect of complement activation during cardiopulmonary bypass. Ann Surg 1988;208:95-103. [Crossref] [PubMed]
- Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1993;55:552-9. [Crossref] [PubMed]
- Colman RW. Platelet and neutrophil activation in cardiopulmonary bypass. Ann Thorac Surg 1990;49:32-4. [Crossref] [PubMed]
- Harker LA. Bleeding after cardiopulmonary bypass. N Engl J Med 1986;314:1446-8. [Crossref] [PubMed]
- Harker LA, Malpass TW, Branson HE, et al. Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: acquired transient platelet dysfunction associated with selective alpha granule release. Blood 1980;56:824-34. [PubMed]
- Ito H, Hamano K, Gohra H, et al. Relationship between respiratory distress and cytokine response after cardiopulmonary bypass. Surg Today 1997.220-5. [Crossref] [PubMed]
- Guidelines and indications for coronary artery bypass graft surgery. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol 1991;17:543-89. [PubMed]
- Finck SJ, Cockerill KJ, Jeter JE, et al. Coronary artery bypass grafting in patients with chronic lymphocytic leukemia. Ann Thorac Surg 1993;55:1192-6. [Crossref] [PubMed]
- Shapira N, Zabatino SM, Ahmed S, et al. Determinants of pulmonary function in patients undergoing coronary bypass operations. Ann Thorac Surg 1990;50:268-73. [Crossref] [PubMed]
- Contini M, Iaco A, Iovino T, et al. Current results in off-pump surgery. Eur J Cardiothorac Surg 1999;16:S69-72. [Crossref] [PubMed]
- LoCicero J III, Ponn RB, Daly BD. Surgical treatment of non-small cell lung cancer. In: Shields TW, LoCicero J III, Ponn RB, editors. General Thoracic Surgery, 5th ed. Philadelphia: Lippincott Williams and Wilkins 2000:1311-41.
- Thomas P, Rubinstein L. Lung Cancer Study Group. Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Ann Thorac Surg 1990;49:242-6. [Crossref] [PubMed]
- Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest 1992;101:1332-7. [Crossref] [PubMed]
- Yano T, Yokoyama H, Fukuyama Y, et al. The current status of postoperative complications and risk factors after a pulmonary resection for primary lung cancer, a multivariate analysis. Eur J Cardiothorac Surg 1997;11:445-9. [Crossref] [PubMed]



