
Vitamin D alleviates acute graft-versus-host disease through promoting the generation of Foxp3+ T cells
Introduction
Vitamin D (VD) refers to a group of fat-soluble secosteroids that increase intestinal absorption of calcium, magnesium, and phosphate and exhibit numerous other biological effects (1). The most important compounds in VD in humans are VD3 (also known as cholecalciferol) and VD2 (ergocalciferol), which can be obtained via diet or supplements (2,3).
Acute graft-versus-host disease (aGVHD) is a medical complication which may result in significant morbidity and mortality after allogeneic hematopoietic stem cell transplantation (4) and organ transplantation (5). Currently, limited success had been made for the strategies to treat or even control aGVHD (6). To simulate the lymphopenic but delayed pathogenic state of graft-versus-host reaction during aGVHD, spleen cells derived from parental origin were injected into F1 mice which may generate donor CD8+ cytotoxic T lymphocytes specific for host spleen cells in 2 weeks (7).
The traditional view of 1,25-dihydroxyvitamin D3 (1α,25(OH)2D3) is a crucial role in maintaining calcium homeostasis and modulating calcium metabolism. The role of 1α,25(OH)2D3 in immune responses to infection has become an exciting topic of research (8,9). 1α,25(OH)2D3 has been shown to regulate cellular proliferation, differentiation, apoptosis, and innate and adaptive immunity (10) in several diseases, such as inflammatory bowel disease (11), diabetes (12), food allergies (13), and tumors (14). However, whether 1α,25(OH)2D3 exerts a therapeutic effect against aGVHD had not been determined. Therefore, we examined the regulative ability of 1α,25(OH)2D3 in protecting aGVHD. Surprisingly, our results consistently demonstrated that administration of 1α,25(OH)2D3 significantly slowed aGVHD progression and improved survival of B6D2F1 recipients of grafted B6 splenocytes. In vitro studies proved that 1α,25(OH)2D3 induces the differentiation of regulatory T cells (Tregs), which regulate, at least in part, immune hemostasis in aGVHD. The results of the study emphatically proved that 1α,25(OH)2D3 treatment could be useful to prevent aGVHD or other autoimmune disease (15).
Methods
Animals
C57BL/6 (H-2Kb) and B6D2F1 (H-2Kb/d) mice (male, 8 weeks old) were obtained from the Animal Resources Center, Nanjing Medical University. The mice were housed with standard rodent diet and water provision. Relevant legal and ethical requirements were followed carefully according to the protocol (number NMU08-092), which was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Fluorescence-activated cell sorting (FACS) analysis
Spleen samples were obtained from recipient mice on the indicated days after cell transplantation. Cells were stained with surface antibody markers before analyzed by FACS. For forkhead box P3 (Foxp3) staining, cells were fixed, permeabilized, and finally stained with Foxp3. For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 0.05 µg/mL) and ionomycin (0.5 µg/mL) for 5 hours, and brefeldin A (5 µg/mL) for four hours under 5% CO2 and 37 °C environment. The stimulated cells were collected, fixed, and permeabilized (85-88-8824-00, eBioscience, San Diego, CA, USA) and stained with FACS-targeted antibodies. Mouse-specific monoclonal antibodies used for flow cytometry included CD8 (APC), CD4 (PE-cy7), CD25 (APC-cy7), IFN-γ (APC), TNF-α (BV421), IL-4 (BV421), TGF-β (AF488), IL-10 (PE), H-2Kb (APC), and H-2Kd (PE) purchased from BioLegend, and Foxp3 (APC)、CD19 (FITC) purchased from BD Pharmingen.
Development of mouse aGVHD models
aGVHD was induced in normal, unirradiated B6D2F1 mice on the same day by intravenous injection of 5×107 B6 mice derived spleen cells, as reported previously (16). Two weeks later, the mice were sacrificed, and splenocytes were stained with anti-mouse-H-2Kb and anti-mouse-H-2Kd to identify the donor and host cells and indicated cell markers (BioLegend, San Diego, CA, USA). Based on the aGVHD model, mice were divided into four groups: control group, 1α,25(OH)2D3 group, 1α,25(OH)2D3 + IgG group, 1α,25(OH)2D3 + PC61 group. Mice in 1α,25(OH)2D3 + PC61 group and 1α,25(OH)2D3 + IgG were injected intraperitoneally with PC61 (250 mg/mouse/day) and IgG (250 mg/mouse/day), respectively for 7 days before they were injected with 50×106 B6 cells. 1α,25(OH)2D3 (0.03 µg/kg/day) (740551, Sigma-Aldrich, St. Louis, MO, USA) was administered intragastrically for 4 weeks (2 weeks before the aGVHD model was established and 2 weeks after establishment).
Naïve T cell isolation and CD4+ Treg generation
Spleen cells from B6 mice were derived, and CD4+CD62L+CD25– T cells were sorted using a magnetic naïve CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch-Gradbach, Germany). The purity of CD4+CD62L+CD25– T cells was tested through FACS and found to be at >98% purity before cell culture.
The above cells were cultured in 48-well plates and stimulated for 3 days with the addition of anti-mouse-CD3/CD28 labeled beads (the ratio of bead to cell is 1:5) in conjunction with IL-2 (20 IU/mL) and TGF-β (10 ng/mL). 1α,25(OH)2D3 (10–7 M) was added in some experiments at the beginning of the culture. The culture medium contained RPMI 1640 medium, 100 U/mL penicillin, 100 mg/mL streptomycin, 10 mM HEPES (Invitrogen Life Technologies, Carlsbad, CA, USA), and 10% heat-inactivated fetal calf serum (Hyclone, Chicago, IL, USA).
In vitro suppression assay
B6 derived CD4+CD25– T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA, USA), and cultured with CD4+ induced Tregs (iTregs) as well as irradiated dendritic cells and anti-CD3 for 3 days at different ratios. The suppressive ability of iTregs was tested through flow cytometry.
Statistical analysis
Results are shown as the mean ± standard error of the mean (SEM). All analysis were performed using Stata software (version 11.0). P values less than 0.05 (two-tailed) was considered statistically significant.
Results
1α,25(OH)2D3 protects against aGVHD in a mouse model
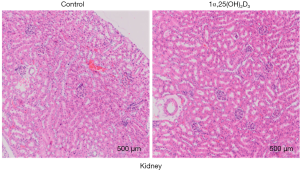
To investigate the protective effect of 1α,25(OH)2D3 during the pathogenesis of aGVHD, 50×106 B6 spleen cells were injected intravenously into B6D2F1 mice (Figure 1A). Survival was monitored daily. 1α,25(OH)2D3 administration significantly prolonged survival ratio compared with the model group (Figure 1B). Additionally, improvement of the kidney injury was also observed in aGVHD mouse model with 1α,25(OH)2D3 treatment (Figure S1).
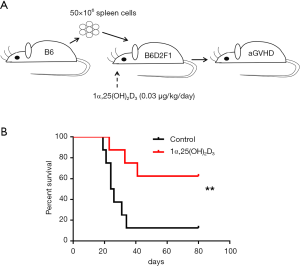
Infusion of 1α,25(OH)2D3 markedly suppresses donor cell engraftment and prevents host cell depletion in aGVHD mice
To assess the effect of 1α,25(OH)2D3 administration on the development of aGVHD, mice were sacrificed on day 14 after the establishment of aGVHD, and donor and host cells in the spleen were identified by flow cytometry following H-2Kd and H-2Kb staining (donor cells: H-2Kb+/d−, host cells: H-2Kb+/d+). Donor cells comprised almost 50% of the cells in the Control group (17). Interestingly, 1α,25(OH)2D3 injection suppressed donor cell engraftment (50% to 10%). The primary pathologic characteristic of aGVHD is a reduction of lymphocytes from the host origin (18). What’s more, although B6 spleen cells transfer led to the lower number of the total number of spleen cells and host cells in F1 mice, 1α,25(OH)2D3 presented strong ability to preserve the proportion of host cells and decrease the expansion of donor cells (Figure 2A).
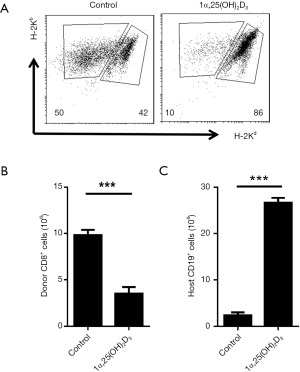
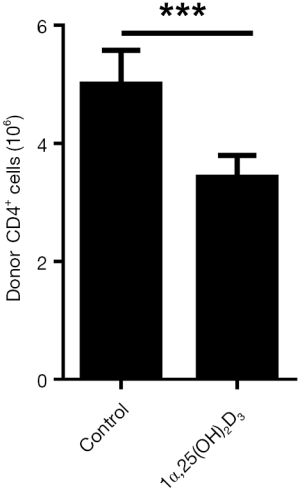
Donor CD8+ cells are important in initiating the pathogenesis of aGVHD in B6-to-F1 control mice (19). We, therefore, tested the frequencies of the donor as well as host cells in the Control and 1α,25(OH)2D3 groups. 1α,25(OH)2D3 markedly suppressed donor CD8+ cells expansion as well as CD4+ cells (Figure 2B,S2). We analyzed the killing effect of donor CD8+ cells as it is another characteristic of aGVHD (19). As shown in Figure 2C, 1α,25(OH)2D3 almost wholly prevented the aGVHD-associated host B cells reduction while without 1α,25(OH)2D3 administered, dramatically B cell decline was observed. Thus, our data demonstrate that 1α,25(OH)2D3 suppresses the expansion and cytotoxic effects of donor CD8+ cells against host CD19+ cells.
1α,25(OH)2D3 increases Foxp3 but reduces cytokine expression in vivo
Next, we examined Foxp3 expression in 1α,25(OH)2D3-treated and -untreated mice. Administration of 1α,25(OH)2D3 led to increasing number of spleen lymphocytes in 1α,25(OH)2D3 group mice, which is almost twice the control (data not shown). Therefore, the absolute number of Foxp3-expressing CD4+ cells increased, whereas no difference was observed in the percentage of Foxp3+ T cells in the spleen (Figure 3A).
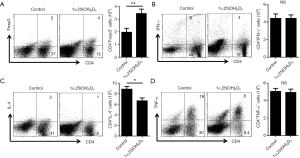
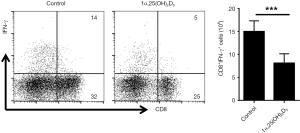
Previous studies suggested the production of inflammatory cytokine contributes to aGVHD induction (20,21). So, we evaluated various cytokines expression in CD4+ T cells with or without 1α,25(OH)2D3 administration. 1α,25(OH)2D3 administration led to down-regulated cytokine expression such as IFN-γ, IL-4, and TNF-α from CD4+ cells (Figure 3B,C,D). Also, the absolute number of CD4+IL-4+ T cells declined following 1α,25(OH)2D3 administration, whereas no difference was observed in the total number of CD4+ cells expressing IFN-γ and TNF-α. In addition, we also found 1α,25(OH)2D3 could decrease the percentage as well as the absolute number of CD8+IFN-γ+ T cells (Figure S3).
1α,25(OH)2D3 increases Foxp3 and IL-10 expression and regulatory function during iTreg generation
To evaluate the regulatory effect of 1α,25(OH)2D3 on the role of Tregs, we first examined Foxp3 expression in 1α,25(OH)2D3-treated and -untreated iTreg subsets. iTregs treated with 1α,25(OH)2D3 exhibited higher Foxp3 expression on day three compared with the control cells treated only with phosphate-buffered saline (PBS) (Figure 4A).
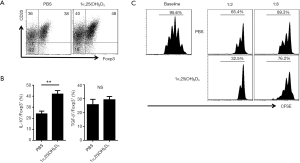
Next, we examined the expression of various cytokines in 1α,25(OH)2D3-treated and -untreated iTreg subsets, as Tregs are known to express anti-inflammatory cytokines such as IL-10 and TGF-β to suppress effector T cell expansion. As expected, 1α,25(OH)2D3 treatment resulted in increased IL-10 expression; however, no significant change was observed in TGF-β expression (Figure 4B). We then directly compared the suppressive effects of both iTreg subsets in vitro. Compared with PBS-treated iTregs, T cell proliferation was suppressed in co-culture with 1α,25(OH)2D3-treated iTregs.
Tregs mediate 1α,25(OH)2D3-associated protection against aGVHD
Finally, a monoclonal antibody against CD25 (CD25MoAb or PC61) was used to abolish the function of Tregs in vivo (22,23). Meanwhile, mice in 1α,25(OH)2D3 + PC61 group and 1α,25(OH)2D3 + IgG were injected intraperitoneally with PC61 (250 mg/mouse/day) and IgG (250 mg/mouse/day), respectively for 7 days before they were injected with 50×106 B6 cells. After 14 days, the effect of 1α,25(OH)2D3 on aGVHD was generally examined by flow cytometry, which showed that the protective ability of 1α,25(OH)2D3 was substantially reversed (Figure 5A), consistent with the results of survival analyses shown in Figure 5B.

Discussion
In this study, we demonstrated that the administration of 1α,25(OH)2D3 reduced the severity of aGVHD in a mouse model. 1α,25(OH)2D3 promoted the expansion of Foxp3-expressing Treg populations and inhibited IFN-γ expressing Th1 and IL-4 expression Th2 cells in vivo during the development of aGVHD.
Several studies have reported that VD has positive effect on the treatment of aGVHD and Tregs are critical regulators of aGVHD (19,24). Silva et al. reported that treatment with VD appears to be effective, safe and inexpensive for the management of patients with chronic GVHD (25). Rosenblatt et al. also demonstrated that VD may have an important role in the prevention and treatment of aGVHD by hindering the maturation of DCs and inducing Foxp3 expressing Treg populations. Zahran et al. reported a strong association between the percentage of Treg phenotypes and VD level in term and preterm pregnant women with VD deficiency (26). Gorman et al. reported an increased proportion of Tregs in VD-treated mice (27). Present data had proved that 1α,25(OH)2D3 successfully increased the number of CD4+Foxp3+ cells both in vitro and vivo, in agreement with previous studies indicating that VD increases Treg populations in vivo. In mice injected with PC61, the effect of 1α,25(OH)2D3 was diminished, confirming that upregulation of Foxp3+ T cells is an important mechanism for 1α,25(OH)2D3 against aGVHD.
In vitro experiments found that 1α,25(OH)2D3 directly enhanced Foxp3 and IL-10 expression as well as the suppressive effects of Tregs 3 days after Treg induction. However, additional studies are needed to elucidate the mechanism by which 1α,25(OH)2D3 regulates the differentiation of iTregs. Such research should also consider other immune cells, including antigen-presenting cells, which exhibit significant cross-talk with Tregs. Dauletbaev et al. demonstrated that VD regulates inflammatory macrophages via downregulation of IL-8 expression (28). In addition, VD was shown to reduce IL-22 expression in innate lymphoid cells (29), and Vanherwegen et al. reported that VD modulates the function of dendritic cells to improve Tregs in glucose metabolism (30), which may indirectly cause the rise of iTregs.
Several previous studies have suggested that increased cytokine production contributes to the pathogenesis of aGVHD. We demonstrated that administration of 1α,25(OH)2D3 modulates the expression of inflammatory cytokines involved in the pathogenesis of aGVHD, such as IFN-γ, IL-4, and TNF-α. These results suggest that VD exerts potent anti-inflammatory activity via suppression of other Th cells. Our data thus indicate that 1α,25(OH)2D3 protects against aGVHD in part via modulation of cytokine expression.
In conclusion, we found that 1α,25(OH)2D3 improves the clinical course of aGVHD in a murine model. 1α,25(OH)2D3 induces the expansion of Treg populations and suppresses that of pro-inflammatory T cells. PC61 injection abolished the protective effect of 1α,25(OH)2D3 against aGVHD. In addition, 1α,25(OH)2D3 inhibits the proliferation of T cells in vivo, which is closely related to the engraftment of donor cells. These data suggest that 1α,25(OH)2D3 is a promising therapeutic candidate for preventing and treating aGVHD.
Acknowledgments
Funding: This work was supported by the National Natural Science Fund Outstanding Youth Fund 81522020, 863 Young Scientists Special Fund (No. 2015AA020932) and National Natural Science Fund 91442117 and 81571564 in China and the foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional materials and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Relevant legal and ethical requirements were followed carefully according to the protocol (number NMU08-092), which was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
References
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80:1678S-88S. [Crossref] [PubMed]
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353-73. [Crossref] [PubMed]
- Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008;88:491S-9S. [Crossref] [PubMed]
- Brennan TV, Rendell VR, Yang Y. Innate immune activation by tissue injury and cell death in the setting of hematopoietic stem cell transplantation. Front Immunol 2015;6:101. [Crossref] [PubMed]
- Sharma A, Armstrong AE, Posner MP, et al. Graft-versus-host disease after solid organ transplantation: a single center experience and review of literature. Ann Transplant 2012;17:133-9. [Crossref] [PubMed]
- Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol 2014;11:536-47. [Crossref] [PubMed]
- Via CS, Shustov A, Rus V, et al. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol 2001;167:6821-6. [Crossref] [PubMed]
- Prietl B, Treiber G, Pieber TR, et al. Vitamin D and immune function. Nutrients 2013;5:2502-21. [Crossref] [PubMed]
- Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets 2013;12:239-45. [Crossref] [PubMed]
- Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci 2018. [Crossref] [PubMed]
- Cantorna MT, McDaniel K, Bora S, et al. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239:1524-30. [Crossref] [PubMed]
- Maddaloni E, Cavallari I, Napoli N, et al. Vitamin D and diabetes mellitus. Front Horm Res 2018;50:161-76. [Crossref] [PubMed]
- Poole A, Song Y, Brown H, et al. Cellular and molecular mechanisms of vitamin D in food allergy. J Cell Mol Med 2018;22:3270-7. [Crossref] [PubMed]
- Ferrer-Mayorga G, Larriba MJ, Crespo P, et al. Mechanisms of action of vitamin D in colon cancer. J Steroid Biochem Mol Biol 2019;185:1-6. [Crossref] [PubMed]
- Abstracts of the 26th Annual Conference of APASL, February 15-19, 2017, Shanghai, China. Hepatol Int 2017;11:1-1093. [Crossref] [PubMed]
- Puliaev R, Nguyen P, Finkelman FD, et al. Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J Immunol 2004;173:910-9. [Crossref] [PubMed]
- Soloviova K, Puliaiev M, Foster A, et al. The parent-into-F1 murine model in the study of lupus-like autoimmunity and CD8 cytotoxic T lymphocyte function. Methods Mol Biol 2012;900:253-70. [Crossref] [PubMed]
- Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol 1987;139:1840-9. [PubMed]
- Gu J, Lu L, Chen M, et al. TGF-β-induced CD4+Foxp3+ T cells attenuate acute graft-versus-host disease by suppressing expansion and killing of effector CD8+ cells. J Immunol 2014;193:3388-97. [Crossref] [PubMed]
- Zeiser R, Zambricki EA, Leveson-Gower D, et al. Host-derived interleukin-18 differentially impacts regulatory and conventional T cell expansion during acute graft-versus-host disease. Biol Blood Marrow Transplant 2007;13:1427-38. [Crossref] [PubMed]
- Nikolic B, Lee S, Bronson RT, et al. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest 2000;105:1289-98. [Crossref] [PubMed]
- Montero E, Nussbaum G, Kaye JF, et al. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun 2004;23:1-7. [Crossref] [PubMed]
- McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol 2002;168:5979-83. [Crossref] [PubMed]
- Pierini A, Strober W, Moffett C, et al. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood 2016;128:866-71. [Crossref] [PubMed]
- Silva F, Pérez-Simón JA, Caballero-Velazquez T, et al. Effect of vitamin D treatment in chronic GVHD. Bone Marrow Transplant 2011;46:1395-7. [Crossref] [PubMed]
- Zahran AM, Zharan KM, Hetta HF. Significant correlation between regulatory T cells and vitamin D status in term and preterm labor. J Reprod Immunol 2018;129:15-22. [Crossref] [PubMed]
- Gorman S, Geldenhuys S, Weeden CE, et al. Investigating the roles of regulatory T cells, mast cells and interleukin-9 in the control of skin inflammation by vitamin D. Arch Dermatol Res 2018;310:221-30. [Crossref] [PubMed]
- Dauletbaev N, Herscovitch K, Das M, et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br J Pharmacol 2015;172:4757-71. [Crossref] [PubMed]
- Lin YD, Arora J, Diehl K, et al. Vitamin D is required for ILC3 derived IL-22 and protection from citrobacter rodentium infection. Front Immunol 2019;10:1. [Crossref] [PubMed]
- Vanherwegen AS, Eelen G, Ferreira GB, et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J Steroid Biochem Mol Biol 2019;187:134-45. [Crossref] [PubMed]


