
Interstitial cystitis/bladder pain syndrome: when part of the posterior fornix syndrome is potentially curable surgically
Highlight box
Key findings
• Interstitial cystitis/bladder pain syndrome (IC/BPS), as defined by the International Continence Society, is likely part of the posterior fornix syndrome (PFS) and therefore, potentially curable surgically.
What is known and what is new?
• IC/BPS has no known pathogenesis and is not considered curable.
• Cure of IC/BPS without Hunner’s lesion by uterosacral/cardinal ligament repair.
What is the implication, and what should change now?
• Following diagnostic and treatment protocols of PFS can help many women who have IC/BPS.
Introduction
The key points of the article are summarized in the video abstract (Video S1) which shows the relationship of interstitial cystitis/bladder pain syndrome (IC/BPS) to posterior fornix syndrome (PFS).
Though IC/BPS is considered a chronic pelvic pain (CPP) syndrome (1), the International Continence Society (ICS) defines IC/BPS more precisely as “persistent or recurrent CPP, pressure, or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom such as an urgent need to void or urinary frequency, diagnosed in the absence of any identifiable pathology which could explain these symptoms” (2).
It is widely believed that the pathogenesis of IC/BPS is unknown and that no cure is possible. Treatment usually consists of bladder distension or installations with various drugs with varied reports of efficacy. The Integral Theory Paradigm (ITP) views the pain and urge components of IC/BPS as different phenotypes of uterosacral ligament (USL) weakness, and not necessarily co-occurring as one condition. The ITP’s view of IC/BPS being one and the same condition as the PFS is further analysed below.
Evidence for IC/BPS and PFS being the one and the same condition
In 2021, Scheffler et al. reported a histologically validated cure of Hunner’s lesion in a 73-year-old woman (3). The report was highly disruptive to existing concepts of IC/BPS, not only because Hunner’s lesion is considered incurable, but also, the patient was managed according to the protocols of the PFS. The PFS comprises CPP, urge, frequency, nocturia, abnormal emptying, post-void residual urine, caused by USL laxity and cured or improved by repair thereof (4). Scheffler et al. repaired the cardinal ligament (CL) and USLs with the Tissue Fixation System (TFS) minisling; Hunner’s ulcer cure was a serendipitous finding. Scheffler et al. hypothesized that IC/BPS was likely part of the PFS.
Goeschen et al. (5) tested the Scheffler hypothesis, that IC/BPS could be part of PFS, for truth or falsity. They revisited clinical data from 198 women who had presented with CPP plus varying degrees of uterine/apical prolapse. The women were treated with a posterior intravaginal slingplasty (IVS), an operation which repaired the USLs using a collagen-producing polypropylene tape (5). Goeschen et al. had applied the same PFS diagnostic and treatment protocols as Scheffler et al. (3), including the diagnostic algorithm and speculum test (Figure 1) (6). Excluding stress urinary incontinence (66 cases), Goeschen et al. found that the 198 women had 313 bladder symptoms (Figure 1) (4). The diagnosis was in accord with descriptions for both IC/BPS and PFS, except that, CPP and bladder symptoms were cured (Table 1). Pain and multiple bladder symptoms were cured (variously) by the USL sling (5), confirming Scheffler et al.’s hypothesis that IC/BPS may be one and the same condition as PFS. Hunner’s ulcer was absent in all 198 of Goeschen et al.’s patients.
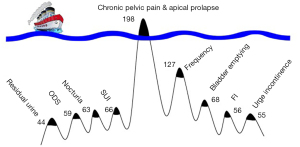
Table 1
| Variables | Incidences before surgery, n [%] | Incidences 12 months after surgery, n [%] |
Comparisons (pre- versus post-incidences) (Z values) |
Odds ratios (95% CI) |
Cure rates 12 months after surgery, % |
|---|---|---|---|---|---|
| Pelvic pain (main symptom) | 198 [100] | 52 [26] | 8.690*** | n.d. | 74.00 |
| Urinary frequency | 127 [64] | 26 [13] | 6.000*** | 0.085 (0.051–0.140) | 79.69 |
| Nocturia | 63 [32] | 13 [7] | 3.690** | 0.151 (0.135–0.493) | 78.13 |
| Bladder emptying difficulties | 68 [34] | 32 [16] | 3.198** | 0.369 (0.228–0.595 | 52.94 |
| SUI | 66 [33] | 4 [2] | 4.266*** | 0.041 (0.015–0.116) | 93.94 |
| ODS | 59 [30] | 12 [6] | 3.596** | 0.152 (0.079–0.294) | 80.00 |
| Urge incontinence | 55 [28] | 11 [6] | 3.412** | 0.153 (0.077–0.303) | 78.57 |
| Residual urine >50 mL | 44 [22] | 20 [10] | 2.490* | 0.393 (0.222–0.696) | 54.55 |
Reused from (5). Copyright 2022, with permission from Karger. *, P<0.05; **, P<0.01; ***, P<0.001. CPP, chronic pelvic pain; ICS, International Continence Society; BPS, bladder pain syndrome; CI, confidence interval; n.d., not detected; SUI, stress urinary incontinence; ODS, obstructive defecation syndrome.
Review of other studies of women who were treated by posterior slings according to PFS protocols (7-16) and who were cured of CPP, urgency, other bladder symptoms, reached the same conclusions as Scheffler et al. (3) and Goeschen et al. (5): IC/BPS and PFS were likely to be the one and the same condition, mainly caused by USL laxity, and could be cured or improved by USL repair.
Unsupported visceral plexus—a likely pathogenesis for CPP and IC/BPS
Reported surgical cure of CPP symptoms by many studies (7-16), support the 1996 hypothesis of Petros (17), that CPP “of unknown origin” may be caused by autonomous afferent impulses originating from visceral nerve axon junctions in the pelvic visceral plexuses (VPs) because of weak USLs. VPs are anatomically supported by USLs. If USLs are weak, the force of gravity or muscle movements acting on the VP junctions may activate autonomous impulses which the cortex interprets as pain originating from the end organ, bladder, rectum, vagina/vulva/muscles, and other sites such as coccyx, lower abdomen. In support of this hypothesis is the common observation that pelvic pain is relieved on lying down, and by multiple different pain manifestations being relieved by USL support by a speculum test (Figure 2) (3,5,6).
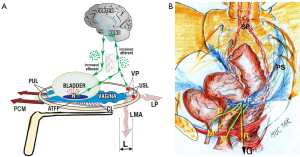
The hypothesis of visceral nerve etiology for pain (17) is consistent with the anatomical studies of Butler-Manuel: the T11–L2 and parasympathetic S2–4 VPs were closely involved with, and supported by, the USLs (18). These visceral nerves carry afferent signals from the organ to the cortex and transmit efferent instructions back to the organ. In normal situations, an injury or infection at the end organ sends afferent signals to the brain. The brain responds by sending efferent signals to hypothesized “sleeper cells” via the visceral efferent pathways; these secrete mast cells, leukocytes and other inflammatory cells to “heal” the perceived injury or infection (19).
The Integral Theory concept for IC and other pain causation in the absence of infection or injury is diagrammatically indicated in Figure 2: unsupported VPs which contain afferent axons from various end organ sites, bladder “B”, vagina/vulva “V”, rectum “R”, muscles “M” are stimulated by gravity “G”, or muscle movement to send autologous signals to the brain via T11–L2 and S2–4 afferent visceral nerves; the brain (wrongly) interprets these signals as coming from the end organs and responds appropriately; it sends efferent signals to hypothesized “sleeper cells” sited in the end organs.
In the case of the urothelium, hypothesized “sleeper cells” create an inflammatory response sufficient to cause Hunner’s ulcer. We hypothesize that the fragility of the urothelium makes it vulnerable to the more florid inflammatory response seen in ulcerating Hunner’s ulcer and end-stage scarring, something not seen in the tissue inflammatory response seen with vulvodynia, which, like IC, exhibits inflammatory cells such as Mast cells and leucocytes and neuroproliferation (19) but no redness (20).
Testing for VP causation of CPP and IC/BPS
The hypothesis of the CPP, part of IC/BPS, being caused by unsupported visceral pelvic plexuses is testable and falsifiable by the speculum test (Figure 2) and the Bornstein local anesthetic (LA) test.
The speculum test
The speculum test (Figure 2) relieves pain and urge by supporting “USL” and stretching the vagina to support the urothelial stretch receptors “N”. The test, if successful, decreases afferent impulses (small green arrows), pain from VPs, and urge from “N”; the patient reports lessening of pain in multiple sites (e.g., “B”, “R”, “M”), and also, simultaneously, urge Figure 2.
The Bornstein test
The Bornstein test is considered the definitive test for VP causation of CPP. It comprises injection of LA into the lower end of the USLs to anesthetize the VPs. Bornstein relieved the hyperesthesia of vulvodynia in 8/10 women bilaterally and 2/10 unilaterally (21). Petros injected LA into the USLs of three IC/BPS women who had multiple sites of CPP with LA. Relief of all the sites of pain was achieved in all three women (22).
Conclusions and future directions
The key research question now, as we see it, is, “What is the prevalence of PFS in IC/BPS women?”. If it is substantial, then it could reasonably be said, that IC/BPS, at least in such women, is part of PFS, and can potentially be improved or cured by mechanical or surgical support of the USLs.
Acknowledgments
We would like to express our gratitude to Editors Professor Peter Petros and Vani Bardetta for their exceptional support in the design and refinement of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1865/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1865/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. Human participation in the video was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tirlapur SA, Birch JV, Carberry CLRoyal College of Obstetricians and Gynaecologists, et al. Management of bladder pain syndrome. BJOG 2016;124:e46-e72.
- Doggweiler R, Whitmore KE, Meijlink JM, et al. A standard for terminology in chronic pelvic pain syndromes: A report from the chronic pelvic pain working group of the international continence society. Neurourol Urodyn 2017;36:984-1008. [Crossref] [PubMed]
- Scheffler K, Hakenberg OW, Petros P. Cure of Interstitial Cystitis and Non-Ulcerating Hunner's Ulcer by Cardinal/Uterosacral Ligament Repair. Urol Int 2021;105:920-3. [Crossref] [PubMed]
- Petros PE, Ulmsten U. The posterior fornix syndrome: a multiple symptom complex of pelvic pain and abnormal urinary symptoms deriving from laxity in the posterior fornix of vagina. Scand J Urol Nephrol 1993;27:89-93.
- Goeschen K, Gold DM, Liedl B, et al. Non-Hunner's Interstitial Cystitis Is Different from Hunner's Interstitial Cystitis and May Be Curable by Uterosacral Ligament Repair. Urol Int 2022;106:649-57. [Crossref] [PubMed]
- Wu Q, Luo L, Petros PP. Mechanical support of the posterior fornix relieved urgency and suburethral tenderness. Pelviperineology 2013;32:55-6.
- Wagenlehner F, Muller-Funogea IA, Perletti G, et al. Vaginal apical prolapse repair using two different sling techniques improves chronic pelvic pain, urgency and nocturia - a multicentre study of 1420 patients. Pelviperineology 2016;35:99-104.
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications - a 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [Crossref] [PubMed]
- Caliskan A, Ozeren M, Goeschen K. Modified posterior intravaginal slingplasty: does the additional bilateral tape attachment to the sacrospinous ligament improve the results? Cent European J Urol 2018;71:326-33. [Crossref] [PubMed]
- Petros P, Swash M, Bornstein J. A Review of Chronic Pelvic Pain in Women. JAMA 2021;326:2207. [Crossref] [PubMed]
- Enache T, Bratila E, Abendstein B. Chronic pelvic pain of unknown origin may be caused by loose uterosacral ligaments failing to support pelvic nerve plexuses - a critical review. Cent European J Urol 2020;73:506-13. [Crossref] [PubMed]
- Petros P, Abendstein B. Pathways to causation and surgical cure of chronic pelvic pain of unknown origin, bladder and bowel dysfunction - an anatomical analysis. Cent European J Urol 2018;71:448-52. [Crossref] [PubMed]
- Petros P. A gynecological perspective of interstitial cystitis/bladder pain syndrome may offer cure in selected cases. Cent European J Urol 2022;75:395-8. [Crossref] [PubMed]
- Inoue H, Kohata Y, Fukuda T, et al. Repair of damaged ligaments with tissue fixation system minisling is sufficient to cure major prolapse in all three compartments: 5-year data. J Obstet Gynaecol Res 2017;43:1570-7. [Crossref] [PubMed]
- Liedl B, Goeschen K, Grigoryan N, et al. The association between pelvic organ prolapse, pelvic pain and pelvic reconstructive surgery using transvaginal mesh: A secondary analysis of a prospective multicenter observational cohort trial. J Clin Gynecol Obstet 2020;9:79-95.
- Petros P, Richardson P. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse. A prospective urodynamic study. Pelviperineology 2010;29:52-5.
- Petros PP. Severe chronic pelvic pain in women may be caused by ligamentous laxity in the posterior fornix of the vagina. Aust N Z J Obstet Gynaecol 1996;36:351-4. [Crossref] [PubMed]
- Butler-Manuel SA, Buttery LD, A'Hern RP, et al. Pelvic nerve plexus trauma at radical and simple hysterectomy: a quantitative study of nerve types in the uterine supporting ligaments. J Soc Gynecol Investig 2002;9:47-56. [Crossref] [PubMed]
- Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology 2014;141:314-27. [Crossref] [PubMed]
- Regauer S, Gamper M, Fehr MK, et al. Sensory Hyperinnervation Distinguishes Bladder Pain Syndrome/Interstitial Cystitis from Overactive Bladder Syndrome. J Urol 2017;197:159-66. [Crossref] [PubMed]
- Bornstein J, Zarfati D, Petros P. Re: Causation of vulvar vestibulitis. Aust N Z J Obstet Gynaecol 2005;45:538-9. [Crossref] [PubMed]
- Petros P. Interstitial cystitis (painful bladder syndrome) may, in some cases, be a referred pain from the uterosacral ligaments. Pelviperineology 2010;29:56-9.


