
Efficacy and safety of camrelizumab plus apatinib as second-line treatment for advanced squamous non-small cell lung cancer
Introduction
Squamous non-small cell lung cancer (NSCLC) accounts for 20–30% of all cases of NSCLC and is associated with poorer prognosis than non-squamous NSCLC (1). Historically, second-line options for squamous NSCLC are almost entirely restricted to cytotoxic chemotherapy (docetaxel) after failure of prior first-line platinum-based doublet chemotherapy; nevertheless, the prognosis remains unsatisfactory (2). Progress in squamous NSCLC still lags behind non-squamous NSCLC, especially due to the lack of an actionable oncogenic driver (3). Furthermore, some agents previously approved for the treatment of non-squamous NSCLC are unfortunately contraindicated in patients with squamous NSCLC due to inadequate efficacy (pemetrexed) or potential safety concerns (bevacizumab) (4,5).
Immunotherapy has revolutionized the therapy landscape of advanced NSCLC, significantly extending the overall survival (OS) in patients with advanced NSCLC (3). To date, several phase III trials have demonstrated that treatment with programmed cell death protein 1 (PD-1) or PD ligand 1 (PD-L1) therapies, including nivolumab, pembrolizumab, or atezolizumab improves OS in comparison with docetaxel in the second-line setting for immunotherapy-naïve patients with advanced NSCLC (6-10). On the basis of these findings, these PD-1/PD-L1 agents have been approved by both the US Food and Drug Administration and the European Medicines Agency in the second-line setting for immunotherapy-naïve patients with advanced NSCLC [nivolumab and atezolizumab for patients regardless of PD-L1 expression; and pembrolizumab only for patients with PD-L1 tumor proportion score (TPS) ≥1%].
Camrelizumab (SHR-1210) is a novel humanized anti-PD-1 IgG4 monoclonal antibody. In June 2020, camrelizumab in combination with chemotherapy (carboplatin and pemetrexed) was approved for the treatment of chemotherapy-naive, advanced non-squamous NSCLC Chinese patients without EGFR and ALK alterations (11). A recent phase II study (NCT03085069) also demonstrated that camrelizumab monotherapy improved efficacy compared with historical data on second-line chemotherapy in pretreated advanced/metastatic NSCLC, and patients with positive PD-L1 expression derived greater benefit from camrelizumab (12).
Apatinib, a small-molecule tyrosine kinase inhibitor that strongly inhibits vascular endothelial growth factor receptor 2 (VEGFR2), has been approved as a third-line agent for the treatment of advanced gastric cancer in China (13). Currently, the combination regimens of anti-PD-1 antibodies plus molecular anti-angiogenic agents have been attracting great interest. A preclinical study has clearly demonstrated that apatinib alleviates hypoxia via modulating the tumor immune micro-environment, enhances tumoral infiltration of CD8+ T cells, and reduces recruitment of tumor-associated macrophages (14). Moreover, preliminary results from the phase Ib trial revealed that the combination of camrelizumab and apatinib, when administrated at the recommended phase II dose (RP2D) of apatinib (250 mg), showed encouraging antitumor activities with an acceptable safety profile in patients with advanced hepatocellular carcinoma or gastric cancer (15). Nevertheless, there are limited data available for the combination regimen of anti-PD-1 inhibitor and anti-angiogenic agents as second-line therapy for the treatment of patients with advanced NSCLC, especially in those with squamous NSCLC. Therefore, we reported the results of Cohort 3 from a phase II dose-expansion trial to explore the efficacy and safety of camrelizumab plus apatinib in advanced squamous NSCLC patients who had failed prior first-line platinum-based chemotherapy (immunotherapy naïve). We present the following article in accordance with the TREND reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4792/rc).
Methods
Study design and patients
This was a phase Ib/II, open-label, multicenter, multicohort study of camrelizumab in combination with apatinib in patients pretreated with NSCLC, conducted at 26 medical centers in China (ClinicalTrials.gov identifier: NCT03083041). This trial consisted of 2 phases: a phase Ib dose-escalation trial designed to assess the tolerability, safety, pharmacokinetics, and pharmacodynamics of camrelizumab in combination with apatinib and to determine the RP2D for apatinib; and a phase II dose-expansion trial to further evaluate the efficacy and safety of camrelizumab plus apatinib at the RP2D. In the current study, results involving patients with squamous NSCLC who received camrelizumab and apatinib at the RP2D (Cohort 3 of a phase II trial) as second-line therapy were reported.
Eligible patients had a histologically or cytologically confirmed diagnosis of advanced non-central squamous NSCLC (not arising from the main stem, and segmental bronchi based on the location of the primary lesion; stage IIIB-IV) and met the following inclusion criteria: (I) 18–70 years old; (II) disease progression after prior first-line platinum-based chemotherapy regimen; (III) at least 1 measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (IV) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (V) a life expectancy ≥12 weeks; (VI) adequate hematologic, hepatic, and renal function. The key exclusion criteria were as follows: (I) active or any history of autoimmune disease, or concurrent usage of immunosuppressive agents or immunosuppressive doses of systemic or local corticosteroids; (II) previously treated with any anti-PD-1/PD-L1 monoclonal antibody or apatinib; (III) newly diagnosed central nervous system metastases; (IV) major blood vessel invasion (16); (V) intratumor cavitation or necrosis; (VI) poorly controlled hypertension; (VII) clinically significant cardiovascular disease; (VIII) bleeding tendency or concurrent treatment with anticoagulation therapies; (IX) hemoptysis (more than 2.5 mL per day); or (X) pulmonary thrombosis, stroke, or deep venous thrombosis within the preceding 6 months. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines. The protocol and all amendments were approved by the institutional review board or independent ethics committee at each study site (see Table S1). All patients provided written informed consent.
Procedures
All participants received a fixed dose of camrelizumab (200 mg intravenously for 20 to 60 min once every 2 weeks) plus the RP2D of apatinib (250 mg once daily orally in 4-week cycles) established by a phase Ib study (17). Treatment with this combination regimen was continued until any of the following occurred, whichever occurred first: disease progression, intolerable toxicity, patient withdrawal, investigator withdrawal, or patients received camrelizumab for 2 years. Camrelizumab dose modification was not allowed and dose interruptions of camrelizumab no longer than 12 weeks were permitted. Dose interruption, dose reduction, modifications in dose frequency of apatinib (initial modification: 5 days on—2 days off; subsequent modification: 1 day on—1 day off) and dose discontinuation were allowed. Tumor responses, as assessed by investigators, were performed every 2 cycles (8 weeks) during the first 6 months of treatment, and every 3 cycles (12 weeks) thereafter according to RECIST version 1.1. Patients who had radiologically progressive disease (PD) were permitted to continue the study treatment at the investigator’s discretion that patients could benefit from, and were tolerant to, further study treatment. Patients who discontinued study treatment for any reason other than confirmed radiographic disease progression were followed up every 3 months for tumor radiological assessment until documented disease progression, start of a new anticancer treatment, or death. Participants were followed up every 2 months after study treatment discontinuation to evaluate survival. The expression of PD-L1 was centrally assessed using immunohistochemistry (IHC) with the PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies, Santa Clara, CA, USA), using archived or fresh tumor tissues harvested prior to study treatment. The expression level of PD-L1 was determined using TPS, which was defined as the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. Specimens were considered PD-L1 positive if the TPS ≥1% (18).
Data on adverse events (AEs) were collected, coded based on the Medical Dictionary for Regulatory Activities Version 23.1, and graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Participants were monitored for AEs until 30 days after the last dose and serious AEs until 90 days after the last dose of camrelizumab or 30 days after the last dose of apatinib, whichever occurred later.
Endpoints
The primary endpoint was objective response rate (ORR) per RECIST version 1.1, defined as the percentage of patients who had a confirmed complete response (CR) or partial response (PR) as the best overall response based on investigators’ assessment. The secondary endpoints of this study included clinical benefit rate [CBR; defined as the proportion of patients with CR or PR, or with stable disease (SD) ≥24 weeks], disease control rate (DCR; defined as proportion of patients who had a CR, PR, or SD as best overall response), duration of response (DoR; defined as time from first documented evidence of CR or PR until disease progression or death due to any cause, whichever occurred first), progression-free survival (PFS; defined as time from the first dose of study treatment to the first documented disease progression per RECIST version 1.1 or death due to any cause, whichever occurred first), OS (defined as time from the first dose of study treatment to death due to any cause), 12-month OS rate, and safety profile. Exploratory endpoints included the correlation between PD-L1 expression and efficacy.
Statistical analysis
Assuming an ORR of 30% and a dropout rate of 20%, a total of 38 patients were required to ensure the 90% confidence interval (CI) for ORR would have an interval width of 0.30.
All patients who received at least 1 dose of study treatment were included in the full analysis set, and those who received at least 1 dose of study treatment and had safety data after dosage administration were included in the safety analysis. The ORR, CBR, and DCR were calculated, and the corresponding 2-sided 95% CIs were also calculated using the Clopper-Pearson method. Median and range of time to response (TTR) were calculated. Median DoR, PFS, and OS were estimated via the Kaplan-Meier method, corresponding 95% CIs were calculated using the Brookmeyer and Crowley method, the 12-month OS rate was also estimated using the Kaplan-Meier method, and 95% CI was calculated using the log-log transformation according to the normal approximation with back transformation to CIs on the untransformed scale. The statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
Demographics and baseline characteristics
Cohort 3 was prematurely terminated on account of slow accrual on 28 February 2019. Between 10 October 2018 and 3 March 2019, a total of 25 patients with squamous NSCLC who received intravenous camrelizumab 200 mg every 2 weeks plus oral apatinib 250 mg once daily as second-line treatment were enrolled into Cohort 3 of the dose-expansion phase II trial (Figure 1).
All participants had failed their prior first-line platinum-based chemotherapy and had received at least 1 dose of camrelizumab or apatinib. The median age was 63.0 (range, 39.0 to 69.0) years. The majority of enrolled patients were male (n=23, 92.0%), had an ECOG performance status of 1 (n=24, 96.0%), had stage IV non-central squamous NSCLC (n=23, 92.0%), and were current or former smokers (n=23, 92.0%). In addition, there were 13 (52.0%) patients with PD-L1 TPS ≥1% and 11 (44.0%) patients with PD-L1 TPS <1%. Demographics and baseline characteristics of participants are shown in Table 1.
Table 1
| Characteristics | Total (n=25) |
|---|---|
| Age, years, median (range) | 63.0 (39.0–69.0) |
| Age category | |
| <65 years | 15 (60.0) |
| ≥65 years | 10 (40.0) |
| Male, n (%) | 23 (92.0) |
| ECOG performance status, n (%) | |
| 0 | 1 (4.0) |
| 1 | 24 (96.0) |
| Disease stage, n (%) | |
| IIIB | 2 (8.0) |
| IV | 23 (92.0) |
| Smoking status, n (%) | |
| Never smoked | 2 (8.0) |
| Current or former smoker | 23 (92.0) |
| No. of organs with metastasis, n (%) | |
| ≤2 | 22 (88.0) |
| >2 | 3 (12.0) |
| Liver metastases | 4 (16.0) |
| Brain metastases | 1 (4.0) |
| PD-L1 TPS, n (%) | |
| <1% | 11 (44.0) |
| ≥1% | 13 (52.0) |
| Not available | 1 (4.0) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
At the time of date cutoff (12 June 2020), the median follow-up was 13.3 (range, 1.6 to 19.2) months. Among all 25 participants, 2 (8.0%) patients were still receiving study treatment at the time of analysis. The reasons for discontinuing study treatment were as follows: disease progression (radiographic or clinical, n=14, 56.0%), AEs (n=4, 16.0%), death (n=3, 12.0%), and patient withdrawal (n=2, 8.0%).
Efficacy
As the time of date cutoff, 25 participants were included in the full analysis set. Best change in sum of target lesion from baseline per patient are presented in Figure 2A, and the magnitude was reduced in most participants. As shown in Table 2, 8 (32.0%) participants achieved PR as their best response, 13 (52.0%) participants had SD, 1 (4.0%) participant had PD, and the overall responses of 3 (12.0%) participants were not evaluable. The ORR assessed by investigators as per RECIST version 1.1 was 32.0% (95% CI: 14.9% to 53.5%). The CBR was 44.0% (95% CI: 24.4% to 65.1%), and the DCR was 84.0% (95% CI: 63.9% to 95.5%). In addition, the ORR was 38.5% (95% CI: 13.9% to 68.4%) in PD-L1 TPS ≥1% patients and 18.2% (95% CI: 2.3% to 51.8%) in TPS <1% patients (Table S2).
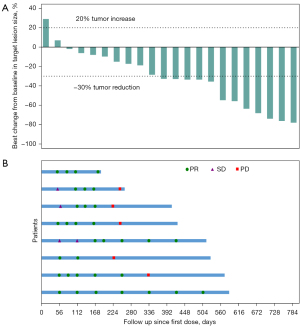
Table 2
| Variables | Total (n=25) |
|---|---|
| Best overall response, n (%) | |
| CR | 0 |
| PR | 8 (32.0) |
| SD | 13 (52.0) |
| PD | 1 (4.0) |
| NE | 3 (12.0) |
| ORR, n (%) (95% CI) | 8 (32.0) (14.9–53.5) |
| CBR (CR/PR/SD ≥24 weeks), n (%) (95% CI) | 11 (44.0) (24.4–65.1) |
| DCR, n (%) (95% CI) | 21 (84.0) (63.9–95.5) |
| PFS, months, median (95% CI) | 6.0 (3.5–8.1) |
| OS, months, median (95% CI) | 13.3 (6.4–18.8) |
| 12-month OS rate, % (95% CI) | 56.0 (34.8–72.7) |
| TTR, months, median (range) | 1.9 (1.6–5.5) |
| DOR, months, median (95% CI) | 6.0 (3.7–NR) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; CBR, clinical benefit rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; TTR, time to response; DOR, duration of response; CI, confidence interval; NR, not reached.
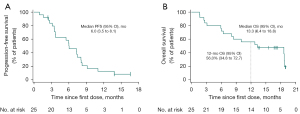
Tumor responses since the first dose of study treatment are shown in Figure 2B, suggesting that the decreased tumor burden could be sustained over several assessments. Response occurred at a median of 1.9 (range, 1.6 to 5.5) months, and the median DoR was 6.0 [95% CI: 3.7–not reached (NR)] months. A total of 22 (88.0%) participants experienced PFS events (documented progressive disease or death), and the median PFS was 6.0 (95% CI: 3.5 to 8.1) months (Figure 3A). As of the cutoff date, 16 (64.0%) participants had died. The median OS was 13.3 (95% CI: 6.4 to 18.8) months (Figure 3B), and the estimated 12-month OS rate was 56.0% (95% CI: 34.8% to 72.7%).
In exploratory analysis, the median PFS was 6.0 (95% CI: 3.5 to 7.5) months in participants with PD-L1 TPS ≥1%, and 5.5 months (95% CI: 2.5 to 8.1) in participants with PD-L1 TPS <1% (Figure S1A). The median OS was 10.1 (95% CI: 5.9–NR) months in participants with PD-L1 TPS ≥1% and 18.7 (95% CI: 2.5–NR) months in participants with TPS <1% (Figure S1B).
Safety
All the 25 participants were included in the safety analysis. The median duration wof camrelizumab exposure was 6.4 (range, 0.9 to 19.6) months, and the median duration of apatinib exposure was 5.5 (range, 0.9 to 19.2) months. Among the 25 participants, 5 (20.0%) had treatment-related adverse events (TRAEs) leading to any treatment discontinuation, 19 (76.0%) had TRAEs leading to any treatment interruption, and 9 (36.0%) had TRAEs leading to apatinib reduction. As illustrated in Table 3, all participants experienced at least 1 TRAE, and the most common TRAEs of any grade were hypertension (72.0%), proteinuria (72.0%), and increased aspartate aminotransferase (AST; 48.0%). The most common ≥ grade 3 TRAEs were hypertension (44.0%) and palmar-plantar erythrodysesthesia (16.0%). Treatment-related serious adverse events (SAEs) were reported in 12 (48.0%) participants, and the most common SAEs were interstitial lung disease [n=2 (8.0%)] and immune-mediated pneumonia [n=2 (8.0%)] (Table S3).
Table 3
| TRAEs | Total (N=25), n (%) | |
|---|---|---|
| Any grade | Grade 3–5 | |
| Any TRAE | 25 (100.0) | 21 (84.0) |
| Hypertension | 18 (72.0) | 11 (44.0) |
| Proteinuria | 18 (72.0) | 2 (8.0) |
| Aspartate aminotransferase increased | 12 (48.0) | 1 (4.0) |
| Palmar-plantar erythrodysesthesia | 10 (40.0) | 4 (16.0) |
| White blood cell count decreased | 10 (40.0) | 2 (8.0) |
| Neutrophil count decreased | 10 (40.0) | 2 (8.0) |
| Alanine aminotransferase increased | 8 (32.0) | 1 (4.0) |
| Anemia | 8 (32.0) | 0 |
| Blood thyroid stimulating hormone increased | 6 (24.0) | 0 |
| Platelet count decreased | 5 (20.0) | 2 (8.0) |
| Asthenia | 5 (20.0) | 2 (8.0) |
| Urinary occult blood positive | 5 (20.0) | 0 |
| Rash | 5 (20.0) | 0 |
| Decreased appetite | 5 (20.0) | 0 |
| Mouth ulceration | 4 (16.0) | 2 (8.0) |
| Diarrhea | 4 (16.0) | 0 |
| Dysphonia | 4 (16.0) | 0 |
| Hypothyroidism | 4 (16.0) | 0 |
| Lymphocyte count decreased | 3 (12.0) | 1 (4.0) |
| Occult blood positive | 3 (12.0) | 0 |
| Blood bilirubin increased | 3 (12.0) | 0 |
| Pruritus | 3 (12.0) | 0 |
| Hypochloremia | 3 (12.0) | 2 (8.0) |
| Hypertriglyceridemia | 3 (12.0) | 0 |
| Pyrexia | 3 (12.0) | 0 |
TRAE, treatment-related adverse event.
As reported by the investigators, 3 participants died due to TRAEs. Of these, 2 deaths occurred because of AEs possibly related to study treatment, and the causality of 1 death was judged as not assessable. A participant (male, 69 years old), who had a previous history of pulmonary infection and pneumothorax, died of interstitial pneumonia 96 days after starting the study treatment; another participant (male, 44 years old) with acquired tracheoesophageal fistula experienced stent loss, which might have subsequently induced life-threatening hemorrhage; the other participant (male, 61 years old) experienced dyspnea during the treatment period, and finally died due to unknown reasons, mainly because he and his family members refused further examination and hospitalization treatment (Table S4).
Immune-mediated AEs regardless of attribution to study treatment occurred in 18 (72.0%) participants, with increased alanine aminotransferase [ALT; n=4 (16.0%)], increased AST [n=4 (16.0%)], increased blood thyroid stimulating hormone [n=4 (16.0%)], and asthenia [n=4 (16.0%)] as the most common events (Table S5).
Discussion
The efficacy of PD-1/PD-L1 monotherapy in advanced pretreated NSCLC patients is modest (6,7,9,19). Therefore, alternative therapies are needed. In recent years, combination regimens of anti-PD-1 antibodies plus molecular antiangiogenic agents have indicated promising antitumor activities and acceptable safety profiles in the treatment of multiple solid tumors (15,20-22). Nevertheless, few clinical trials have focused on this combination regimen as a second-line therapy in patients with squamous NSCLC. To our knowledge, this was the first phase II trial investigating a combination strategy of anti-PD-1/PD-L1 antibody (camrelizumab) plus anti-angiogenic agent (apatinib) as a second-line treatment in patients with advanced squamous NSCLC.
Several studies have demonstrated that PD-1/PD-L1 monotherapy (nivolumab, pembrolizumab, and atezolizumab) as second or later-line therapy improves the antitumor effects in patients with NSCLC, including both squamous and non-squamous NSCLC, with an ORR of 14.0–20.0% (6,7,9,19,23,24). Although direct comparisons might be challenging due to different study designs and population enrichment across trials, the ORR with this combination regimen as a second-line treatment was 32.0% in squamous NSCLC patients, which is close to the reported rate for the same regimen in patients with advanced non-squamous NSCLC previously treated with chemotherapy (30.9%) (21), and numerically higher than the rate achieved with camrelizumab monotherapy in patients with previously treated advanced and/or metastatic squamous cell carcinoma (25.8%) (12). Overall, 20 (80.0%) participants achieved a shrinkage of their target lesions from baseline. In our phase II trial, all enrolled patients had failed prior first-line platinum-based chemotherapy, and the efficacy of the further available treatment regimen (PD-1/PD-L1 monotherapy or chemotherapy alone) might have been insufficient. Successful disease control might be crucial for this patient population, as disease progression can be rapid. This combination regimen also achieved an impressive DCR of 84.0%, and the CBR with this combination regimen was 44.0%, indicating that a high percentage of patients obtained sustained disease control.
Additionally, survival benefits with camrelizumab plus apatinib as a second-line therapy indicated clinically relevant survival times (median PFS, 6.0 months; median OS, 13.3 months), which were numerically longer than those observed with nivolumab monotherapy in patients with advanced, previously treated squamous-cell NSCLC (median PFS, 3.5 months; median OS, 9.2 months) (19). Our survival data highlighted that this combination regimen might have its place in the setting of squamous NSCLC, providing some support for ongoing and future clinical trials on combination regimens of anti-PD-1 inhibitor and anti-angiogenic agents for the treatment of advanced NSCLC (both squamous and non-squamous NSCLC).
Phase III trials consistently show that enriched benefits of these anti-PD-1/PD-L1 agents as second-line treatment can be achieved in NSCLC patients with higher PD-L1 expression compared with those with less or no PD-L1 expression (6,7,9,19,23,24). In our preliminary study, this combination regimen was shown to improve ORRs and PFS in both patients with PD-L1 TPS ≥1% and TPS <1%. Despite patients with PD-L1 TPS ≥1% achieving numerically higher ORRs and longer median PFS than patients with PD-L1 TPS <1%, only 13 (52.0%) and 3 (12.0%) patients harbored PD-L1 TPS ≥1% and TPS ≥50%, respectively. Therefore, this finding might have resulted from bias introduced by small sizes in these subgroups and precludes a definitive conclusion from being drawn.
Hemoptysis restricts the application of anti-angiogenic agents in the context of lung squamous cell carcinoma. However, no patient with this combination regimen in our phase II trial experienced grade 3 or higher hemoptysis, thus providing a basis for the further exploration of this combination regimen in NSCLC patients. The safety profile indicated no unanticipated or unexpected AEs, which mirrors the results on individual camrelizumab or apatinib monotherapy (11,25). Increased AST and ALT were likely related to camrelizumab (11), while the occurrence of hypertension, proteinuria, and palmar-plantar erythrodysesthesia were probably associated with apatinib (25). We found that the incidences of grade 3 or higher hypertension, proteinuria, and palmar-plantar erythrodysesthesia were slightly higher than those observed in apatinib monotherapy (25), which might be attributed to this combination regimen. This combination regimen might also result in increased occurrences of common hematotoxicity (decreased platelet count, decreased neutrophil count, and decreased white blood cell count) and asthenia, which could be explained partially by the overlapped AEs spectrums from either study treatment (camrelizumab or apatinib).
Interestingly, reactive cutaneous capillary endothelial proliferation (RCCEP), a common skin AE induced by camrelizumab, occurred in only 2 (8.0%) patients with this combination regimen, and all these RCCEPs were grade 1 or 2, which was considerably reduced compared with treatment with camrelizumab monotherapy (74.0%) (26) or camrelizumab plus chemotherapy (77.6%) (27). Our results indicated that RCCEP incidence was decreased when combined with apatinib, which is also in line with findings obtained regarding multiple solid tumors, suggesting that that the VEGFA/VEGFR-2 signaling pathway may be involved in the pathogenesis of RCCEP (15,21). As reported by the investigators, 3 participants died due to TRAEs. Of these, 2 deaths occurred because of AEs possibly related to study treatment, and the causality of 1 death was judged as not assessable. One patient died of interstitial pneumonia 96 days after starting the study treatment, which is similar to the previously reported studies of camrelizumab (15,28); another patient with acquired tracheoesophageal fistula experienced stent loss, which induced life-threatening hemorrhage; the other patient experienced dyspnea during the treatment period and finally died for unknown reasons, most likely because he and his family members refused further examination and hospitalization treatment.
The current study has several limitations. First, our results demonstrated the antitumor activity of camrelizumab plus apatinib as second-line therapy, but the trial was limited by its single-arm phase II design, with no PD-1/PD-L1 monotherapy or chemotherapy arm as a control. Second, Cohort 3 was prematurely terminated due to slow accrual after 25 patients had been enrolled, and the planned sample size of this cohort (38 patients) was not achieved. As we only enrolled immunotherapy naïve patients with squamous NSCLC, and nivolumab has been approved for second-line treatment of NSCLC in China since June 2018, the enrollment rate of patients in Cohort 3 was below expectation. Fortunately, the dropout rate of Cohort 3 was not high, and the ORR with this combination regimen achieved 32.0% in 25 patients, with the lower limit for 90% CI being 17.0%, which was higher than the prespecified lower limit of the 90% CI (15.0%). Third, potential bias might have been present regarding investigator assessment of the ORR and PFS.
In summary, Cohort 3 from a phase II dose-expansion trial demonstrated that camrelizumab in combination with apatinib as second-line therapy shows satisfactory antitumor activity in patients with non-central squamous NSCLC, regardless of tumor PD-L1 expression. Camrelizumab plus apatinib have a manageable safety profile in this patient population, and the toxic reactions observed were generally consistent with those in previously reported studies. Interstitial pneumonia and hemorrhage are important risks requiring careful monitoring and prompt intervention. A phase III randomized trial (NCT04203485) is currently underway to further assess the efficacy and safety of this combination regimen as the first-line therapy in NSCLC patients.
Acknowledgments
We thank the patients and their families and acknowledge the contributions of all investigators in this trial. We would like to acknowledge Lin Dong (PhD, Medical Writer, Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for medical writing support according to Good Publication Practice Guidelines. We would also like to thank AME Editing Service for the help in polishing our paper.
Funding: The study was sponsored by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4792/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4792/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4792/coif). Quanren Wang, Weixia Li, and Xinfeng Yang report that they are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guideline. The protocol and all amendments were approved by the institutional review board or independent ethics committee of each study site. All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii27-39. [Crossref] [PubMed]
- Lim SM, Hong MH, Kim HR. Immunotherapy for Non-small Cell Lung Cancer: Current Landscape and Future Perspectives. Immune Netw 2020;20:e10. [Crossref] [PubMed]
- Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64-70. [Crossref] [PubMed]
- Socinski MA, Obasaju C, Gandara D, et al. Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer. J Thorac Oncol 2018;13:165-83. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1-Positive, Advanced Non-Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol 2020;38:1580-90. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Fehrenbacher L, von Pawel J, Park K, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1156-70. [Crossref] [PubMed]
- Wu F, Gao G, Zhou C, et al. A phase III, randomized, open-label, multicenter study of SHR-1210 (anti-PD-1 antibody) in combination with pemetrexed and carboplatin as first line therapy in subjects with advanced/metastatic non-squamous non-small cell lung cancer. Ann Oncol 2018;29:viii545.
- Yang JJ, Huang C, Fan Y, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: a phase II study. Cancer Immunol Immunother ; Epub ahead of print. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res 2019;25:515-23. [Crossref] [PubMed]
- Reck M, Barlesi F, Crinò L, et al. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Ann Oncol 2012;23:1111-20. [Crossref] [PubMed]
- Zhou C, Gao G, Wu F, et al. A phase Ib study of SHR-1210 plus apatinib for heavily previously treated advanced non-squamous non-small cell lung cancer (NSCLC) patients. J Clin Oncol 2018; [Crossref]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Xu J, Shen J, Gu S, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res 2021;27:1003-11. [Crossref] [PubMed]
- Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Wu F, Zhang S, Xiong A, et al. A Phase II Clinical Trial of Apatinib in Pretreated Advanced Non-squamous Non-small-cell Lung Cancer. Clin Lung Cancer 2018;19:e831-42. [Crossref] [PubMed]
- Wu Y, Huang C, Fan Y, et al. A Phase II Umbrella Study of Camrelizumab in Different PD-L1 Expression Cohorts in Pre-Treated Advanced/Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:S128. [Crossref]
- Zhou C, Chen G, Huang Y, et al. A Randomized Phase 3 Study of Camrelizumab plus Chemotherapy as 1st Line Therapy for Advanced/Metastatic Non-Squamous Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:S215-6. [Crossref]
- Mo H, Huang J, Xu J, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer 2018;119:538-45. [Crossref] [PubMed]



