
SIX1/EYA1 are novel liver damage biomarkers in chronic hepatitis B and other liver diseases
Introduction
SIX1 is a member of the sine oculis homeobox transcription factor family (SIX1-6) in humans (1,2). It interacts with the transcriptional coactivator, EYA1 (eyes absent 1) and EYA1 phosphatase to form a transcriptional complex that drives the expression of particular genes, thereby controlling cell proliferation and differentiation in numerous organs including the brain, auditory system, lung, muscle, kidney, and craniofacial structures, etc. (3,4). Aberrant SIX1 over-expression in adult tissue is associated with the initiation and progression of numerous types of cancer, including breast, ovarian, cervical, and liver cancer (5,6). Over-expression of SIX1 in hepatocellular carcinoma is significantly correlated with the proliferation and metastasis of tumor cells, resulting in a reduced 5-year survival rate in patients whose tumor cells overexpress this gene (7-9).
Chronic hepatitis B (CHB) is a viral disease that is particularly common in developing countries, and can cause liver fibrosis or the development of hepatocarcinoma in affected patients (10,11). In the present study, we collected serum and liver tissue samples from patients with CHB and other liver diseases in order to investigate the clinical significance and physiopathological role of SIX1 and EYA1 in these diseases.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-21-2526).
Methods
Patients
In total, 313 active CHB patients, 153 patients with other active liver diseases, and 33 healthy controls (all ≥18 years old) were recruited from among out- or in-patients in the Department of Infectious Diseases and Health Management Center from December 8, 2017 to September 13, 2018, and were enrolled in this study. CHB and other liver diseases were diagnosed according to the American Association for the Study of Liver Diseases (AASLD) 2018 hepatitis B guidance and other pertinent diagnostic criteria.
Collection of serum samples and clinical biochemical tests
Serum samples were allowed to clot for 2 hours at room temperature (RT), centrifuged at 1,000 ×g for 20 minutes, and the supernatants were stored at −80 °C for later analysis. Clinical serological biochemical tests were carried out in the clinical laboratory of the Department of Infectious Diseases, Southwest Hospital.
Dynamic observation of SIX1 and EYA1 serum levels in selected CHB patients before and after comprehensive treatment
Serum samples collected from CHB patients whose alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were over 100 IU/L before antivirus regimens or non-specific therapy and below 100 IU/L after treatment were tested for SIX1 and EYA1 levels by enzyme linked immunosorbent assay (ELISA). As such, these samples were different from previous samples (even from the same patient).
Assessment of SIX1 and EYA1 serological levels by ELISA
Both SIX1 and EYA1 ELISA kits were purchased from Shanghai Jianglai Industrial Limited by Share Ltd. (Shanghai, China) and were used according to the manufacturer’s instructions. Briefly, 50 µL of the standard or sample was added to the appropriate wells, and then 100 µL of enzyme conjugate was added to the wells for 60 minutes at 37 °C. Plates were then washed four times, and 50 µL each of substrate A and B were added to each well for 15 minutes at 37 °C protected from light. Next, 50 µL of stop solution was added to each well, and the optical density (OD) of each well was measured at 450 nm using a microplate reader (Multiskan Spectrum 51118650, Thermo Scientific, Waltham, MA, USA) within 15 minutes.
Liver tissues
Liver biopsy samples were obtained from a CHB inpatient. Human healthy liver tissues were provided by Xi’an Alenabio Inc. (Xi’an, Shanxi, China) and were used as a positive control. B6 mouse liver was used as a negative control.
Immunofluorescent staining for SIX1, EYA1, human serum albumin (HSA), and glial fibrillary acidic protein (GFAP) in frozen liver sections
Frozen sections were fixed with 100% ethanol for 15 minutes and permeabilized using 0.05% Tween20 twice (2 minutes per treatment). Blocking was performed using 3% bovine serum albumin for 30 minutes at RT. After washing with phosphate belanced solution (PBS), sections were incubated with rabbit anti-human SIX1 primary antibody (1:100 dilution) (HPA001893, Sigma, St. Louis, MO, USA), rabbit anti-human EYA1 primary antibody (1:100 dilution) (ab85009, Abcam, Cambridge, MA, USA), and mouse anti-human HSA primary antibody (1:1,000 dilution) (MAB1455, R&D Systems, Minneapolis MN, USA) or mouse anti-human GFAP primary antibody (1:100 dilution) (MA5-12023, ThermoFisher, Waltham, MA, USA) at 4 °C overnight. Sections were then incubated with Alexa Fluor®488 donkey anti-mouse immunoglobulin G (IgG) and Alexa Fluor®568 donkey anti-rabbit IgG (1:1,000 dilution) (A21202, A10042, ThermoFisher, Waltham, MA, USA) for one hour at RT. After washing with PBS, sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI). Finally, coverslips were mounted with anti-fade mounting medium, and immunofluorescent signals were visualized and recorded using an Olympus DP72 microscope and the cellSens Standard 1.5 software. (Olympus Corporation, Tokyo, Japan)
Statistical analysis
Statistical analyses were carried out using the SPSS v17.0 statistical package (SPSS Inc., Chicago, IL, USA), with comparisons being made via Student’s t-tests. P<0.05 was considered to be statistically significant.
Ethics
The authors declare that this study fully complied with all relevant ethical standards. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by ethics board of Southwest Hospital, Third Military Medical University. Individual consent for this retrospective analysis was waived.
Results
Increased serum SIX1 and EYA1 levels in patients with CHB and other liver diseases
An ELISA assessment of serum SIX1 and EYA1 levels revealed that the levels in 313 CHB patients (Table 1) were significantly higher than those in healthy controls (P<0.05). Serum SIX1 levels were also significantly increased in patients with numerous other liver diseases (Table 2), including liver fibrosis, hepatocellular carcinoma, fatty liver disease, alcoholic liver disease, fulminant hepatic failure, autoimmune liver disease, and hepatitis C (P<0.05). Levels were not increased in patients with drug-induced liver injury or with inherited liver diseases, including Wilson’s disease, hemochromatosis, and hereditary hyperbilirubinemia. Serum EYA1 levels were significantly elevated in patients with all assessed liver diseases relative to healthy controls (P<0.05).

Full table

Full table
Dynamic observation of serum SIX1 and EYA1 levels in 35 CHB patients
We subsequently selected 35 CHB patients for the dynamic observation of serum SIX1 and EYA1 over time. We found that the serum SIX1 and EYA1 levels remained significantly elevated in these patients relative to the healthy controls, while both ALT and AST levels decreased to below 100 IU/L in these individuals at intervals of 34 to 193 days (Table 3). Serum EYA1 levels in patients in the convalescent phase increased significantly relative to the post-treatment recovery phase (P<0.05).

Full table
HSC-specific elevated expression of SIX1 and EYA1 in CHB liver tissues
GFAP is an intermediate filament protein that is expressed by numerous cell types including astrocytes in the nervous system and hepatic stellate cells (HSCs) in the liver. In this study, we found that both SIX1 and EYA1 staining was detectable in GFAP-positive HSCs, but not in HSA-positive hepatocytes, based on immunofluorescent staining (Figure 1). The expression of SIX1 and EYA1 in the livers of CHB patients was elevated compared to the normal liver controls, in which only 1–4 positive cells were detectable per microscopy field. These findings revealed that both SIX1 and EYA1 were only expressed in HSCs.
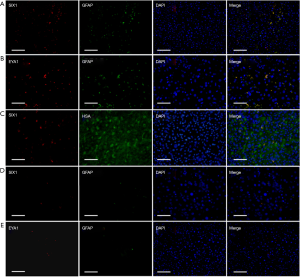
Discussion
CHB is a form of chronic hepatophagocytic viral infection in humans, which induces deleterious protracted immune responses, ultimately leading to progressive liver damage, fibrosis, or cirrhosis. CHB is also known to be a significant risk factor for the eventual development of hepatocellular carcinoma (12,13). The accurate estimation of CHB staging and severity is extremely important in clinical settings in order to allow for the correct selection of appropriate therapeutic interventions. Currently, such estimations are made largely based upon serial serological biochemical tests that are intended to reflect the liver injury and repair responses in patients, however these tests are limited in their ability to fully reflect the entirety of liver function in patients (14,15).
Recent studies have found that some new biomarkers in serum have good potential application value in the evaluation of liver injury: (I) animal experimental studies have found that microRNA has a significant relationship with liver injury (16). During liver injury, the expression of miR-122 in serum of the experimental group was significantly increased, and the increase of miR-122 was 15 times of the control, But AST/ALT is only about 4 times of the control (17). After liver injury, the expression of miR-122 in serum has a good correlation with liver enzymes, such as ALT, AST, glutamic acid dehydrogenation (18). Through the study of some muscle injury patients (19), it was found that the expression of miR-122 did not change, However, ALT was significantly increased in the experimental group. This indicates that miR-122 as a marker of liver injury has better specificity than ALT; (II) Keratin 18 (K-18) is one of the main intermediate silk proteins that constitute the liver cytoskeleton, When the liver is injured, the fragmented and full-length K-18 can be released into the serum and detected, which is closely related to the time of cell death in histology and is more sensitive than ALT (20). in addition, the study of exercise-induced renal injury shows that compared with ALT, K-18 will still maintain a relatively stable level, this shows that K-18 has good specific (21); (III) high mobility group box 1 (HMGB 1) is a highly conserved and widely distributed non-histone DNA binding protein with a variety of biological functions (22) as stabilizing nucleic acid structure, regulating transcription, and gene expression. Some studies (23,24) found that the serological HMGB1 level in patients with chronic hepatitis virus (HBV) infection was significantly higher than that of healthy control, and its content is closely related to the degree of liver inflammation and fibrosis; (IV) Golgi membrane protein 73 (GP73), is usually considered to be a serum marker for early liver cancer screening. Xu et al. (25) found that the content of GP73 in serum is positively correlated with the severity of hepatitis caused by HBV. In some patients with normal ALT level, the expression level of GP73 in patients with significant liver inflammatory injury and liver fibrosis is significantly higher than those without liver injury and fibrosis. The content of GP73 in serum is closely related to the progress of liver disease. It was positively correlated with the grade and stage of liver pathology (26).
SIX1 is a DNA binding protein that interacts with its coactivator, EYA1, to form a transcriptional complex regulating cell proliferation and differentiation (1,2). It is also involved in various tumors including those associated with liver cancer, and its expression is associated with increased mortality (27,28). At present it is unclear as to whether SIX1 and EYA1 are involved in CHB and other liver diseases. In this study we found, for the first time, that increased serum SIX1 and EYA1 were detectable in patients with CHB and other diseases relative to healthy controls. This novel finding strongly suggests that both SIX1 and EYA1 may be involved in the pathological development and progression of CHB and other liver diseases. HSCs have been well documented to be involved in liver fibrosis; once activated, these cells serve as the primary cellular source of matrix protein-secreting myofibroblasts (29,30). They are also known to be involved in liver repair and regeneration (31,32). HSC-specific expressions of SIX1 and EYA1 in this study indicated that both of these proteins could participate in liver repair and regeneration in the context of CHB virus infection and other liver diseases. Dynamic observation of serological SIX1 and EYA1 in CHB patients also allowed us to confirm this finding. Up-regulation of SIX1/EYA1 did not correlate with disease status, possibly due to chronic liver damage and repair in the livers of CHB patients. However, further study will be needed to confirm these findings, as the results of the present study remain relatively superficial.
Conclusions
Our study found elevated serum SIX1 and EYA1 levels in patients with CHB and other liver diseases. Both SIX1 and EYA1 expressions were HSC-specific and significantly increased in the liver tissue of CHB samples. These results indicate that both SIX1 and EYA1 are novel biomarkers that may be useful for the clinical diagnosis of CHB and other liver diseases, or for determining patient prognosis.
Acknowledgments
Funding: The present study was financially supported by the Talent Start-up Fund 4174DG (to JD) and the Clinical Innovation Project SWH2016BZGFKJ-40 (to BX) from Southwest Hospital, Third Military Medical University.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-2526
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-2526
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-2526). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by ethics board of Southwest Hospital, Third Military Medical University (No. KY201845). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kumar JP. The sineoculishomeobox (SIX) family of transcription factors as regulators of development and disease. Cell Mol Life Sci 2009;66:565-83. [Crossref] [PubMed]
- Ruf RG, Xu PX, Silvius D, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A 2004;101:8090-5. [Crossref] [PubMed]
- Zhang T, Xu J, Maire P, et al. Six1 is essential for differentiation and patterning of the mammalian auditory sensory epithelium. PLoS Genet 2017;13:e1006967 [Crossref] [PubMed]
- Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 2012;139:1965-77. [Crossref] [PubMed]
- Micalizzi DS, Christensen KL, Jedlicka P, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest 2009;119:2678-90. [Crossref] [PubMed]
- Xu HX, Wu KJ, Tian YJ, et al. Expression profile of SIX family members correlates with clinic-pathological features and prognosis of breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4085 [Crossref] [PubMed]
- Kong J, Zhou X, Liu S, et al. Overexpression of sineoculis homeobox homolog 1 predicts poor prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol 2014;7:3018-27. [PubMed]
- Feng GW, Dong LD, Shang WJ, et al. HDAC5 promotes cell proliferation in human hepatocellular carcinoma by up-regulating Six1 expression. Eur Rev Med Pharmacol Sci 2014;18:811-6. [PubMed]
- Ng KT, Man K, Sun CK, et al. Clinicopathological significance of homeoprotein Six1 in hepatocellular carcinoma. Br J Cancer 2006;95:1050-5. [Crossref] [PubMed]
- Mysore KR, Leung DH. Hepatitis B and C. Clin Liver Dis 2018;22:703-22. [Crossref] [PubMed]
- Mak LY, Cruz-Ramón V, Chinchilla-López P, et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book 2018;38:262-79. [Crossref] [PubMed]
- Weinberger B, Haks MC, de Paus RA, et al. Impaired Immune Response to Primary but Not to Booster Vaccination Against Hepatitis B in Older Adults. Front Immunol 2018;9:1035. [Crossref] [PubMed]
- Nitschke K, Luxenburger H, Kiraithe MM, et al. CD8+ T-Cell Responses in Hepatitis B and C: The (HLA-) A, B, and C of Hepatitis B and C. Dig Dis 2016;34:396-409. [Crossref] [PubMed]
- Portilho MM, Mendonça ACDF, Bezerra CS, et al. Usefulness of in-house real time PCR for HBV DNA quantification in serum and oral fluid samples. J Virol Methods 2018;256:100-6. [Crossref] [PubMed]
- Kim H, Hur M, Bae E, et al. Performance evaluation of cobas HBV real-time PCR assay on Roche cobas 4800 System in comparison with COBAS AmpliPrep/COBAS TaqMan HBV Test. Clin Chem Lab Med 2018;56:1133-9. [Crossref] [PubMed]
- Ward J, Kanchagar C, Veksler-Lublinsky I, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci USA 2014;111:12169-74. [Crossref] [PubMed]
- Nagano T, Higashisaka K, Kunieda A, et al. Liver-specific microRNAs as biomarkers of nanomaterial-induced liver damage. Nanotechnology 2013;24:405102 [Crossref] [PubMed]
- Sharapova T, Devanarayan V, LeRoy B, et al. Evaluation ofmiR-122 as a Serum Biomarker for Hepatotoxicity in Investigative Rat Toxicology Studies. Vet Pathol 2016;53:211-21. [Crossref] [PubMed]
- Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 2010;56:1830-38. [Crossref] [PubMed]
- Schutte B, Henfling M, Kölgen W, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res 2004;297:11-26. [Crossref] [PubMed]
- Thulin P, Nordahl G, Gry M, et al. Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int 2014;34:367-78. [Crossref] [PubMed]
- Anggayasti WL, Mancera RL, Bottomley S, et al. The self-association of HMGB1 and its possible role in the binding to DNA and cell membrane receptors. FEBS Lett 2017;591:282-94. [Crossref] [PubMed]
- Zhou RR, Zhao SS, Zou MX, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol 2011;11:21. [Crossref] [PubMed]
- Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med 2014;40:1-116. [Crossref] [PubMed]
- Xu Z, Liu L, Pan X, et al. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore) 2015;94:e659 [Crossref] [PubMed]
- Xu Z, Pan X, Wei K, et al. Serum Golgi protein 73 levels and liver pathological grading in cases of chronic hepatitis B. Mol Med Rep 2015;11:2644-52. [Crossref] [PubMed]
- Ventura-Holman T, Mamoon A, Subauste MC, et al. The effect of oncoprotein v-erbA on thyroid hormone-regulated genes in hepatocytes and their potential role in hepatocellular carcinoma. Mol Biol Rep 2011;38:1137-44. [Crossref] [PubMed]
- Ng KT, Lee TK, Cheng Q, et al. Suppression of tumorigenesis and metastasis of hepatocellular carcinoma by shRNA interference targeting on homeoprotein Six1. Int J Cancer 2010;127:859-72. [Crossref] [PubMed]
- Thi Thanh Hai N, Thuy LTT, Shiota A, et al. Selective overexpression of cytoglobin in stellate cells attenuates thioacetamide-induced liver fibrosis in mice. Sci Rep 2018;8:17860. [Crossref] [PubMed]
- Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017;121:27-42. [Crossref] [PubMed]
- Langiewicz M, Graf R, Humar B, et al. JNK1 induces hedgehog signaling from stellatecells to accelerate liver regeneration in mice. J Hepatol 2018;69:666-75. [Crossref] [PubMed]
- Cook D, Achanta S, Hoek JB, et al. Cellular network modeling and single cell gene expression analysis reveals novel hepatic stellate cell phenotypes controlling liver regeneration dynamics. BMC Syst Biol 2018;12:86. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


