
18F-fluorodeoxyglucose positron emission tomography—based prediction for splenectomy in patients with suspected splenic lymphoma
Introduction
Splenic lymphoma is a rare form of malignant lymphoma that affects only the spleen and splenic hilar lymph nodes. It accounts for less than 1% of non-Hodgkin’s lymphoma cases (1). However, diagnosing splenic lymphomas often proves challenging due to a lack of lymphadenopathy or solid tissue masses amenable to biopsy.
For patients with lymphoma, fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT at the time of initial staging can help to give us a hint for potential biopsy (2). For patients with splenic lymphoma, splenomegaly or a high standardized uptake value (SUV) of the spleen on FDG-PET/CT may be the only diagnostic indicator, and some patients may also fail to show a significant increase in uptake values on PET-CT. Splenectomy is one of the effective treatment for patients with lymphoma confined to the spleen. Moreover, lymphoma is a systemic disease, and splenectomy is not carried out with eradicative intent. Clinically, splenectomy is not really a treatment, but rather a step toward a definitive diagnosis. Unfortunately, 18F-FDG PET tracer is not specific to malignant lymphomas. Tumors, sarcoidosis, malaria, and many other inflammatory diseases show increased 18F-FDG uptake (3). Whether to conduct splenectomy according to PET-CT result remains challenging.
The spleen has adoptive and innate immune functions, and can produce lymphocytes and secrete a large quantity of antibodies. It maintains homeostasis by removing aged cells and microbial antigens (4). Although laparoscopic splenectomy, which is being increasingly applied, may cause fewer complications than open splenectomy, it is still associated with significant morbidity and mortality (5,6). Therefore, we need a good prediction model to avoid unnecessary splenectomy on the basis of PET-CT results.
PET has been widely accepted as a beneficial imaging modality in the assessment of several inflammatory and neoplastic diseases. Although PET with 18F-FDG is widely used for the diagnosis and staging of lymphomas, limited data have been reported on its capability to detect splenic lymphoma. In the present study, we investigated the correlation and indicators of preoperative PET-CT and splenic pathology after splenectomy. We also developed a model to predict the need for splenectomy with accuracy.
We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/atm-21-2790).
Methods
Study participants
After obtaining approval from the ethics committee of Huadong Hospital affiliated with Fudan University (the registration number of ethics board No. 2019K071), we viewed the pathology database of Huadong and Huashan Hospital. A total of 175 cases of splenectomy from February 2013 to September 2019 were identified. The specific inclusion criteria for patients were as follows: (I) diffuse, increased uptake of 18F-FDG in the spleen, but not in the lymph nodes; (II) no peripheral lymphadenopathy detected (i.e., node diameter >10 mm); and (III) underwent bone marrow biopsy or bone marrow aspiration, with no lymphoma detected. Patients who had previously been diagnosed with lymphoma and had undergone diagnostic splenectomy (with the purpose of identifying lymphoma transformation) were excluded. In all cases, the operation was performed concurrently with the utilization of PET/CT. Cases meeting these criteria were reviewed, and 60 patients with suspected splenic lymphoma who underwent FDG-PET examination followed by splenectomy were identified. None of the cases had any adverse events at the time of receiving PET/CT.
All methods were carried out in accordance with the relevant guidelines and regulations (7,8). Informed consent was obtained from each participant, or, if the participant was under 18, from a parent and/or legal guardian. This study was performed in adherence with the Declaration of Helsinki (as revised in 2013).
PET/CT image acquisition and analysis
The 18F-FDG PET/CT scans were acquired on a Biograph 64 PET/CT scanner (Siemens, Munich, Germany). All patients fasted for at least 6 hours before the scan, and all had blood glucose levels <130 mg/dL (8.7 mmol/L). A whole-body scan was acquired at approximately 1 hour after the administration of 18F-FDG (200–400 MBq, depending on the patient’s body weight). The PET scanner had an axial field width of 16.2 cm, a spatial resolution of 4 mm full-width at half-maximum, and a pixel size of 2.04×2.04 mm2. The CT scans were acquired first (120 kVp,150 mAs) using a slice thickness of 3.27 mm and then reconstructed to 512×512 matrix (voxel size: 0.98×0.98×3.27 mm3). Each PET image measured 128×128×207 voxels, had an anisotropic resolution of 5.47×5.47×3.27 mm3, and was acquired using a three-dimensional row-action maximization likelihood algorithm. The PET images were converted into SUV units through normalizing the activity concentration to the dosage of injected 18F-FDG and the patient’s body weight. All PET examinations were carried out on patients at the same center using the same equipment and methods.
Volume-based image analysis
The three-dimensional regions of interest (ROIs) of the enlarged spleens were segmented on the merged PET/CT images based on the 18F-FDG-PET data by a skilled nuclear medicine physician using ITK-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php). To avoid including areas with physiological FDG uptake within the ROIs, a joint reading of both the fusion CT and the 18F-FDG PET scans was performed simultaneously. The volumes of interest (VOIs) were recalculated and validated by an independent senior nuclear medicine physician, who was blind to the results of the patients’ any other tests and clinical informations. MATLAB version R2015b (Math Works, USA) was employed to generate standard uptake value (SUV) maps from the original 18F-FDG-PET Digital Imaging and Communications in Medicine (DICOM) data. For each lesion, the maximum standardized uptake value (SUVmax), SUVmean, and metabolic tumor volume (MTV) based on first-order features were extracted using PyRadiomics (https://github.com/Radiomics/pyradiomics) (9). The total lesion glycolysis (TLG) of the spleen was equal to the product of the MTV and the SUVmean.
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses in this study. Comparisons of age, SUVmax of the spleen, SUVmean of the spleen, SUVmax of the liver and bone marrow, MTV, TLG, and the 18F-FDG SUVmax ratios of the spleen/liver, spleen/bone marrow, and liver/bone marrow between the lymphoma and benign disease groups were performed using Student t tests. All the variance between the groups were statistically compared. Sex was compared using the chi-squared test. All statistical tests were two-tailed, with a P value <0.05 was considered significant. Receiver operating characteristic (ROC) curve analysis was used to evaluate the cutoff point for predicting lymphoma before splenectomy. The positive and negative predictive values (PPV and NPV, respectively), sensitivity, and specificity were computed. Serial and parallel tests were compared to enhance the sensitivity and specificity.
Results
Clinical characteristics of patients scanned by 18F-FDG PET-CT
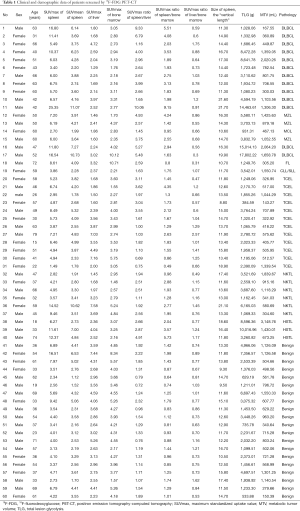
The demographic and clinical characteristics of the 60 patients with suspected splenic lymphoma who underwent FDG-PET examination followed by splenectomy (lymphoma cases, n=40; benign cases, n=20) are summarized in Table 1.
Full table
Among the lymphoma cases, there were 21 patients with T-cell lymphoma (11 men and 10 women; age range, 15–79 years; mean age, 56.17 years) and 19 patients with B-cell lymphoma (9 men and 10 women; age range, 31–72 years; mean age, 55.62 years). There were no significant differences in sex (x2=0.1, P=0.752>0.05) or mean age (t=−1.168, P=0.243>0.05) between the two groups.
For the 20 benign cases, splenectomy revealed the following pathology: reactive lymphocyte hyperplasia (n=12); congestive splenomegaly (n=3); sarcoidosis (n=2); amyloidosis (n=1); arteritis (n=1); and tuberculosis (n=1).
There was no significant difference in mean age or sex between the benign and lymphoma groups (P=0.582 and P=0.536, respectively). Figure 1 shows the specific classification of the lymphoma groups.
Differences in the SUVmax, SUVmean, MTV, and TLG of the spleen between the lymphoma and benign disease groups
In the lymphoma group, the median SUVmax, SUVmean, MTV, and TLG were 7.49, 4.31, 898.33 mL, and 4,048.0 g, respectively; in the benign group, the corresponding values were 5.17, 3.29, 619.60 mL, and 2,706.7 g, respectively (Tables 1 and 2). Thus, no significant differences were noted between the two groups with respect to SUVmax, SUVmean, MTV, or TLG (P=0.051, P=0.074, P=0.322, and P=0.133, respectively) (Figure 2A). However, the difference in SUVmax between the B-cell lymphoma and benign groups was significant (P=0.027). The median SUVmax values of the liver and bone marrow were also compared between the lymphoma and benign groups, but no significant differences were noted (P=0.080 and P=0.055, respectively).

Full table

Differences in the SUVmax ratio distribution of the spleen/liver and spleen/bone marrow between the lymphoma and benign disease groups
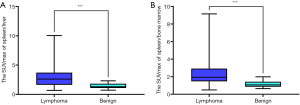
The SUVmax ratio of the spleen/liver in the lymphoma group was significantly different from that in the benign group (3.05±2.08 vs. 1.40±0.46, P=0.001; Figure 2B). The SUVmax ratio of the spleen/bone marrow in the lymphoma group was also significantly different from that in the benign group, (2.46±1.63 vs. 1.16±0.38, P=0.001; Figure 2C). However, no difference was found in the liver/bone marrow SUVmax ratios of the two groups (0.91±0.42 vs. 0.86±0.25, P=0.61; Figure 2D).
The average spleen size of the 40 patients in the lymphoma group was 14.57±3.15 cm, and that of the 20 patients with benign disease was 12.95±2.24 cm (Table 3), which translated to a significant difference in spleen size between the 2 groups (P=0.044; Figure 2E).

Full table
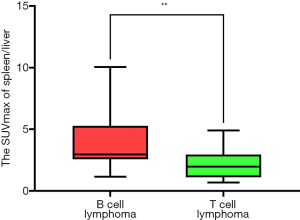
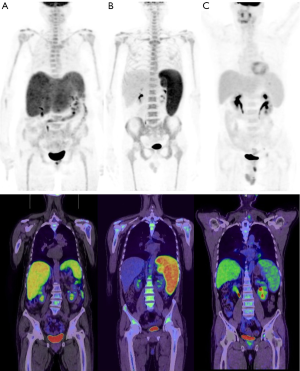
Box plots for the SUVmax ratios of the spleen/liver and spleen/bone marrow in the lymphoma and benign groups are shown in Figure 3. The differences in the SUVmax ratios of the liver/bone marrow and spleen/bone marrow between the B-cell and T-cell lymphoma groups were not significant, nor was the difference in spleen size (P=0.157, P=0.057, and P=0.112, respectively; Figure 2C,D,E). However, the SUVmax ratio of the spleen/liver was significantly different between the two groups (P=0.009) (Figure 4). Patients with T-cell lymphoma had a high SUVmax ratio of the spleen/bone marrow but a low SUVmax ratio of the spleen/liver, whereas patients with B-cell lymphoma had high SUVmax ratios of both the spleen/liver and spleen/bone marrow. In patients with benign disease, the SUVmax ratios of the spleen/liver and spleen/bone marrow were low (Figure 5).
ROC curve to calculate the threshold for predicting lymphoma before splenectomy
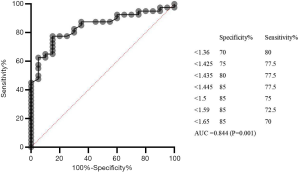
Next, we sought to develop a model to predict lymphoma before splenectomy. Using ROC curve analysis, we determined the optimal threshold for detecting lymphoma before splenectomy. The area under the ROC curve (AUC) value of the SUVmax ratio of the spleen/liver was 0.809 (95% confidence interval: 0.702–0.917; P<0.01). The optimal cutoff value of the spleen/liver SUVmax ratio for distinguishing lymphoma from benign disease was 2.42 (sensitivity: 62.5%; specificity: 100.0%) (Figure 6). The AUC value of the SUVmax ratio of the spleen/bone marrow was 0.844 (95% confidence interval: 0.745–0.943; P<0.01). The optimal cutoff value of the spleen/bone marrow SUVmax ratio was 1.45 (sensitivity: 77.5%; specificity: 85.0%) (Figure 7). Therefore, a spleen/liver SUVmax ratio of >2.42 and a spleen/bone marrow SUVmax ratio of >1.45 might be able to predict lymphoma before splenectomy. Table 3 shows the diagnostic specificity, sensitivity, PPV, and NPV of the spleen/liver and spleen/bone marrow SUVmax ratios.

Testing in series and parallel
We obtained the cutoff values for two predictors of lymphoma: an SUVmax ratio of the spleen/liver >2.42 and an SUVmax ratio of the spleen/bone marrow >1.45, as per the FDG-PET results. Next, we calculated the mean values to improve the specificity and sensitivity of the diagnostic test. SUVmax ratio of the spleen/liver >2.42 and SUVmax ratio of the spleen/bone marrow >1.45 were compared both in series (applied if SUVmax ratio of the spleen/liver >2.42 and SUVmax ratio of the spleen/bone marrow >1.45) and in parallel (applied if SUVmax ratio of the spleen/liver >2.42 or SUVmax ratio of the spleen/bone marrow >1.45). The diagnostic specificity and sensitivity of the tests applied in parallel were 85.0 and 82.5%, respectively, and the PPV and NPV were 91.7 and 70.8%, respectively. For the tests applied in series, the specificity and sensitivity were 100 and 57.5%, respectively, and the PPV and NPV were 100% and 54.1%, respectively (Table 3).
Discussion
To the best of our knowledge, this study includes the largest group of patients with suspected splenic lymphoma who were evaluated by FDG-PET before splenectomy reported in the literature so far. All patients showed diffuse, increased uptake of 18F-FDG in the spleen, bone marrow, and liver, but not in the lymph nodes. None of the patients had peripheral lymphadenopathy (i.e., node diameter >10 mm). Our findings provide rationale for the use of FDG-PET in patients intended to undergo diagnostic splenectomy. In the literature, only the study of Rutherford et al., who analyzed 10 cases of splenectomy with suspected lymphoma, including 6 cases with previous diagnoses, is similar to ours. They classified PET/CT scans as low, intermediate, or high splenic metabolic activity based on the SUV. Patients with low splenic SUVs were found to be less likely to have splenic involvement of lymphoma, whereas intermediate and high SUVs suggested the presence of lymphoma (10). However, their work was unable to guide splenectomy very well. Therefore, as splenectomy can be traumatic, the main objective of our study was to find a non-traumatic preliminary screening method for patients with suspected splenic lymphoma.
Diffuse, increased uptake of 18F-FDG in the spleen, liver, and bone marrow, with no significant lymphadenopathy is consistently observed in patients with lymphoma and other inflammatory conditions (11,12). However, 18F-FDG is a non-specific tracer for malignant lymphomas (3), and it is unwise to make a decision on splenectomy on the basis of the splenic SUV value on PET-CT. In our study, there were no significant differences in SUVmax, MTV, or TLG between the lymphoma and benign disease groups. Yamanaka et al. also studied the prognostic value of MTV, in patients with diffuse large B-cell lymphoma, and found that analysis of the MTVs of the spleen and bone marrow failed to yield any prognostic value (13).
The spleen is the largest blood filtering and lymphoid organ, and it also plays an important role in defending against invading pathogens (14,15). Increased splenic uptake of 18F-FDG can indicate elevated glucose usage by the spleen during inflammation. However, a diffuse and increased 18F-FDG uptake may also reflect lymphomatous infiltration of the spleen (10). The liver is surveyed by antigen-presenting cells and lymphocytes. Hepatic lymphocytes are rich in natural-killer cells and T-lymphocytes, which are members of the innate immune system defending against circulating lymphocyte recruitment, liver injury, and invading pathogens (16). In healthy individuals, the splenic uptake of 18F-FDG is less than that of the liver and does not alter remarkably with age (17,18). Greater uptake by the spleen than the liver has been considered abnormal (19). Similar results were obtained in the present study, in which we found a statistically significant difference in the SUVmax ratio of the spleen/liver between patients in the lymphoma and benign groups. Therefore, we hypothesize that the diffuse and increased uptake of 18F-FDG by the spleen and liver under various inflammatory conditions may result from the activation of the splenic and hepatic immune systems. Further, an increase in the activity of the spleen compared with the liver is sometimes associated with invasive lymphoma (10). Interestingly, the SUVmax ratio of the spleen/liver is also a valuable prognostic indicator in lymphoma. For instance, Albano et al. investigated the prognostic value of PET/CT for splenic marginal zone lymphoma and found that the SUVmax ratio of the spleen/liver was an independent prognostic factor for progression-free survival (20).
The present study also showed that the SUVmax ratio of the spleen/liver can be used to distinguish lymphoma types. B-cell lymphoma has a higher ratio than T-cell lymphoma, and T-cell lymphoma is more likely to involve the liver, especially γδHSTL, which may result in 18F-FDG PET/CT hepatomegaly with increased FDG uptake (21).
A previous study found histologic evidence of lymphoma in the bone marrow in approximately 50–80% and 25–40% of patients with indolent and aggressive non-Hodgkin lymphoma, respectively (22). It has been reported that as many as 44% of patients with lymphoma could have splenic involvement (23). Furthermore, for 35.4% of individuals with non-Hodgkin lymphoma, the spleen was found to be the only subdiaphragmatic site affected during the clinical stages of lymphoma. In their study, Sundaram et al. concluded that PET-CT could not replace bone marrow aspiration or biopsy in lymphoma (24). All of the patients in our study received bone marrow biopsy or bone marrow aspiration, and they did not undergo splenectomy if lymphoma was found in the bone marrow examination. In our study, we found that the SUVmax ratio of the spleen/bone marrow differed significantly between the lymphoma and benign disease groups. The SUVmax value of the spleen in the lymphoma group was significantly higher than the SUVmax value of bone marrow, compared with benign disease group. We hypothesize that this was partly attributable to the low incidence of bone marrow involvement among patients with splenic lymphoma.
Splenomegaly is a common finding and is often unrelated to tumors; thus, it is an example of non-lymphoma-specific involvement (25). In fact, lymphomatous spleens are usually of a normal size (26). With the exception of cases with obvious, massive enlargement of an organ, organ size cannot predict tumor involvement. Castellino et al. reported that approximately 30% of all cases of splenic enlargement were attributable to nonmalignant causes (27). In our study, the difference in spleen size between the lymphoma and benign disease groups was significant, which may be connected with the small sample size (n=60).
We found that two ratios (the SUVmax ratios of the spleen/liver and spleen/bone marrow) derived from PET/CT were closely related to the postoperative diagnosis of lymphoma. Two cutoff values were determined by ROC curve analysis. Tests conducted in parallel (an SUVmax ratio of the spleen/liver >2.42 or an SUVmax ratio of the spleen/bone marrow >1.45) were beneficial in increasing the sensitivity and specification of the diagnostic test. Our study is the first to report that the SUVmax ratios of the spleen/liver and spleen/bone marrow carry diagnostic value for splenic lymphoma.
Our study has some limitations. First, it was a retrospective study. We controlled selective bias by strict inclusion criteria. All of our patients came from the same medical history application platform, which was used to ensure that medical history information was truthful. Our criteria for judgment are pathological diagnosis and experienced attending doctors on PET/CT to ensure the authenticity of information. Second, the sample size was not very large, especially because of the strict inclusion criteria essential to accomplish our primary goal.A larger, prospective clinical trial is currently underway to verify the relationship between these two values and splenic lymphoma.
Acknowledgments
Funding: This work was supported by the Elite Project of Huadong Hospital (grant no. H1439).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-2790
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-2790
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-2790). All authors report that this work was supported by the Elite Project of Huadong Hospital (grant no. H1439). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study obtained approval from the ethics committee of Huadong Hospital affiliated with Fudan University (the registration number of ethics board NO. 2019K071). Informed consent was obtained from each participant, or, if the participant was under 18, from a parent and/or legal guardian. This study was performed in adherence with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han SM, Teng CL, Hwang GY, et al. Primary splenic lymphoma associated with hemophagocytic lymphohistiocytosis complicated with splenic rupture. J Chin Med Assoc 2008;71:210-3. [Crossref] [PubMed]
- Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology 2004;232:823-9. [Crossref] [PubMed]
- Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 1999;94:429-33. [Crossref] [PubMed]
- Tarantino G, Savastano S, Capone D, et al. Spleen: A new role for an old player? World J Gastroenterol 2011;17:3776-84. [Crossref] [PubMed]
- Uranues S, Alimoglu O. Laparoscopic surgery of the spleen. Surg Clin North Am 2005;85:75-90. ix. [Crossref] [PubMed]
- Schlachta CM, Poulin EC, Mamazza J. Laparoscopic splenectomy for hematologic malignancies. Surg Endosc 1999;13:865-8. [Crossref] [PubMed]
- Bonnet S, Guédon A, Ribeil JA, et al. Indications and outcome of splenectomy in hematologic disease. J Visc Surg 2017;154:421-9. [Crossref] [PubMed]
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program 2009;523-31. [Crossref] [PubMed]
- van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77:e104-7. [Crossref] [PubMed]
- Rutherford SC, Andemariam B, Philips SM, et al. FDG-PET in prediction of splenectomy findings in patients with known or suspected lymphoma. Leuk Lymphoma 2008;49:719-26. [Crossref] [PubMed]
- Liu Y. Clinical significance of diffusely increased splenic uptake on FDG-PET. Nucl Med Commun 2009;30:763-9. [Crossref] [PubMed]
- Herrinton LJ, Liu L, Abramson O, et al. The incidence of hepatosplenic T-cell lymphoma in a large managed care organization, with reference to anti-tumor necrosis factor therapy, Northern California, 2000-2006. Pharmacoepidemiol Drug Saf 2012;21:49-52. [Crossref] [PubMed]
- Yamanaka S, Miyagawa M, Sugawara Y, et al. The prognostic significance of whole-body and spleen MTV (metabolic tumor volume) scanning for patients with diffuse large B cell lymphoma. Int J Clin Oncol 2021;26:225-32. [Crossref] [PubMed]
- Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol 1992;132:31-74. [Crossref] [PubMed]
- de Porto AP, Lammers AJ, Bennink RJ, et al. Assessment of splenic function. Eur J Clin Microbiol Infect Dis 2010;29:1465-73. [Crossref] [PubMed]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54-62. [Crossref] [PubMed]
- Kotzerke J, Guhlmann A, Moog F, et al. Role of attenuation correction for fluorine-18 fluorodeoxyglucose positron emission tomography in the primary staging of malignant lymphoma. Eur J Nucl Med 1999;26:31-8. [Crossref] [PubMed]
- Meier JM, Alavi A, Iruvuri S, et al. Assessment of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography. Semin Nucl Med 2007;37:154-72. [Crossref] [PubMed]
- Rini JN, Manalili EY, Hoffman MA, et al. F-18 FDG versus Ga-67 for detecting splenic involvement in Hodgkin's disease. Clin Nucl Med 2002;27:572-7. [Crossref] [PubMed]
- Albano D, Camoni L, Giubbini R, et al. Prognostic Value of 18F-FDG PET/CT Metabolic Parameters in Splenic Marginal Zone Lymphoma. Clin Lymphoma Myeloma Leuk 2020;20:e897-904. [Crossref] [PubMed]
- Cho MW, Chin BB. 18F-FDG PET/CT findings in hepatosplenic Gamma-Delta T-cell lymphoma: case reports and review of the literature. Am J Nucl Med Mol Imaging 2018;8:137-42. [PubMed]
- Chen YK, Yeh CL, Tsui CC, et al. F-18 FDG PET for evaluation of bone marrow involvement in non-Hodgkin lymphoma: a meta-analysis. Clin Nucl Med 2011;36:553-9. [Crossref] [PubMed]
- Bangerter M, Moog F, Buchmann I, et al. Whole-body 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin's disease. Ann Oncol 1998;9:1117-22. [Crossref] [PubMed]
- Sundaram S, Jizzini M, Lamonica D, et al. Utility of bone marrow aspirate and biopsy in staging of patients with T-cell lymphoma in the PET-Era - tissue remains the issue. Leuk Lymphoma 2020;61:3226-33. [Crossref] [PubMed]
- Castellino RA. Hodgkin disease: practical concepts for the diagnostic radiologist. Radiology 1986;159:305-10. [Crossref] [PubMed]
- Partridge S, Timothy A, O'Doherty MJ, et al. 2-Fluorine-18-fluoro-2-deoxy-D glucose positron emission tomography in the pretreatment staging of Hodgkin's disease: influence on patient management in a single institution. Ann Oncol 2000;11:1273-9. [Crossref] [PubMed]
- Castellino RA. Imaging techniques for staging abdominal Hodgkin's disease. Cancer Treat Rep 1982;66:697-700. [PubMed]
(English Language Editor: J. Reynolds)



