Autologous endothelial progenitor cells improve allograft survival in porcine lung transplantation with prolonged ischemia
Introduction
Lung transplantation is a viable therapeutic option for severe end-stage and non-malignant lung disease. Despite advances in its surgical and medical management, the survival of lung transplant remains the worst amongst solid organ transplantations. Ischemia reperfusion injury (IRI) following lung transplant is the most common cause of respiratory failure, which contributes to 30% of perioperative mortality, and is also responsible for postoperative primary graft failure (1,2). The optimal ischemic time of donor lung is about 6 to 8 h and is significantly associated with worse graft survival when the ischemic time exceeds 330 min (3). IRI is associated with overproduction of reactive oxygen species during non-hypoxic lung ischemia by activation of NADPH oxidases and inducible nitric oxide synthase in the pulmonary vasculature and lung inflammatory cells (1,4,5). The release of proinflammatory cytokines from the activated macrophage injures the integrity of pulmonary endothelial cells and alveolar epithelial cells (6-9). Activation of cell adhesion molecules also mediates important roles in the process of IRI (10,11). These interacting processes lead to damage of the vascular endothelium, increased cellular gap junctions, cell apoptosis, and resulting in increased alveolar-capillary permeability, infiltration of interstitial inflammatory cells, and lung hemorrhage (1,4,12-15). Although a number of pulmonary endothelium- and alveolar epithelium-targeting therapeutic strategies have been extensively investigated, acute primary graft failure secondary to IRI remains a major cause of the postoperative morbidity and mortality for lung transplantation.
During the past two decades, the discovery of endothelial progenitor cells (EPCs) has promoted our understanding in postnatal vasculogenesis and endothelial protection, particular during vascular activation or injury (16-20). Administration of EPCs in experimental animal models or patients provides therapeutic function as an endogenous repair mechanism in maintaining integrity of the endothelial monolayer by replacing denuded parts of the injured artery and/or forming new vessels by direct incorporation and paracrine-mediated effects (21-24). In our laboratory, we have demonstrated that autologous EPCs preserves pulmonary endothelial function and maintains the integrity of pulmonary alveolar-capillary barrier in rabbit models of acute lung injury (ALI) (16,25). Transplantation of EPCs not only improved gas exchange and degree of pulmonary edema in the rabbits with ALI, but also attenuated inflammatory responses in the lung. Furthermore, our unpublished data also suggested that administration of EPCs ameliorated the development of IRI in experimental model of bowel ischemia. Logistically extended from our previous investigation in ALI, we hypothesized that the high re-endothelization and anti-inflammatory capability of EPCs might preserve the function of lung graft during the acute reperfusion period of lung implantation, and tested the therapeutic potential of EPCs in a porcine model of lung transplantation with prolonged graft ischemia.
Methods
Piglets and anesthesia
Yorkshire piglets aged 2–3 months were obtained from the National Taitung Animal Propagation Station, Taiwan. All animals were housed under pathogen-free and controlled temperature conditions at the Animal Center of the National Cheng Kung University Medical College. All procedures were performed in accordance with the guidelines, and the protocol for the research project was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Cheng Kung University, Taiwan (IACUC Approval No. 99024). The induction of anesthesia was performed by intramuscular injection of a cocktail anesthetic consisted of Zoletil (Virbac Taiwan Co., Ltd., 12.5 mg/kg), Rompun (Bayer, German, 0.2 mL/kg), and atropine (Taiwan Biotec, Taiwan, 0.05 mg/kg). A 6-mm endotracheal tube was intubated by direct laryngoscopy and the piglets were mechanically ventilated in the volume-control ventilation mode (Siemens Servo 900B; Siemens; Elema, Sweden) throughout the study period. Tidal volume was set at 8–10 mL/kg and minute ventilation was adjusted to achieve normocapnia. Anesthesia was maintained with isoflurane (0.5% to 1% in oxygen). Rocuronium (NV Organon, Netherlands, 5 mg/h) was administered intravenously to facilitate mechanical ventilation. Hydration was maintained by infusing isotonic saline solution via an ear vein at a rate of 5 mL/kg/h. Standard electrocardiography and ear pulse oxymetry were monitored continuously.
Porcine model of lung transplantation
Donor lung procurement
Following median sternotomy, the pleural cavities of donor piglet were dissected open and the inferior pulmonary ligaments were released. The superior and inferior vena cava were dissected free. The left hemiazygos vein was divided in order to maintain a bloodless operation field. Following full heparinization (300 U/kg, i.v.), the vena cava was ligated and the lung was perfused via a direct pulmonary artery cannulation. The pulmonary circulation was then perfused with 4 °C lactate ringer solution (60 mL/kg) and followed by 4 °C Perfadex solution (Vitrolife Sweden, 1,000 mL). The perfusates were drained from the left atrial appendage. When the perfusion was completed, both lungs were procured en bloc from the thoracic cavity and stored in 4 °C Perfadex solution before implantation. The total ischemic time of lung block was recorded from the initiation of pulmonary perfusion to the resume of blood flow after implantation.
Lung implantation
Left thoracotomy was performed at the 5th intercostal space of the recipient animals to expose the hilar structure. The inferior pulmonary ligament was released and the left hemi-azygos vein was divided. The hilar structures were cross-clamped in the order of pulmonary artery, pulmonary vein and left main bronchus, and followed by left pneumonectomy. After removal of the left lung of recipient, the donor lung was implanted. The bronchus, pulmonary vein, and then pulmonary artery were orderly anastomosed with 4-0, 5-0, and 6-0 Prolene (Ethicon Inc., Somerville, NJ). Immediately before restoration of pulmonary blood flow, autologous EPCs or plain culture medium were delivered into the left pulmonary artery. Methylprednisolone (Pfizer, Belgium, 500 mg) was also administered intravenously before reperfusion. The pulmonary arteries were de-clamped, followed by declamping of pulmonary veins. The donor lung was then ventilated adequately to re-expand the collapsed lung units. Thirty minutes after reperfusion, the grafted left lung function was evaluated by blocking of right pulmonary circulation through ligation of the right pulmonary artery. The overall survival time of recipients was recorded from the time point of right pulmonary artery occlusion and animals that survived from the operation were sacrificed by intravenous infusion of pentobarbital (Ta Fong, Taiwan, 250 mg/kg) 4 h later.
Hemodynamic measurement and blood gas analysis
Catheters were inserted into the pulmonary trunk and descending aorta to directly measure the pulmonary artery and systemic arterial pressures, respectively. The cardiac output was also monitored simultaneously by connecting the aortic blood pressure to a cardiac output system (VigileoTM FlowTrac Monitor). Blood samples for gas analysis were drawn from the aortic arterial line and analyzed by a blood gas analyzer (ABL 520, Radiometer) within 10 min of sampling.
Isolation, characterization and delivery of EPCs
Peripheral blood (3 mL/kg) was obtained from the recipient piglets via the femoral artery under intramuscular anesthesia. Mononuclear cells (MNCs) were isolated using density gradient centrifugation with Ficoll-Plaque Plus (Amersham Biosciences). MNCs were then washed and plated on 6-well plates coated with human fibronectin (Sigma-Aldrich), supplemented with endothelial growth medium-2 (Cambrex). On day 7 of culture, the adherent cells (known as early EPCs) were harvested for analysis or transplantation. The early EPCs were incubated subsequently with acetyl-LDL (acLDL 10 µg/mL, Molecular Probes, Carlsbad, CA, USA) and isolectin (5 µg/mL, Molecular Probes, Carsbad, CA, USA). The staining of acLDL and isolectin in cultured EPCs was detected under fluorescence confocal microscopy at the absorption wavelengths of 555 and 495 nm, respectively. EPCs (~3×105 cells) were harvested by trypsinization 7 days after plating for transplantation. Immediately before transplantation, a fluorescent cell tracker CM-DiI (Molecular Probes) was used to label the cells and to track their homing in the pulmonary vasculature. The surgeons were blinded to treatment solution (1 mL of culture medium consisted of autologous EPCs or plain medium).
Histological examination
Frozen lung tissues were sectioned and examined under fluorescence microscopy for the detection of DM-DiI-labeled cells. Biopsies of formalin-fixed lung tissues were embedded in paraffin wax and sectioned (5 µm). Sectioned tissues were stained with Hemotoxylin and Eosin. Lung histology was observed under a light microscope.
Statistical analysis
Results are presented as the mean ± SD. Data were compared by an unpaired t-test or ANOVA, as appropriate. Survival curves were constructed using Kaplan-Meier analysis. Statistical significance was accepted at a level of P<0.05.
Results
EPC characterization
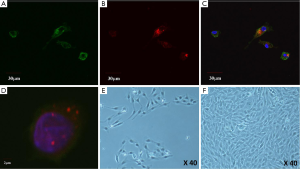
Cultured day-7 EPCs exhibited phenotyping of endothelial cells, including incorporation of AcLDL and isolectin (Figure 1). About 2 weeks after culturing in EGM-2, outgrowth colonies were formed in the typical monolayer, cobblestone appearance of endothelial cells, as shown at ×40 magnification (Figure 1).
Subject characterization and ischemic time
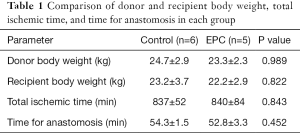
The total body weights of the donor and recipient subjects were matched (Table 1). The total ischemic time for lung graft was approximately 14 h, and time durations were similar between the two treatment groups (Table 1). There were also no differences in operation time and intraoperative blood loss during lung implantation was minimal.
Full table
General outcomes of lung transplantation
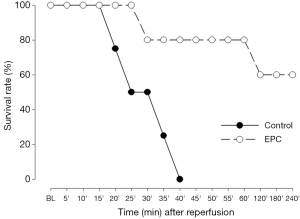
There was no perioperative mortality in the recipient subjects for both treatment groups until the ligation of right pulmonary artery. However, all animals receiving plain medium died within 40 min after occlusion of right pulmonary artery (Figure 2), but 3 out of 5 (60%) piglets receiving autologous transplantation of EPCs survived up to 4 h after diversion of the entire cardiac output of right ventricle into the graft lung (Figure 2).
Hemodynamic measurements
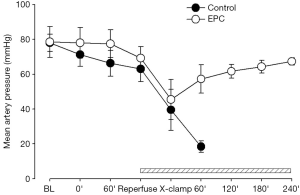
Restoration of pulmonary arterial flow into the graft lung induced drop of systemic blood pressure in the two treatment groups, indicating the development of IRI during lung implantation (Figure 3). In the control group, all hemodynamic parameters started to deteriorate following occlusion of right pulmonary artery and eventually shut down within 40 min (Figures 3-5). The mean aortic pressure decreased, and the cardiac out and mean pulmonary artery pressure elevated after right pulmonary artery occlusion in the EPC-treated piglets that survived after lung transplantation. However, these parameters were gradually compensated and returned to the baseline levels at the end of experiment (Figures 3-5).
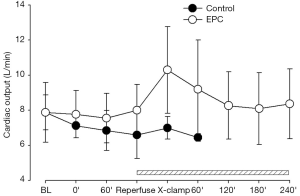
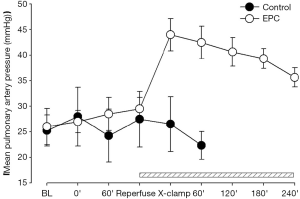
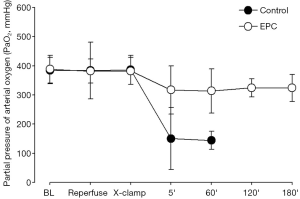
Pulmonary gas exchange
The partial pressure of oxygen in the systemic arterial blood (PaO2) decreased dramatically and PaCO2 accumulated following cessation of right pulmonary arterial blood flow in the animals receiving plain culture medium, suggesting the failure of lung graft function for maintaining efficient gas exchange (Figure 6). There was a marginally reduction of PaO2 in the EPC-treated group after cross-clamping of right pulmonary artery, but levels of PaO2 and PaCO2 were maintained within normal ranges throughout the observation period (Figure 6).
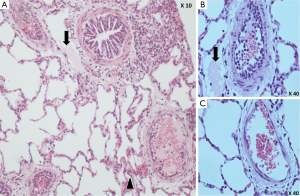
Histological examination
Freshly frozen lung sections were examined under a fluorescent microscope to identify EPCs labeled with DM-DiI dye. However, no typical pulmonary endothelial enhancement of fluorescent staining was detected in the examined lung sections.
Under light microscopy, sloughing of endothelium with thrombi formation in the terminal pulmonary arterioles, diffuse interstitial hemorrhage and collapsed alveoli with formation of hyaline membrane were observed in the lung biopsies of control piglets (Figure 7). Most strikingly, disseminated intravascular thrombosis was formed in the pulmonary arterioles of these animals (Figure 7). On the other hand, most of the terminal pulmonary arterioles in the biopsies of EPC-treated lungs were patent with minor degree of endothelial injury and less thrombi formation, hence the degree of lung injury was less severe, although patchy lung collapse and pulmonary hemorrhage were also found (Figure 7).
Discussion
We presented the first study demonstrating that transplantation of autologous EPCs significantly enhanced the function of allogeneic lung graft of piglets with prolonged ischemic time up to 14 h. Transplantation of circulating EPCs attenuates the damage of alveolar-capillary membrane secondary to IRI during lung transplantation, and thereby potentiating the cardiopulmonary performance and improving the overall survival of the recipient subjects. The improvements in hemodynamics and gas exchange of EPC-transplanted animals are most likely derived from the paracrine effects of the endothelial progenitors rather than a direct cell fusion response.
Isolation and characterization of porcine circulating EPCs are uncommonly reported in the literature, most likely due to the unavailability of antibodies for specific EPC markers. Nevertheless, the isolated cells, derived from peripheral MNCs, exhibited typical phenotypes of endothelial cells and formed outgrowth cobblestone-like colonies following culturing in the endothelial culture medium. In this study, we utilized a clinically feasible approach for therapeutic cell transplantation. EPCs isolated from the recipient piglets were administered immediately before reperfusion of pulmonary blood flow into the allogeneic lung graft. The therapeutic effects of autologous EPC transplantation were then assessed by diversion of the entire pulmonary blood flow into the allogeneic lung graft following ligation of the right pulmonary artery of the recipients.
The functions of lung graft with prolonged ischemic time up to 14 h were determined by measuring changes in the systemic/pulmonary hemodynamics and pulmonary gas exchange. Resume of blood flow in the implanted lung graft resulted in abrupt drop of systemic blood pressure, which was compatible with the development of reperfusion injury. Prolonged ischemia of the lung graft accumulated metabolic waste and lactate, and reperfusion following declamping of pulmonary artery caused a significant fall in systemic blood pressure due to acidosis-induced vasodilation. In addition, prolonged cold ischemia of lung graft may also engender a “no-reflow phenomenon” demonstrated by significant microvascular damages, pulmonary blood flow obstruction and increased pulmonary vascular resistance (1). In our experiment, diversion of the entire pulmonary blood flow into the lung graft resulted in elevated pulmonary artery pressure. The high pulmonary artery resistance further depressed pulmonary venous flow and reduced left ventricular preload, as evidenced by significant drop of cardiac output measured in the descending aorta. Therefore, the synergism of reduced systemic vascular resistance and low cardiac output resulted in profound hypotension following reperfusion of allogeneic lung graft.
IRI of the lung graft also led to respiratory distress and hypoxemia secondary to disruption of the alveolar-capillary membrane, activation of adhesion molecules, and massive infiltration of PMNs (1,4,6,7,11,12). The net effect of increased protein permeability across the alveolar-capillary barrier and enhanced pulmonary inflammatory response provoke non-cardiogenic pulmonary edema, hyaline membrane formation, and lung hemorrhage after lung implantation (26). The post-mortem autopsies of the lung graft tissue obtained from the control piglets showed pulmonary endothelial necrosis and sloughing with thrombi formation in the terminal arterioles, which were consistent with the characteristic changes of lung IRI. In addition, increased neutrophil aggregation was also distinctive along the detached necrotic endothelium. Diffuse lung interstitial hemorrhage and collapsed alveoli with formation of hyaline membrane were also significant featured in these animals. On the other hand, most of the terminal pulmonary arterioles in the EPC-treated lungs were patent with less severe degrees of endothelial injury and less thrombi formation, although patchy lung collapse and pulmonary hemorrhage were also observed.
In order to identify the fate of transplanted EPCs, cells were tracked by fluorescence-conjugated tracers before delivery into the pulmonary artery. In contrast to our previous report, the DM-DiI-labeled EPCs, however, were not found in the pulmonary vasculature after careful examination of frozen sections of the lung. We believed that identification of EPCs in the pulmonary vessel wall was technically challenging due to the dispersed large surface area of pulmonary circulation and the washout effect following intravascular administration. In addition, potential immune responses between allogeneic graft and autologous cells may further attenuate the retention of transplanted cells in the lung tissue. Nevertheless, our results support the well-recognized concept that apart from direct cell fusion, EPCs may mediate vascular protective response via their paracrine effects and the high expression of intrinsic antioxidant capacity.
The endothelial regenerative potential of EPCs has been demonstrated in cardiovascular pathological conditions. Results of larger clinical trials in patients with myocardial infarction or congestive heart failure showed that transplantation of bone marrow-derived endothelial progenitors (CD133 cells) significantly improved the global and regional left-ventricular ejection fraction (27-29). We have demonstrated that transplantation of EPCs significantly suppressed the protein levels of iNOS in pulmonary artery (16), where induction of iNOS is commonly suggested to be responsible for diminished vasoconstriction of pulmonary artery due to overproduction of nitric oxide (30,31). On the other hand, previous reports indicated that activating the PI3k/Akt in endothelial cells can prevent cell death (32), resulting in increasing cell survival and activating eNOS, leading to NO production (33). As a result, EPCs play a pivotal role in homeostasis of endothelial integrity, and thus maintain a normal physiological function of the barrier between pulmonary microcirculation and gas exchange units. The salvaged pulmonary microcirculation maintained gas exchange at the alveolar-capillary membrane, lowered the pulmonary vascular resistance, and provided adequate tissue oxygenation. Transplantation of circulating EPCs attenuates the damage of alveolar-capillary membrane secondary to IRI during lung transplantation, potentiating the cardiopulmonary performance and improving the overall survival of the recipient subjects.
There are several major limitations in our present study. This was a pilot study in large animals aimed to determine the efficacy of transplantation of EPCs in allogeneic lung implantation with prolonged graft ischemia. Therefore, head-to-head comparisons of the hemodynamics and other biochemical measurements were infeasible, as the survival time of the two experimental groups were distinct. This major limitation in the study design hinders mechanistic investigation into the beneficial effects of EPCs in the lung transplantation, such as measurement of systemic pro-inflammatory titers and vascular function of allogeneic pulmonary artery. Furthermore, the fate of EPCs was unable to be traced by DM-DiI labeling following intrapulmonary artery delivery. More sensitive scanning tools, such as magnetic resonance tracking system, should be employed to track the implanted cells. It is therefore essential to perform a follow-up comparative experiment with less severe degree of IRI (i.e., shorter ischemic time) to confirm the therapeutic application of EPCs in a more controlled and quantitative fashion.
Collectively, this is the first pilot study demonstrating that transplantation of autologous EPCs enhances the function of allogeneic lung graft of piglets with prolonged ischemic time, and most significantly administration of these endothelial progenitors reduces overall mortality of animals throughout the study period. Transplantation of circulating EPCs attenuates the damage of endothelium secondary to IRI during lung transplantation, thereby potentiates the cardiopulmonary performance and improves the overall survival of the recipient subjects. The improvements in hemodynamics and gas exchange of EPC-transplanted animals are most likely derived from the paracrine effects of the EPCs.
Acknowledgements
Funding: This research was funded by the grant of National Cheng Kung University Hospital (NCKUH-20100090).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures were performed in accordance with the guidelines, and the protocol for the research project was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Cheng Kung University, Taiwan (IACUC Approval No. 99024).
References
- de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490-511. [Crossref] [PubMed]
- King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg 2000;69:1681-5. [Crossref] [PubMed]
- Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med 2005;171:786-91. [Crossref] [PubMed]
- den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 2010;299:H1283-99. [Crossref] [PubMed]
- Al-Mehdi AB, Zhao G, Dodia C, et al. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ Res 1998;83:730-7. [Crossref] [PubMed]
- Fiser SM, Tribble CG, Long SM, et al. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg 2001;71:1134-8. [Crossref] [PubMed]
- Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg 2001;121:1069-75. [Crossref] [PubMed]
- Novick RJ, Gehman KE, Ali IS, et al. Lung preservation: the importance of endothelial and alveolar type II cell integrity. Ann Thorac Surg 1996;62:302-14. [Crossref] [PubMed]
- Hidalgo MA, Shah KA, Fuller BJ, et al. Cold ischemia-induced damage to vascular endothelium results in permeability alterations in transplanted lungs. J Thorac Cardiovasc Surg 1996;112:1027-35. [Crossref] [PubMed]
- Demertzis S, Langer F, Graeter T, et al. Amelioration of lung reperfusion injury by L- and E- selectin blockade. Eur J Cardiothorac Surg 1999;16:174-80. [Crossref] [PubMed]
- Moore TM, Khimenko P, Adkins WK, et al. Adhesion molecules contribute to ischemia and reperfusion-induced injury in the isolated rat lung. J Appl Physiol 1995;78:2245-52. [PubMed]
- Ross SD, Tribble CG, Gaughen JR Jr, et al. Reduced neutrophil infiltration protects against lung reperfusion injury after transplantation. Ann Thorac Surg 1999;67:1428-33. [Crossref] [PubMed]
- Ng CS, Wan S, Yim AP. Pulmonary ischaemia-reperfusion injury: role of apoptosis. Eur Respir J 2005;25:356-63. [Crossref] [PubMed]
- Stammberger U, Gaspert A, Hillinger S, et al. Apoptosis induced by ischemia and reperfusion in experimental lung transplantation. Ann Thorac Surg 2000;69:1532-6. [Crossref] [PubMed]
- Fischer S, Maclean AA, Liu M, et al. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med 2000;162:1932-9. [Crossref] [PubMed]
- Lam CF, Liu YC, Hsu JK, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology 2008;108:392-401. [Crossref] [PubMed]
- Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266-72. [Crossref] [PubMed]
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 2004;287:C572-9. [Crossref] [PubMed]
- Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 2001;7:1035-40. [Crossref] [PubMed]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7. [Crossref] [PubMed]
- Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;9:1370-6. [Crossref] [PubMed]
- Burnham EL, Taylor WR, Quyyumi AA, et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854-60. [Crossref] [PubMed]
- Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004;109:1543-9. [Crossref] [PubMed]
- Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004;94:678-85. [Crossref] [PubMed]
- Lam CF, Roan JN, Lee CH, et al. Transplantation of endothelial progenitor cells improves pulmonary endothelial function and gas exchange in rabbits with endotoxin-induced acute lung injury. Anesth Analg 2011;112:620-7. [Crossref] [PubMed]
- Suresh R, Kupfer Y, Tessler S. Acute respiratory distress syndrome. N Engl J Med 2000;343:660-1. [Crossref] [PubMed]
- Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17. [Crossref] [PubMed]
- Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107:2294-302. [Crossref] [PubMed]
- Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45-6. [Crossref] [PubMed]
- Griffiths MJ, Liu S, Curzen NP, et al. In vivo treatment with endotoxin induces nitric oxide synthase in rat main pulmonary artery. Am J Physiol 1995;268:L509-18. [PubMed]
- Holzmann A, Manktelow C, Taut FJ, et al. Inhibition of nitric oxide synthase prevents hyporesponsiveness to inhaled nitric oxide in lungs from endotoxin-challenged rats. Anesthesiology 1999;91:215-21. [Crossref] [PubMed]
- Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res 2000;86:24-9. [Crossref] [PubMed]
- Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999;399:597-601. [Crossref] [PubMed]


