
Risk factors and strategies for recovery quality, postoperative pain, and recurrent fractures between percutaneous kyphoplasty and percutaneous vertebroplasty in elderly patients with thoracolumbar compression fractures: a retrospective comparative cohort study
Highlight box
Key findings
• The clinical efficacy of PKP in elderly patients with thoracolumbar vertebral compression fracture is superior to that of PVP.
What is known and what is new?
• Osteoporosis is a clinically obvious metabolic bone disease which can be diagnosed when a person’s bone mineral density (BMD) is 2.5 standard deviations (SD) lower than the average of the general population of the same race and sex
• To research the risk factors and countermeasures of recovery quality, postoperative pain, and recurrent fracture after percutaneous kyphoplasty (PKP) in elderly patients based on a large sample, single center, retrospective study.
What is the implication, and what should change now?
• This study only analyzed objective quantitative indicators, and did not evaluate the influence and role of subjective emotional factors. It is necessary to establish relevant influence models for further analysis in the future.
Introduction
Osteoporosis is a clinically obvious metabolic bone disease which can be diagnosed when a person’s bone mineral density (BMD) is 2.5 standard deviations (SD) lower than the average of the general population of the same race and sex (1). In patients with osteoporosis, bone metabolism is unbalanced, characterized by decreased bone production by osteoblasts or increased bone resorption by osteoclasts (2,3). Osteoporosis can be subdivided into primary, secondary, and idiopathic osteoporosis. It is associated with a reduction in estrogen levels and a weakening of the inhibitory effect on osteoclasts. Secondary osteoporosis is frequently connected with the administration of corticosteroids, diabetes mellitus, and secondary amenorrhea. Osteoporotic vertebral compression fracture (OVCF) is a common type of spinal fracture. The incidence of OVCF is usually high in patients with osteoporosis. Improper treatment can easily cause serious deformation of the spine, which affects spinal function and thereby impacting life and work. OVCF is often treated by surgery; percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) are 2 common surgical methods for OVCF.
For asymptomatic OVCF patients, the treatment plan can involve appropriate rest and reduction of activity (4). The conservative treatment is mainly bed rest, painkillers such as non-steroidal anti-inflammatory drugs, and physiotherapy. Although the overwhelming majority of patients can achieve a satisfactory outcome, some patients still experience no pain relief (4). Prolonged bed rest and inactivity can also increase the risk of pneumonia, bed sores, and deep vein thrombosis. In addition, long-term use of pain medication can lead to other conditions such as gastrointestinal reactions and cardiovascular risks. The statistics demonstrate that 75% of the pain caused by acute OVCF is relieved within 12 weeks after conservative treatment (4,5). For other patients, conservative treatment may be ineffective, and surgical intervention becomes necessary (6). The traditional surgical scheme is open surgery for internal fixation of the spine, which is a lengthy, traumatic procedure that prolongs the patient recovery time. The surgical treatment of OVCF has entered a new era with the introduction of a surgical technique in which bone cement material is injected into 1 or more vertebrae under X-ray and/or computed tomography (CT) monitoring to help relieve pain and provide mechanical stability to prevent further collapse of the vertebral body (7). PVP was first developed by French scholars Galibert and Deramond in 1984. They carried out PVP in a 54-year-old female patient with C2 vertebral hemangioma (7). Polymethyl methacrylate (PMMA) is administered into the unstable vertebra during PVP, a microsurgical procedure, to enhance it. Subsequently, 2 French scholars performed PVP on an additional 6 similar patients with satisfactory results. Thereafter, the PVP technique gradually entered the minds of spinal surgeons. By the mid-1990s, it had been widely adopted in Europe in the treatment of patients with spinal hemangioma or metastatic disease (8). After a follow-up of 3 years, encouraging short-term results were observed, which increased the use of PVP surgery when treating vertebral fractures (9). PKP has been widely used when treating vertebral compression fractures and lytic lesions caused by bone metastasis, multiple myeloma, hemangioma, and other diseases (10). The purpose of PKP is to strengthen the bone, lessen or eliminate the discomfort incurred by a fractured spine, and, using a specialized instrument, reconstruct all or part of the loss vertebral body elevation as a result of the compression fracture. At present, the mechanism of pain relief is not completely clear, and there is still a lack of high-level evidence of clinical prospective research results.
In addition, the incidence of recurrent fractures of adjacent vertebrae after PKP is 3–20% (11,12). Meanwhile, with the increase of clinical cases and the improvement of operation, we found that recurrent fracture of the adjacent vertebral body is a common long-term complication of PKP. This problem has gradually attracted the attention of orthopedic surgeons and researchers, and the mechanism of re-fracture of adjacent vertebrae after PKP has not been unified. Hence, 313 cases with osteoporotic spinal fracture treated from December 2019 to May 2021 in Tianjin Hospital were analyzed retrospectively. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6475/rc).
Methods
Patient clinical information
Prior to conducting, the sample size was estimated via epidemiological data. A total of 313 patients with osteoporotic spinal fracture between December 2019 and May 2021 were compared retrospectively. Depending on the operation modes, cases were classified into a PVP cohort (n=130) and a PKP cohort (n=183). There were 68 males and 62 females in the PVP cohort and 92 males and 91 females in the PKP cohort. The average age of the PKP group was 61.93±8.71 years, and that of the PVP cohort was 77.88±9.42 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of Tianjin Hospital (ethics approval ID: AF-IRB-032-07). Individual consent for this retrospective analysis was waived.
The selection criteria were as follows: (I) the patient had a new fracture with an intact posterior vertebral wall and normal spinal canal; there were no remarkable compressions of the spinal-cord/nerve roots; (II) the patient had no cognitive, language, or intellectual impairment; (III) cases met “Chinese expert consensus of Osteoporosis diagnosis (third draft 2014 Edition)” (13) and “guidelines for the treatment of OVCF” (14); (IV) the patients agreed to be followed up for 12 months and could accept and answer telephone follow-up; (V) patients without non-operative treatment such as pain relief, anti-osteoporosis, physiotherapy, and brace; (VI) the patients with good compliance, regular reexamination, and complete follow-up data.
The exclusion criteria were as follows: (I) preoperative CT had indicated that the degree of vertebral collapse was more than 75% or indicated symptoms of nerve injury; (II) cases complicated with other systemic multiple or visceral injuries, such as brain trauma and chest trauma; (III) cases complicated with uncontrollable diabetes, cardiovascular and cerebrovascular diseases, renal insufficiency, blood coagulation dysfunction, or other surgical taboos; (IV) concurrent malignant tumors, tuberculosis, or other infectious diseases; (V) allergy to bone cement and developer materials; (VI) Incomplete medical records, irregular reexaminations or incomplete follow-up.
Therapeutic methods
Preoperative preparation
All patients were admitted to Tianjin Hospital and preoperative tests were completed. All patients were evaluated comprehensively for their medical and psychological status. The risks and implications of the procedure were explained and the patient signed a consent form for the procedure. Anterior and lateral X-ray examination of thoracolumbar vertebrae before operation confirmed the involved vertebral body segments, vertebral body CT, and 3-dimensional (3D) reconstruction to determine the fracture shape and whether the spinal canal was involved, and thoracolumbar vertebrae magnetic resonance imaging (MRI) was conducted to determine whether it was a new fracture. At the same time, the related complications of heart, brain, lung, and other important organs were treated, surgical contraindications were excluded, and the operation was performed after the vital signs of the patients had stabilized. Preoperative prone posture exercise, 4–6 times a day, for at least 30 minutes each time, was encouraged, as it is conducive to intraoperative fracture reduction.
Treatment methods
PKP group: patients underwent circular shadow localization and body surface marking of the affected vertebrae. The endplate was punctured at about 3 cm beside the spinous process outside the arch ring, and the needle tip was located at about 1/3 depth in the anterior middle of the vertebral body and did not exceed the spinous process. The puncture needle was removed, the sleeve was replaced, and the working catheter was inserted into the expansive balloon. Injection of contrast was finished and the balloon was removed. Under C-arm fluoroscopy, the adjusted bone cement was injected slowly, and the injection was stopped when the vertebral-body edge was reached. When the bone cement displayed a shape similar to that of toothpaste, it was completely hardened, and the puncture needle was removed.
PVP group: the patient was placed in the prone position and elevated at the waist. The position of the round shadow of the affected vertebra and the body surface were marked under C-arm machine fluoroscopy, and the collapsed endplate was punctured at 3 cm next to the spinous process outside the arch ring. A guide needle was inserted, and a hollow catheter was entered into the diseased cone. The position and depth were confirmed by C-arm machine fluoroscopy, and the contrast agent was injected. The C-arm machine confirmed the height recovery of the vertebral fracture. The patients in the 2 groups were strictly immobilized after surgery and were unable to twist the body. At 24 hours after operation, patients were dressed in protective gear and encouraged to walk on the ground for an appropriate amount of time. After 2 weeks, the patient received lumbar and back muscle training, but continued to avoid weight-bearing actions, bending, and turning. Patients were followed up for 12 months.
Clinical data collection
Data and references were collected, and a uniform questionnaire on risk factors for re-fracture was designed. A combination of questionnaires and consultation of clinical information was utilized, including gender, age, body mass index (BMI), degree of fracture, number of injured vertebrae, BMD, and surgical status, with postoperative telephone follow-up or review (15,16). The degree of fracture was evaluated by Genant semi-quantitative method, including mild fracture, moderate fracture, and severe fracture (17). Double copies were used to independently enter clinical data, with strict quality control, and timely completion or correction of missing or misreported items to ensure the accuracy and integrity of data. Values were assigned to each high-risk factor, and a risk prediction equation was established through multiple logistic regression.
Pain evaluation and patient grouping
The quantitative evaluation criteria of patients’ pain degree were based on visual analogue scale (VAS). The specific evaluation method of VAS score was as follows: a 10 cm horizontal line was drawn on the paper, in which one end was defined as 0, representing none pain; the other end was defined as 10, being severe pains; the middle segment of the horizontal line in turn represented different degrees of pain. In accordance with the feeling of self-pain, the corresponding score value was calculated (Table 1).
Table 1
| Project | PKP group (n=183) | PVP group (n=130) | t/χ2 | P value |
|---|---|---|---|---|
| Gender (male/female) | 92/91 | 68/62 | 0.126 | 0.723 |
| Age (years) | 76.93±8.71 | 77.88±9.42 | ||
| BMI (kg/m2) | 21.74±1.66 | 22.18±1.84 | ||
| Fracture site, n (%) | 1.077 | 0.299 | ||
| Lumbar vertebrae | 99 (54.10) | 78 (60.00) | ||
| Thoracic vertebrae | 84 (45.90) | 52 (40.00) | ||
| Number of fractured vertebrae, n (%) | 5.543 | 0.063 | ||
| Single segment | 89 (48.63) | 74 (56.92) | ||
| Double segment | 68 (37.16) | 48 (36.92) | ||
| Multi-segment | 26 (14.21) | 8 (6.15) | ||
| Degree of osteoporosis, n (%) | 1.423 | 0.491 | ||
| Mild | 81 (44.26) | 64 (49.23) | ||
| Moderate | 78 (42.62) | 54 (41.54) | ||
| Heavy weight | 24 (13.11) | 12 (9.23) |
Data are presented as n, mean ± SD, or n (%). PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty; BMI, body mass index; SD, standard deviation.
Observation index
Clinical data collection table
According to the medical and surgical records, we collected the information of all patients when all cases were enrolled, such as age, sex, involved vertebral segment, cause of injury, time from injury to operation, BMI, past history of low back pain, surgical puncture approach, operation time, and amount of bone cement injected.
Evaluation standard of curative effect
The curative effect was evaluated according to the reference (18). Efficacy assessment was performed 2 months after surgery. The efficacy was classified into 3 levels. Significant efficacy was defined as the return of most of the compressed vertebral body to a normal state and complete or basic restoration of function. Effective was defined as fracture healing, basic disappearance of low back pain, and improvement in the morphology of the lumbar vertebrae compared to the preoperative period. Ineffective was defined as a patient with localized pain and no change in deformity.
VAS score, height ratio, Cobb angle, and dysfunction index
Using VAS, a score of 0 indicated no pain; <3 indicated mild pain, which is tolerable; 4–6 indicated pain, which interferes with sleep; and 7–10 indicated intense pain, which is intolerable (19). Height of the anterior border of vertebral body, height of the midline of vertebral body, and Cobb angle (angle formed by the lower edges of 2 vertebral bodies at the top of the spinal curvature) were measured by X-ray. The Oswestry disability index (ODI) was used to evaluate activity capacity with a score of 0.5 for each project, with a total of 9 projects: 0 indicated normal activity function; 5 indicated the most serious limitation of activity function.
Short-term complications
The X-ray and MRI of thoracolumbar vertebrae were reexamined regularly after operation. If the T1 weighted imaging (T1WI) and T2 weighted imaging (T2WI) of MRI indicated that the edema signal in the operative segment persisted 3 months after operation, it indicated that the fracture was not healed. If the early postoperative MRI showed that there was no edema signal around the bone cement of the operative vertebral body, and the later reexamination showed that the edema signal appears again, or the edema signal on T1WI and T2WI appeared in other vertebral bodies, it indicated the occurrence of recurrent fracture, which was commonly seen in the adjacent vertebral body.
Preoperative BMD
The BMD was measured by dual energy X-ray absorptiometry (DXA) (GE Healthcare, Chicago, IL, USA), and the right hip was used as the reference standard. The measured result was t-value, which represented the result of comparison with the average BMD and standard deviation (SD) of the normal reference population.
Preoperative lumbar and back soft tissue injury
The low back soft tissue injuries of all patients were evaluated according to the findings of MRI fat suppression (FS) sequence before operation. If there was obvious high signal in FS sequence, it was considered soft tissue injury. The MRI images of the same patient were evaluated separately by 2 professional radiologists, and if they disagreed, the images were referred to another high-level radiologist.
Preoperative vertebral height compression ratio
According to the preoperative standard thoracolumbar X-ray lateral film, B = fractured vertebral-body height of anterior edge; A = upper height of the anterior edge; and C = lower vertebral-body height of the anterior edge. Then the original height of the vertebral body was calculated as B0 = (Aspirc)/2. Preoperative vertebral height compression ratio = (B0murB)/B0 × 100%. The data of the same patient were measured separately by 2 researchers who did not participate in the operation, and the final results were averaged.
Postoperative vertebral height recovery rate
According to the standard thoracolumbar X-ray lateral film after operation, B1 = fractured vertebral-body height of the anterior edge. Then the postoperative vertebral height recovery rate = (B1murB)/B1 × 100%. The data of the same patient were measured separately by 2 researchers who did not participate in the operation, and the final results were averaged.
Improvement rate of sagittal Cobb angle
The Cobb angle was analyzed by Phillips method. Two straight lines were made from the upper edge of the upper segment of the fractured vertebral body and the lower edge of the lower segment, and then the vertical lines of the 2 lines were made. The preoperative Cobb angle was measured as a, and the postoperative Cobb angle was recorded as b, then the improvement rate of Cobb angle was = (Amurb)/b × 100%. The measurements were carried out separately by 2 researchers who did not participate in the operation and did not know the specific grouping of patients, and the results were averaged. Before operation, and following 1-day, 1-week, 1-, 3-, and 12-month operation, the data of Cobb angle were collected.
Dispersion degree of bone cement
The 10×10 rectangular grid was constructed on the standard thoracolumbar X-ray films and lateral X-ray films respectively, covering the range of fractured vertebrae. The number of grids occupied by bone cement and vertebral body was counted respectively, in which those accounting for less than half of the grid were not counted, and those more than half of the grid were recorded as 1 grid. On the positive X-ray film, the number of bone cement in the grid is recorded as E, and the number of vertebral bodies in the grid is recorded as F, so the dispersion coefficient of bone cement in the positive position is K1 = E/F × 100%. In the same way, the dispersion coefficient K2 of bone cement in the lateral film and the total dispersion coefficient of bone cement K = K1 × K2 were obtained. The count of the grid was measured separately by 2 researchers who did not participate in the operation and did not know the specific grouping of the patients, and the final results were averaged.
Statistical analysis
The study data were processed using SPSS 26.0 statistical software (IBM Corp., Armonk, NY, USA). Count data were expressed as [n (%)], and chi-square test was used. Measured data were expressed as mean ± SD, and t-test was applied. Logistic regression was conducted to analyze the impact factors. Pearson correlation analysis was used for normal distribution data, and Spearman correlation analysis was performed for skewness distribution data and grade data. Logistic regression was used to analyze the influencing factors. P<0.05 indicated that the difference between groups is statistically significant.
Results
Comparison of general data between 2 groups
First, the patients’ sex, age, BMI, location of fracture, the number of fracture vertebrae, the degree of osteoporosis, and so on were compared (Table 1, P>0.05).
The clinical efficacy between the 2 groups
Secondly, we compared clinical efficacy between the 2 groups. The number of effective, Significantly effective, and ineffective cases in the PVP group were 76, 48, and 16 cases, respectively. The total effective rate of PVP group was 86.69%. The total effective rate of PKP group was 98.36% (Figure 1). The total effective rate in the PKP group was higher than that in the PVP group (χ2=4.325, P<0.05).

VAS score comparison
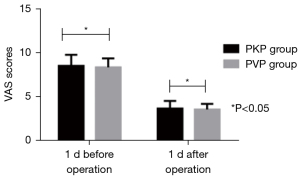
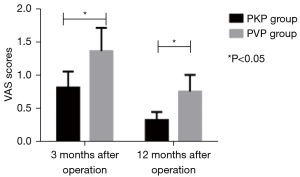
We compared the VAS scores between 2 groups. VAS scores decreased after operation, and the VAS scores of the PKP group at 1 week, 1, 3, and 12 months after operation were remarkably lower than those of the PVP group (P<0.05, Figures 2-4).

ODI score comparison
We compared the ODI scores between 2 groups. No remarkable differences were discovered in ODI scores before surgery. The ODI scores decreased after operation, and the ODI scores of 1 week, 1, 3 and 12 months after surgery in the PKP group were lower than those in the PVP group (P<0.05, Table 2).
Table 2
| Group | N | Before operation | 1 d after operation | 1 week after operation | 1 month after operation | 3 months after operation | 12 months after operation |
|---|---|---|---|---|---|---|---|
| PKP group | 183 | 3.39±1.38 | 2.24±1.26 | 1.54±0.73 | 1.06±0.58 | 0.83±0.32 | 0.44±0.23 |
| PVP group | 130 | 3.44±1.52 | 2.11±1.03 | 1.69±0.36 | 1.33±0.54 | 1.04±0.65 | 0.75±0.28 |
| t | – | 0.303 | 0.969 | 2.163 | 4.175 | 3.775 | 10.727 |
| P | – | 0.762 | 0.334 | 0.031 | 0.000 | 0.000 | 0.000 |
Data are presented as mean ± SD. ODI, Oswestry disability index; PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty.
The height ratio of anterior edge of vertebral body between 2 groups
The height ratio of the anterior vertebral body in the PKP group was remarkably lower than that in the PVP group (P<0.05, Table 3).
Table 3
| Group | N | Before operation | 1 d after operation | 1 week after operation | 1 month after operation | 3 months after operation | 12 months after operation |
|---|---|---|---|---|---|---|---|
| PKP group | 183 | 52.18±3.31 | 79.73±3.61 | 75.64±3.52 | 76.35±3.62 | 75.68±3.55 | 76.27±3.51 |
| PVP group | 130 | 52.37±3.44 | 79.66±3.44 | 76.25±3.58 | 76.53±3.41 | 76.34±3.25 | 75.17±3.54 |
| t | – | 0.492 | 0.172 | 1.500 | 0.444 | 1.678 | 2.723 |
| P value | – | 0.623 | 0.863 | 0.135 | 0.657 | 0.094 | 0.007 |
Data are presented as mean ± SD. PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty; SD, standard deviation.
Comparison of Cobb angle between the 2 groups
We compared the Cobb angle between the 2 groups. Compared to before surgery, 1 day, 1 week, 1, 3, and 12 months after operation, the Cobb angle decreased. In addition, the decrease of PKP was more obvious, but the difference was not significant (P>0.05, Table 4).
Table 4
| Group | N | Before operation | 1 d after operation | 1 week after operation | 1 month after operation | 3 months after operation | 12 months after operation |
|---|---|---|---|---|---|---|---|
| PKP group | 183 | 22.35±3.34 | 12.28±1.36 | 11.96±1.14 | 11.65±1.19 | 11.74±1.25 | 11.73±1.28 |
| PVP group | 130 | 22.41±3.47 | 12.35±1.23 | 11.88±1.15 | 11.73±1.17 | 11.75±1.16 | 11.64±1.32 |
| t | – | 0.154 | 0.467 | 0.610 | 0.590 | 0.072 | 0.605 |
| P value | – | 0.878 | 0.641 | 0.543 | 0.556 | 0.943 | 0.546 |
Data are presented as mean ± SD. PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty; SD, standard deviation.
Univariate analysis of factors related to re-fracture after PKP
Univariate analysis was conducted to analyze the related factors of recurrent fracture after PKP. The results indicated that age, number of injured vertebrae, history of complicated fracture, number of operative vertebrae, and BMD of patients were remarkably correlated with the incidence of recurrent fracture after PKP (P<0.05, Table 5).
Table 5
| Items | Unfractured group (n=151), n (%) | Re-fracture group (n=32), n (%) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 5.543 | 0.019 | ||
| >75 | 74 (49.01) | 23 (71.88) | ||
| ≤75 | 77 (50.99) | 9 (28.13) | ||
| Gender | 2.753 | 0.097 | ||
| Male | 81 (53.64) | 12 (37.50) | ||
| Female | 70 (46.36) | 20 (62.50) | ||
| BMI (kg/m2) | 2.662 | 0.103 | ||
| ≥25 | 80 (52.98) | 22 (68.75) | ||
| <25 | 71 (47.02) | 10 (31.25) | ||
| Course of disease (d) | 2.924 | 0.087 | ||
| ≥30 | 47 (31.13) | 15 (46.88) | ||
| <30 | 104 (68.87) | 17 (53.13) | ||
| Before operation Cobb angle (°) | 0.837 | 0.360 | ||
| ≥25 | 81 (53.64) | 20 (62.50) | ||
| <25 | 70 (46.36) | 12 (37.50) | ||
| Number of injured vertebrae (unit) | 12.437 | 0.000 | ||
| 1–2 | 75 (49.67) | 5 (15.63) | ||
| >2 | 76 (50.33) | 27 (84.38) | ||
| Fracture history | 9.739 | 0.002 | ||
| Yes | 31 (20.53) | 15 (46.88) | ||
| None | 120 (79.47) | 17 (53.13) | ||
| BMD (g/cm2) | 16.803 | 0.000 | ||
| 1–2.5 | 93 (61.59) | 7 (21.88) | ||
| >2.5 | 58 (38.41) | 25 (78.13) | ||
| Operation site | 0.029 | 0.865 | ||
| Thoracic vertebrae | 78 (51.66) | 16 (50.00) | ||
| Lumbar vertebrae | 73 (38.34) | 16 (50.00) | ||
| Amount of bone cement injected (mL) | 1.526 | 0.217 | ||
| 1–5 | 96 (63.58) | 24 (75.00) | ||
| >5 | 55 (36.42) | 8 (25.00) | ||
| Number of operative vertebrae (unit) | 11.961 | 0.001 | ||
| 1–2 | 79 (53.32) | 6 (18.75) | ||
| >2 | 72 (47.68) | 26 (81.25) | ||
PKP, percutaneous kyphoplasty; BMI, body mass index; BMD, bone mineral density; SD, standard deviation.
Multivariate logistic regression analysis of postoperative recurrent fracture
The single factors in this study included age, number of injured vertebrae, fracture history, BMD, and number of operated vertebrae. Whether the patient had a recurrent fracture (“no” =0 and “yes” =1) was the dependent variable for logistic regression analysis. The results showed that age, surgical vertebral bodies, BMD, and the number of injured vertebral bodies were independent risk factors for recurrent fractures after PKP (P<0.05) (Table 6).
Table 6
| Risk factors | Β value | S.E. | Wald value | P value | OR value (95% CI) |
|---|---|---|---|---|---|
| Age | 1.911 | 0.803 | 5.664 | 0.017 | 6.760 (1.401–32.619) |
| Number of injured vertebrae | 2.213 | 0.358 | 38.212 | 0.000 | 9.143 (4.533–18.443) |
| Fracture history | 1.246 | 0.552 | 5.095 | 0.024 | 3.476 (1.178–10.257) |
| BMD | 2.334 | 0.316 | 54.554 | 0.000 | 10.319 (5.555–19.170) |
| Number of operative vertebrae | 1.803 | 0.764 | 5.569 | 0.018 | 6.068 (1.357–27.125) |
BMD, bone mineral density; S.E., standard error; OR, odds ratio; CI, confidence interval.
Analysis of baseline data, operation-related parameters, and imaging measurement indexes of patients with residual pain group and non-residual pain group
We compared the baseline data, operation-related parameters, and imaging measurement indexes (Table 7). The results indicated remarkable differences in BMI, BMD, complicated with low back soft tissue injury, history of low back pain, short-term complications, fracture nonunion, postoperative vertebral height recovery rate, sagittal Cobb angle improvement rate, and total diffusion coefficient of bone cement (P<0.05).
Table 7
| Project | Non-residual pain group (n=155) | Residual pain group (n=28) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 78.83±7.35 | 78.54±7.12 | 0.193 | 0.847 |
| Gender (male/female) | 67/78 | 15/13 | 0.478 | 0.490 |
| BMI (kg/m2) | 24.57±2.91 | 21.64±2.33 | 5.040 | 0.000 |
| BMD (T value) | −3.11±0.51 | −3.93±0.66 | 7.463 | 0.000 |
| Time from injury to operation (days) | 6.52±3.65 | 6.33±2.71 | 0.262 | 0.793 |
| Fracture site of vertebral body (thoracic vertebrae/lumbar vertebrae) | 68/87 | 16/12 | 1.682 | 0.195 |
| Soft tissue injury of low back (with/without) | 77/78 | 20/8 | 4.504 | 0.034 |
| Past history of low back pain (with/without) | 56/99 | 18/10 | 7.806 | 0.005 |
| Cause of injury, n (%) | 8.704 | 0.013 | ||
| Fall injury from height | 40 (25.81) | 15 (53.57) | ||
| Fall injury on flat ground | 87 (56.13) | 10 (35.71) | ||
| Other | 28 (18.67) | 3 (10.71) | ||
| Preoperative VAS score (score) | 7.24±0.92 | 7.08±1.11 | 0.820 | 0.414 |
| Surgical puncture approach (unilateral/bilateral) | 87/78 | 15/13 | 0.007 | 0.932 |
| Operation time (min) | 40.32±12.73 | 38.56±11.27 | 0.684 | 0.495 |
| Amount of cement injected into bone (mL) | 4.55±0.92 | 4.48±0.87 | 0.373 | 0.709 |
| Short-term complications (example), n (%) | 4 (2.58) | 13 (46.43) | 54.111 | 0.000 |
| Fracture nonunion (example), n (%) | 2 (1.29) | 11 (39.29) | 51.881 | 0.000 |
| Recurrent fracture, n (%) | 4 (2.58) | 28 (100.00) | 155.993 | 0.000 |
| Preoperative vertebral height compression ratio (%) | 34.78±9.83 | 36.21±10.56 | 0.700 | 0.485 |
| Postoperative vertebral height recovery rate (%) | 48.66±13.24 | 39.72±11.41 | 3.353 | 0.001 |
| Improvement rate of sagittal Cobb angle (%) | 25.83±6.92 | 21.66±5.43 | 3.023 | 0.003 |
| Total dispersion coefficient of bone cement | 0.36±0.05 | 0.22±0.03 | 14.337 | 0.000 |
Data are presented as mean ± SD, n, or n (%). BMI, body mass index; BMD, bone mineral density; VAS, visual analogue scale; SD, standard deviation.
Logistic regression analysis of related risk factors
We compared the potential risk factors of BMI, BMD, low back soft tissue injury, cause of injury, history of low back pain, short-term complications, nonunion, recurrent fracture, postoperative vertebral height recovery rate, sagittal Cobb angle improvement rate, and total diffusion coefficient of bone cement in univariate analysis, and applied logistic regression for multivariate analysis (Table 8). We concluded that BMI, BMD, low back soft tissue injury, postoperative vertebral body height recovery rate, sagittal Cobb angle improvement rate, total diffusion coefficient of bone cement, short-term complications, nonunion, and recurrent fracture are the residual low back major risk factors for pain after PKP (P<0.05).
Table 8
| Risk factors | Β value | S.E. | Wald value | P value | OR value (95% CI) |
|---|---|---|---|---|---|
| BMI | −0.941 | 0.424 | 4.925 | 0.026 | 0.390 (0.170–0.896) |
| BMD | −1.532 | 0.341 | 20.184 | 0.000 | 0.216 (0.111–0.442) |
| Soft tissue injury of low back | 0.424 | 0.118 | 12.911 | 0.000 | 1.528 (1.213–1.926) |
| Cause of injury | 0.136 | 0.045 | 9.134 | 0.003 | 1.146 (1.049–1.251) |
| Past history of low back pain | 0.088 | 0.014 | 39.510 | 0.000 | 1.092 (1.062–1.122) |
| Short-term complications | 1.173 | 0.278 | 17.804 | 0.000 | 3.232 (1.874–5.573) |
| Fracture nonunion | 2.325 | 0.487 | 22.792 | 0.000 | 10.227 (3.937–25.563) |
| Recurrent fracture | 1.348 | 0.313 | 18.548 | 0.000 | 3.850 (2.084–7.110) |
| Postoperative vertebral height recovery rate | −0.823 | 0.258 | 10.176 | 0.001 | 0.43 (0.265–0.728) |
| Improvement rate of sagittal Cobb angle | −0.746 | 0.037 | 406.513 | 0.000 | 0.474 (0.441–0.510) |
| Total dispersion coefficient of bone cement | −1.263 | 0.439 | 8.277 | 0.004 | 0.283 (0.120–0.669) |
BMI, body mass index; BMD, bone mineral density; S.E., standard error; OR, odds ratio; CI, confidence interval.
Discussion
OVCF is an obvious complication of osteoporosis. It has become a severe problematic disease which threatens the life and health of the elderly. Epidemiological data has shown that about 88 million people in China have osteoporosis of varying degrees. Osteoporosis can easily lead to vertebral compression fractures, local pain, and dyskinesia (2). Nowadays, PKP has become one of the effective methods for the treatment of thoracolumbar compression fractures. It can not only quickly relieve pain and stabilize the vertebral body, but also partially reduce the collapsed vertebral body and correct kyphosis. According to World Health Organization (WHO) estimates, the number of people over the age of 60 in the world was about 900 million (20). It is found that when BMD decreases by 1 SD, the risk of OVCF increases by a factor of almost 2 (21). More than 25% of women over the age of 65 have experienced OVCF more than once (22). The prevalence of OVCF is 4.7% in the 50–54 age group and 32.2% in the 75–79 age group, with a lifetime risk of 16% for white females and 5% for white males (23). OVCF will not only cause severe low back pain in patients, but also affect daily life and activities, and even lead to disability, as well as accompanying psychological disorders such as depression and anxiety (4). Previous research has indicated that patients with OVCF visit primary health care institutions 4 times more often than the controls in the year following fracture. In addition, once the first osteoporotic fracture occurs, the risk of a second fracture is 5 times higher than that of people with no history of fracture (24). In the UK, the National Health Service (NHS) spends about £942 million a year on osteoporotic vertebral fractures, of which only £12 million is spent on fracture treatment and the rest on fracture-related long-term complications (25). Therefore, OVCF remains also a major social health problem.
The treatment of PVP involves the injection of bone cement into the vertebral body with a pressure syringe to support the collapsed vertebral body, rapidly solidify the vertebral vessels, relieve pain quickly, and restore the function of thoracolumbar vertebrae in the early stage (26). It has the characteristics of good safety, quick effect, and quick recovery after operation. PKP treatment aims to restore the original anatomical structure of the fractured vertebral body by dilating an inserted balloon (26). Our study has indicated that compared with those before operation, the VAS, ODI, and Cobb angles of patients treated with PVP and PKP decrease remarkably after 12 months.
Our data showed that the total efficacy in the PKP group was higher than that in the PVP group. The VAS scores after surgery in the PKP group were lower than those in the PVP group. The ODI score in the PKP group was considerably lower than that in the PVP group after the operation.
PVP is different from PKP in that PVP restores the height of vertebral body according to the reduction of body position during operation (24). The operation of PVP is relatively simple: percutaneous pedicle or lateral injection, correct kyphosis, and prevent the aggravation of collapse. In the current study, the clinical data of 383 elderly patients with OVCF were analyzed retrospectively.
After PKP, the injured vertebrae fusion may increase the internal pressure in the vertebral body, reduce flexibility of the spinal joints, and lead to new fractures (26). In the present study, 32 cases of thoracolumbar compression fractures occurred after PKP, accounting for 17.49%, which was consistent with previous reports (26), suggesting that there is a higher risk of new fractures after PKP. Therefore, the analysis of the risk factors of recurrent fracture after PKP is helpful to reduce the incidence of postoperative recurrent fracture. In our study, age, the number of operative vertebrae, BMD, the number of injured vertebrae, and the history of fracture were the risk factors of new fracture, which was consistent with most of the previous research results (27-29). With the increase of the age of patients, the probability of postoperative re-fracture increases remarkably, and it is considered that age is an independent risk factor for re-fracture after PKP (28). It was found that the proportion of age >75 years in the re-fracture group after PKP was 1.45 times higher than that of the non-fracture group. The author asserted that the main reason is that with the increase of age, the loss of calcium in the body increases, BMD decreases remarkably, bone brittleness is high, and re-fracture can easily to occur after surgery, so it is necessary to provide anti-osteoporosis treatment. Meanwhile, our study found that BMD is also an independent risk factor for re-fracture after PKP, which further validates the above analysis. In addition, it is worth noting that patients with a large number of injured vertebrae and surgical vertebrae are also prone to postoperative re-fractures. Currently, the proportion of injured vertebrae and operative vertebrae >2 in the re-fracture group was remarkably higher than the non-fracture group, which was consistent with the results of He et al. (30). Moreover, it was highlighted that the number of injured vertebral bodies and the number of operated vertebral bodies >2 were independent risk factors for re-fracture after PKP, suggesting that the ultimate load of adjacent vertebral bodies decreased, but the stress increased after PKP.
Currently, PKP is considered to have definite efficacy and high safety, and has become the standard surgical option for OVCF, providing remarkable pain relief in 78–95% of OVCF patients. However, residual pain after PKP is not uncommon in clinical practice and the efficacy is uncertain (27). In this clinical study, we defined VAS ≥4 as the critical value of postoperative residual pain to distinguish whether the pain was satisfactorily relieved or not. Because the VAS score >4 is classified as moderate pain, it can affect people’s sleep and interfere with normal life and work. Among the 183 patients treated with PKP in this study, 28 patients developed residual pain after surgery, with an incidence of 15.30%, which was consistent with the data in the previously published literature (26). In terms of the mechanism of bone tissue-related pain, the skeletal cortex and internal innervation play an important role. Nerve axons can extend to the cancellous bone, and these axons enter the periosteal surface from the bone marrow. In bone and joint diseases, inflammatory mediators in the synovial sac and surrounding soft tissue may stimulate the end of the injury receptor and aggravate pain, which is the theory of inflammatory mediators produced by pain (31). Other studies have found that the end of nociceptive receptors in bones can be directly activated by changes in biomechanics, such as that osteoporosis increases the mechanical force on bones, resulting in more sensitive receptors in the bone matrix to injury (29). Essentially, the nociceptive signals generated by synovial and periosteal membranes are different from those induced in the bone matrix by nociceptive terminals from bone marrow input. However, the body usually cannot effectively distinguish between the 2, and low back pain in patients after PKP may be the result of superimposed multiple stimulation pathways. The mechanism of pain relief caused PKP is also closely related to the destructive effect of bone cement on nerve nociceptor. Some researchers (32) have studied the possible risk factors of residual pain after PKP, including rib fracture, bone cement leakage compression of spinal cord or nerve, infection, and adjacent vertebral fracture. In this study, univariate analysis and subsequent multivariate logistic regression indicated that body mass index, BMD, low back soft tissue injury, postoperative vertebral height recovery rate, sagittal Cobb angle improvement rate, total diffusion coefficient of bone cement, short-term complications including postoperative infection, bone cement leakage, fracture nonunion, and recurrent fracture were the main risk factors of low back residual pain. We summarized these influencing factors as low back soft tissue injury, spinal instability, postoperative recurrent fracture, fracture nonunion, and short-term complications.
Collectively, the occurrence of low back residual pain and re-fracture after PKP is a common clinical obstacle in patients’ postoperative rehabilitation. Clinically, attention should be paid to identifying these high-risk patients and carrying out targeted prevention and intervention measures to help reduce the incidence of postoperative residual pain and enhance patients’ satisfaction with treatment.
This study has some limitations. Firstly, the number of cases in this clinical trial was small. Additionally, all cases were from a single center, with relatively shorter follow-up, so it remains necessary to perform large-sample multicenter prospective studies in the future. Secondly, there may be interactions and interference between the analyzed factors. Therefore, it is better to further analyze the main effects and interactions of risk factors in the future to make the research results more comprehensive. Finally, pain is a complex emotional experience. This study only analyzed objective quantitative indicators, and did not evaluate the influence and role of subjective emotional factors. It is necessary to establish relevant influence models for further analysis in the future.
Conclusions
In conclusion, the clinical effects of PKP in elderly patients with thoracolumbar compression fracture is better compared to VAS, ODI, and bone cement leakage after PVP. Age, the number of vertebral bodies, BMD, and the number of injured vertebrae is the risk factors of re-fracture after PKP. Low back soft tissue injury, spinal instability, recurrent fracture, fracture nonunion, and short-term complications are risk factors for residual pain after PKP. Clinically, more attention should be paid to identify high-risk patients and implement preventive measures to help reduce the occurrence of recurrent fracture and residual pain after operation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6475/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6475/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6475/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of Tianjin Hospital (ethics approval ID: AF-IRB-032-07). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li Y, Feng X, Pan J, et al. Percutaneous Vertebroplasty Versus Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures in Patients with Distant Lumbosacral Pain. Pain Physician 2021;24:E349-56.
- Shan L, Wang L. Progress in diagnosis and treatment of osteoporosis. Medical review 2019;25:3652-3656,3661.
- Chen Y, Yin P, Hai Y, et al. Is Osteoporotic Thoracolumbar Burst Fracture a Contraindication to Percutaneous Kyphoplasty? A Systematic Review. Pain Physician 2021;24:E685-92.
- Abdelgawaad AS, Ezzati A, Govindasamy R, et al. Kyphoplasty for osteoporotic vertebral fractures with posterior wall injury. Spine J 2018;18:1143-8. [Crossref] [PubMed]
- Li M, Zhang Y, Jin P, et al. Percutaneous vertebral augmentation using drill rotation for osteoporotic vertebral compression fractures with intravertebral vacuum cleft. Skeletal Radiol 2020;49:1459-65. [Crossref] [PubMed]
- Fang YP, Lu YJ, Gan MF, et al. Percutaneous kyphoplasty for a patient of thoracolumbar osteoporotic vertebral compression fractures with distal lumbosacral pain: a case report. Ann Palliat Med 2021;10:4944-9. [Crossref] [PubMed]
- Xue YD, Zhang ZC, Dai WX. Investigation of Preoperative Traction Followed by Percutaneous Kyphoplasty Combined with Percutaneous Cement Discoplasty for the Treatment of Severe Thoracolumbar Osteoporotic Vertebral Compression Fractures. Int J Gen Med 2021;14:6563-71. [Crossref] [PubMed]
- Huang D, Ying J, Xu D, et al. Comparison of Percutaneous Kyphoplasty with or without Pedicle Screw Fixation in Osteoporotic Thoracolumbar Vertebral Fractures: A Retrospective Study. Dis Markers 2021;2021:4745853. [Crossref] [PubMed]
- Wang C, Zhang Y, Chen W, et al. Comparison of percutaneous curved kyphoplasty and bilateral percutaneous kyphoplasty in osteoporotic vertebral compression fractures: a randomized controlled trial. BMC Musculoskelet Disord 2021;22:588. [Crossref] [PubMed]
- Liu X, Wang H, Zhang Y, et al. The analgesic efficacy of extracorporeal shock wave combined with percutaneous vertebroplasty in the treatment of osteoporotic thoracolumbar compression fractures in postmenopausal women. Biomed Eng Online 2021;20:58. [Crossref] [PubMed]
- Dai C, Liang G, Zhang Y, et al. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern Asia: a systematic review and meta-analysis. J Orthop Surg Res 2022;17:161. [Crossref] [PubMed]
- Gu YT, Zhu DH, Liu HF, et al. Minimally invasive pedicle screw fixation combined with percutaneous vertebroplasty for preventing secondary fracture after vertebroplasty. J Orthop Surg Res 2015;10:31. [Crossref] [PubMed]
- Wen Z, Mo X, Zhao S, et al. Comparison of Percutaneous Kyphoplasty and Pedicle Screw Fixation for Treatment of Thoracolumbar Severe Osteoporotic Vertebral Compression Fracture with Kyphosis. World Neurosurg 2021;152:e589-96. [Crossref] [PubMed]
- Yin P, Ma Y, Ma X, et al. The clinical guideline for osteoporotic compression fractures. Chin J Osteoporos 2015;21:643-8.
- Tao W, Hu Q, Nicolas YSM, et al. Is unilateral transverse process-pedicle percutaneous kyphoplasty a better choice for osteoporotic thoracolumbar fractures in the old patients? BMC Surg 2021;21:252. [Crossref] [PubMed]
- Li J, Xiang Z, Zhou J, et al. Three-Dimensional Reconstruction of a CT Image under Deep Learning Algorithm to Evaluate the Application of Percutaneous Kyphoplasty in Osteoporotic Thoracolumbar Compression Fractures. Contrast Media Mol Imaging 2022;2022:9107021. [Crossref] [PubMed]
- Zou D, Dong S, Du W, et al. Risk factor analysis of pulmonary cement embolism during percutaneous vertebroplasty or kyphoplasty for osteoporotic vertebral compression fractures. J Orthop Surg Res 2021;16:312. [Crossref] [PubMed]
- Li K, Li Y, Zeng J. Comparison of short-term and long-term effects of PVP and PKP in the treatment of osteoporotic thoracolumbar compression fracture. Tissue Engineering and Reconstruction Surgery 2021;17:46-9.
- Li Y, Chen B. comparison of kyphoplasty in the treatment of thoracolumbar compression fractures in the elderly. Chinese Journal of Gerontology 2019;39:1611-4.
- Al-Agha AE, Kabli YO, AlBeiruty MG, et al. Quantitative ultrasound screening of bone mineral density on children with short stature. Saudi Med J 2020;41:597-601. [Crossref] [PubMed]
- Bruno AG, Burkhart K, Allaire B, et al. Spinal Loading Patterns From Biomechanical Modeling Explain the High Incidence of Vertebral Fractures in the Thoracolumbar Region. J Bone Miner Res 2017;32:1282-90. [Crossref] [PubMed]
- Gourlay ML, Overman RA, Ensrud KE. Bone Density Screening and Re-screening in Postmenopausal Women and Older Men. Curr Osteoporos Rep 2015;13:390-8. [Crossref] [PubMed]
- Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women's Health Initiative. Semin Reprod Med 2014;32:454-62. [Crossref] [PubMed]
- Chin KY. A review on the performance of osteoporosis self-assessment tool for Asians in determining osteoporosis and fracture risk. Postgrad Med 2017;129:734-46. [Crossref] [PubMed]
- Imai K. Vertebral fracture risk and alendronate effects on osteoporosis assessed by a computed tomography-based nonlinear finite element method. J Bone Miner Metab 2011;29:645-51. [Crossref] [PubMed]
- Xu J, Wu G, Liao X. Comparison of the efficacy of multi-segment simultaneous puncture and segmental vertebroplasty in the treatment of multi-vertebral osteoporotic fractures. Chinese Journal of Orthopaedic Trauma 2016;18:532-5.
- Zhu S, Liu Y, Zhang M, et al. Analysis of risk factors of re-fracture after PKP. Chinese Journal of Orthopaedic Surgery 2016;24:1445-8.
- Xu R, Li S, Chen G, et al. The effect of additional facet joint block for analgesia in patients with thoracolumbar compression fracture undergoing percutaneous kyphoplasty surgery: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e29034. [Crossref] [PubMed]
- Hou W, Sun X, Zhang C, et al. analysis of the incidence and related risk factors of adjacent vertebral fracture after percutaneous vertebroplasty in elderly patients with vertebral compression fracture. Chinese Journal of Orthopaedic surgery 2016;24:1909-11.
- He H, Tan Y, Yang S, et al. Study of Unilateral Extrapedicular and Bilateral Pedicle Approach Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture. J Coll Physicians Surg Pak 2022;32:924-7. [Crossref] [PubMed]
- Huang WC, Lin MH, Lee MH, et al. Percutaneous dorsal root ganglion block for treating lumbar compression fracture-related pain. Acta Neurochir (Wien) 2018;160:1283-9. [Crossref] [PubMed]
- Bian F, Bian G, Zhao L, et al. Risk factors for recollapse of new vertebral compression fractures after percutaneous kyphoplasty in geriatric patients: establishment of a nomogram. BMC Musculoskelet Disord 2022;23:458. [Crossref] [PubMed]
(English Language Editor: J. Jones)


