
Prognostic role of immune microenvironment in pleural metastases from breast and lung adenocarcinomas
Introduction
The pleural space, a mesothelium-lined cavity can be the site of many diseases, inflammatory, reactive, neoplastic, all often being manifested as a pleural effusion (1). Malignant pleural effusion is a frequent condition with 150,000 cases per year in USA (2). Among previously undiagnosed malignant pleural effusions, lung and breast cancer are the most common causes, accounting for almost 45% and 25% of the cases, while the primary counterpart, malignant mesothelioma (MM), is less frequent, about 12% (3). Malignant pleural effusion is associated with poor prognosis showing a median overall survival of 11 months (3). Apart from the histological type and the patient’s performance status, another factor found to be associated with poor prognosis in patients with malignant pleural effusion is the higher blood neutrophil-to-lymphocyte ratio (3), providing one of the first clues for the prognostic role of the patient’s immune system even in this advanced state of disease.
The importance of the immune system in the tumor microenvironment has been proven repeatedly in many forms of cancer, including MM (4). Several studies in MM, most using immunohistochemistry in tissue samples to search for innate and adaptive immune cells, showed that the immune microenvironment of pleural MM is mainly immune suppressive/tolerant (4). Regarding pleural metastatic disease, data are originate mostly from studies in pleural fluid studying CD4+, CD8+ cells, regulatory lymphocytes, tumor-associated macrophages (TAMs) (2,5-13), while studies in pleural tissue and with correlation to clinical data are largely lacking, especially in both small-cell and non-small-cell lung carcinomas, as the pleural metastasis microenvironment might be different from the primary tumor (14). Also, tumor microenvironment is important as novel molecular therapies target specifically its components and therefore its knowledge may totally transform the therapeutic strategy in these patients and their prognosis (14).
Thus, the aim of this study is to analyze the principal immune cell types in pleural metastases and to search for their prognostic role. The primary endpoint of the study was to investigate if the main immune cell populations are present in pleural metastases and if they have any prognostic role. Secondary endpoints are to detect any differences in their presence between lung and breast primaries and to search for any correlation with the macroscopic (thoracoscopic) findings. We present the following article in accordance with the REMARK reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6326/rc).
Methods
Study design—population
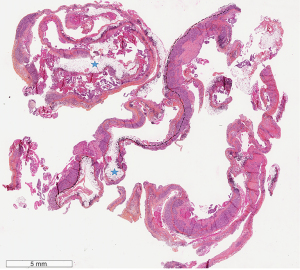
This is a monocentric retrospective study of patients diagnosed with pleural adenocarcinoma (ADC) metastasis from 01/2016 to 12/2018. Inclusion criteria included: (I) thoracoscopy biopsies to assure sufficient sample size; (II) biopsies which included the underlying adipose tissue of the parietal pleura to assure the tumor-host tissue interface (Figure 1); (III) lung or breast cancer metastasis (3 ovarian primaries, 3 metastases of unknown primary, 2 gastrointestinal primaries, 2 renal primaries, and 1 head and neck primary, all diagnosed during the same time period were excluded) for homogeneity reasons; (IV) at least 3 years of follow-up or until death. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Terre d’Ethique (IRBN582021/CHUSTE). Patients’ consent was waived by the institutional review board given the retrospective nature of the study and the anonymization of all data.
Patients’ characteristics are shown in Table 1. The study included 50 lung cancer and 20 breast cancer metastasis patients, with a median age of 71 (range, 36–91) years. Most patients (90%) were deceased of disease. Baseline clinicopathological characteristics, such as age, sex, tobacco history, pleural fluid macroscopic aspect at thoracentesis, macroscopic aspect of the pleura cavity during thoracoscopy, cytological diagnosis after thoracocentesis and histological diagnosis after thoracoscopy were retrieved from medical records.
Table 1
| Variable | Total (n=70, 100%) | Lung cancer (n=50, 71.4%) | Breast cancer (n=20, 28.6%) |
|---|---|---|---|
| Age, median [range] | 71 [36–91] | 70 [36–89] | 72 [55–91] |
| Sex, n (%) | |||
| Female | 36 (51.4) | 17 (34.0) | 19 (95.0) |
| Male | 34 (48.6) | 33 (66.0) | 1 (5.0) |
| Smoker, n (%) | |||
| Yes, current | 20 (28.6) | 16 (32.0) | 4 (20.0) |
| Yes, former | 22 (31.4) | 20 (40.0) | 2 (10.0) |
| No | 28 (40.0) | 14 (28.0) | 14 (70.0) |
| Pack years, median [range] | 30 [5–100] | 30 [5–100] | 30 [20–40] |
| Right pleural effusion, n (%) | 36 (51.4) | 26 (52.0) | 10 (50.0) |
| Left pleural effusion, n (%) | 27 (38.6) | 20 (40.0) | 7 (35.0) |
| Both sides, n (%) | 7 (10.0) | 4 (8.0) | 3 (15.0) |
| Hemorrhagic pleural fluid (n=68), n (%) | |||
| Yes | 41 (60.3) | 29 (58.0) | 12 (66.7) |
| No | 27 (39.7) | 21 (42.0) | 6 (33.3) |
| Purulent pleural fluid (n=68), n (%) | |||
| Yes | 8 (11.7) | 8 (16.0) | 0 |
| No | 60 (88.2) | 42 (84.0) | 18 (100.0) |
| Serous pleural fluid (n=68), n (%) | |||
| Yes | 27 (39.7) | 20 (40.0) | 7 (38.9) |
| No | 41 (60.3) | 30 (60.0) | 11 (61.1) |
| Cytology of pleural fluid (n=68), n (%) | |||
| Positive for malignancy | 52 (76.5) | 38 (77.6) | 14 (73.7) |
| Negative for malignancy | 16 (23.5) | 11 (22.4) | 5 (26.3) |
| Nodules in thoracoscopy* (n=67), n (%) | |||
| Yes | 55 (82.1) | 37 (75.5) | 18 (100.0) |
| No | 12 (18.9) | 12 (24.5) | 0 |
| Masses in thoracoscopy* (n=67), n (%) | |||
| Yes | 10 (14.9) | 9 (18.4) | 1 (5.6) |
| No | 57 (85.1) | 40 (81.6) | 17 (94.4) |
| Pachypleuritis in thoracoscopy (n=67), n (%) | |||
| Yes | 24 (35.8) | 23 (46.9) | 1 (5.6) |
| No | 43 (64.2) | 26 (53.1) | 17 (94.4) |
| Inflammation in thoracoscopy (n=67), n (%) | |||
| Yes | 17 (25.4) | 13 (26.5) | 4 (22.2) |
| No | 50 (74.6) | 36 (73.5) | 14 (77.8) |
| Patients state at one year, n (%) | |||
| Deceased | 51 (72.9) | 39 (78.0) | 12 (60.0) |
| Alive | 19 (27.1) | 11 (22.0) | 8 (40.0) |
| Patients state at two years, n (%) | |||
| Deceased | 58 (82.9) | 45 (90.0) | 13 (65.0) |
| Alive | 12 (17.1) | 5 (10.0) | 7 (35.0) |
| Patients state at three years, n (%) | |||
| Deceased | 63 (90.0) | 47 (94.0) | 16 (80.0) |
| Alive | 7 (10.0) | 3 (6.0) | 4 (20.0) |
*, nodules vs. masses using a 3 cm cut off. No statistically significant difference was found in patients’ age or pack years consumption between the two primaries according to the Mann Whitney U test (P=0.7 and P=0.9, respectively).
Immunohistochemistry
Immunohistochemistry was performed to evaluate the immune cell populations using the following markers: CD8 for cytotoxic T cells, CD4 for helper T cells, CD20 for B cells, CD163 for M2 macrophages, and S100 for dendritic cells. Whole-tumor tissue sections were studied for CD8 (C8/144B, Dako Agilent, Santa Clara, CA, USA, 1/100), CD4 (SP35, Abcam, Cambridge, UK, 1/50), CD20 (L26, Dako Agilent, 1/200), CD163 (10D6, Novocastra, Newcastle upon Tyne, UK, 1/200), S100 (Polyclonal, Dako Agilent, 1/2,500) using an automated staining system (OMNIS, Dako-Agilent) and the EnVision FLEX kit (OMNIS, Dako, Glostrup, Denmark) according to the manufacturer’s protocol.
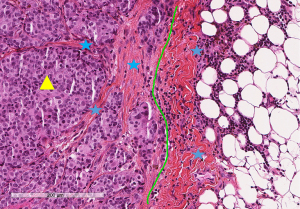
Immunohistochemical evaluation of each immune cell marker was recorded as a continuous variable in a semi-quantitative manner evaluating the percentage of area occupied by the immune cells in the tumor (% of intratumoral immune cells) and stroma (% of stromal immune cells) area, according to the proposed guidelines for solid tumors (15) which suggest that: “the denominator used to determine the % stromal immune cells is the area of stromal tissue (i.e., area occupied by inflammatory cells over total stromal area), not the number of stromal cells (i.e., fraction of total stromal nuclei that represent inflammatory cell nuclei). Similarly, for intra-tumoral immune cells the tumor cell area is the denominator” (15). The evaluation was performed by two pathologists until final agreement; the whole slide was studied with full assessment of the tumor area and its invasive margin (Figure 2) without focusing on hotspots, as suggested (15).
Statistical analysis
We used the Fisher’s exact test to explore any relationship between two groups for categorical data and the Mann Whitney U non-parametric test for the comparison of continuous variables. Survival probability was estimated by Kaplan-Meier analysis with log-rank product limit estimation. Survival was calculated from the diagnosis of pleural disease to death or to last follow-up. The cut off values for the Kaplan-Meier analysis were defined using the receiver operating characteristic (ROC) curves. Multivariate survival analysis was performed using the Cox proportional hazards model. For all analyses, statistical significance was indicated at a P value of <0.05. Data were analyzed using the StatView© software (version 5, Abacus Concepts, Berkley, CA, USA).
Results
Primary endpoints
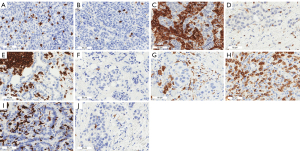
Immunohistochemical findings are shown in Table 2. The most prominent immune cell population in the stroma compartment of the whole cohort was CD4+ immune cells, followed by similar populations of CD20+ and CD163+ immune cells, CD8+ and S100+ immune cells. While CD4, CD8, CD163 and S100 were found in the stroma of the central tumor and its invasive margin, CD20 were predominantly found at the invasive margin. In the intratumoral compartment, CD4+ cells were also more prominent followed by S100+ cells, CD163+ cells, and CD8+ cells, while CD20+ cells in this compartment were virtually absent and no further comparisons were made for this population (Figure 3). Comparisons between the two primaries with the Mann Whitney U test are also shown in Table 2. Significant higher values for the stromal CD163 group (P=0.04) and for the intratumoral S100 group (P=0.006) were found in lung metastases in comparison to breast.
Table 2
| Variable | Total (n=70), mean [range] | Lung ADC (n=50), mean [range] | Breast ADC (n=20), mean [range] | P |
|---|---|---|---|---|
| CD8 % stromal | 15.19 [0–60] | 14.30 [0–60] | 17.40 [0–50] | 0.45 |
| CD8 % intratumoral | 3.20 [0–20] | 3.34 [0–20] | 2.85 [0–10] | 0.69 |
| CD4 % stromal | 41.14 [5–80] | 39.30 [5–80] | 45.75 [5–80] | 0.32 |
| CD4 % intratumoral | 12 [0–80] | 11.86 [0–80] | 12.35 [0–50] | 0.38 |
| CD20 % stromal | 18.57 [0–80] | 18.10 [0–80] | 19.75 [0–60] | 0.63 |
| CD20 % intratumoral | 0.13 [0–5] | 0.16 [0–5] | 0.05 [0–1] | 0.93 |
| CD163 % stromal | 18.49 [0–80] | 21.26 [0–80] | 11.55 [0–30] | 0.04 |
| CD163 % intratumoral | 5.43 [0–50] | 6.94 [0–50] | 1.65 [0–10] | 0.13 |
| S100 % stromal | 5.06 [0–50] | 5.96 [0–50] | 2.80 [0–10] | 0.11 |
| S100 % intratumoral | 6.76 [0–60] | 8.76 [0–60] | 1.75 [0–20] | 0.006 |
ADC, adenocarcinoma.
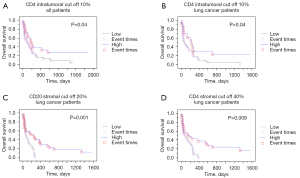
Median overall survival was 151 days. Only 9 (12.8%) patients (5 patients with breast and 4 with lung cancer) were still alive at the time of the analysis. For lung cancer patients’ median survival was 114 days while it was 205 days for breast cancer patients (P=0.041). Kaplan-Meier survival analysis results using with the ROC defined cut off values are shown in Table 3. The infiltration of CD8 and CD163 immune cells did not correlate with prognosis. Higher stromal and intratumoral CD4 counts, as well as higher stromal CD20 cells were positive prognostic factors for lung cancer metastases, while higher S100 intratumoral counts were positive prognostic factors in lung and marginally breast cancer metastases (Figures 4,5).
Table 3
| Variable | Total (n=70) | P1 | Lung cancer (n=50) | P2 | Breast cancer (n=20) | P3 |
|---|---|---|---|---|---|---|
| CD8 stromal ≤15 | 134 | 0.07 | 79 | 0.2 | 205 | 0.2 |
| CD8 stromal >15 | 169 | 132 | 201 | |||
| CD8 intratumoral ≤1 | 134 | 0.1 | 79 | 0.9 | 201 | 0.07 |
| CD8 intratumoral >1 | 171 | 49 | 782 | |||
| CD4 stromal ≤40 | 128 | 0.03 | 79 | 0.009 | 201 | 0.9 |
| CD4 stromal >40 | 235 | 169 | 346 | |||
| CD4 intratumoral ≤10 | 123 | 0.04 | 71 | 0.04 | 205 | 0.4 |
| CD4 intratumoral >10 | 229 | 215 | 503 | |||
| CD20 stromal ≤20 | 114 | 0.08 | 71 | 0.001 | 205 | 0.8 |
| CD20 stromal >20 | 234 | 235 | 204 | |||
| CD163 stromal ≤10 | 171 | 0.4 | 123 | 0.9 | 205 | 0.4 |
| CD163 stromal >10 | 128 | 47 | 128 | |||
| CD163 intratumoral ≤5 | 169 | 0.4 | 114 | 0.7 | 234 | 0.1 |
| CD163 intratumoral >5 | 128 | 71 | 128 | |||
| S100 stromal ≤3 | 123 | 0.02 | 47 | 0.0008 | 205 | 0.7 |
| S100 stromal >3 | 260 | 260 | 201 | |||
| S100 intratumoral ≤2 | 128 | 0.04 | 59 | 0.02 | 176 | 0.07 |
| S100 intratumoral >2 | 235 | 171 | Not reached |
Cut off values defined using ROC curves. P1, P value between median days referring in the Total column; P2, P value between median days referring in the Lung cancer column; P3, P value between median days referring in the Breast cancer column. ROC, receiver operating characteristic.
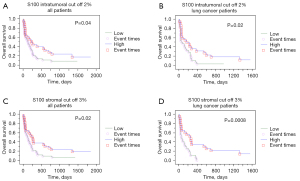
Multivariate survival analysis showed that factors retaining their negative prognostic role were for lung ADC (Table 4) low intratumoral CD4, S100 and stromal CD20 counts, while only low intratumoral CD4 for breast ADC (Table 5).
Table 4
| Variable | HR | CI | P |
|---|---|---|---|
| CD8 stromal ≤15 | 1.106 | 0.527–2.324 | 0.79 |
| CD8 intratumoral ≤1 | 0.643 | 0.282–1.463 | 0.29 |
| CD4 stromal ≤40 | 1.032 | 0.420–2.537 | 0.94 |
| CD4 intratumoral ≤10 | 2.380 | 1.006–5.626 | 0.048 |
| CD20 stromal ≤20 | 2.228 | 1.005–4.936 | 0.048 |
| CD163 stromal ≤10 | 0.623 | 0.308–1.262 | 0.18 |
| CD163 intratumoral ≤5 | 0.944 | 0.435–2.046 | 0.88 |
| S100 stromal ≤3 | 1.817 | 0.798–4.136 | 0.15 |
| S100 intratumoral ≤2 | 1.45 | 0.602–3.486 | 0.040 |
HR, hazard ratio, CI, confidence interval.
Table 5
| Variable | HR | CI | P |
|---|---|---|---|
| CD8 stromal ≤15 | 0.231 | 0.40–1.332 | 0.10 |
| CD8 intratumoral ≤1 | 5.47 | 0.660–45.35 | 0.11 |
| CD4 stromal ≤40 | 0.266 | 0.470–1.511 | 0.13 |
| CD4 intratumoral ≤10 | 29.72 | 1.032–856.3 | 0.047 |
| CD20 stromal ≤20 | 6.829 | 0.794–58.73 | 0.080 |
| CD163 stromal ≤10 | 0.15 | 0.017–1.321 | 0.087 |
| CD163 intratumoral ≤5 | 0.548 | 0.21–14.55 | 0.71 |
| S100 stromal ≤3 | 0.550 | 0.116–2.596 | 0.44 |
| S100 intratumoral ≤2 | 14.76 | 0.986–221.01 | 0.51 |
HR, hazard ratio; CI, confidence interval.
Secondary endpoints
Regarding the thoracoscopic intrapleural findings, the presence of nodules was not associated with the degree of immune cells infiltration except for CD20 stromal infiltration (P=0.02, higher in the presence of nodules, 42.092 vs. 26.538). The presence of masses was negatively associated with CD8 stromal (P=0.02, higher in the absence of masses 42.598 vs. 26.808) and CD20 stromal infiltration (P=0.003, higher in the absence of masses 42.854 vs. 22.731) and positively with CD163 stromal infiltration (Mann Whitney test, P=0.01, higher in the presence of masses 53.192 vs. 36.762). The thoracoscopic aspect of pachypleuritis was also negatively associated with CD20 stromal infiltration (P=0.02 higher in the absence of pachypleuritis 43.890 vs. 31.661); it was positively associated with CD163 stromal infiltration (P=0.02, higher in the presence of pachypleuritis 47.411 vs. 35.070). The macroscopic aspect of an inflammatory pleura was associated only with S100 intratumoral infiltration (P=0.02 higher in the absence of an inflammatory aspect 42.661 vs. 29.684). The thoracoscopic aspect showed statistically significant difference between the two primaries only for the presence of pachypleuritis (Fisher’s P=0.001) which was more frequent in lung cancer primary. The macroscopic aspect was not associated with prognosis; only a strong tendency of P=0.07 was seen for nodules: median survival 176 vs. 49 days for the presence vs. the absence of nodules.
Discussion
This is the first study in pleural metastases tissues examining the principal immune cell populations and comparing them with the clinical characteristics. Primarily, we show that CD4+ T cells, CD8+ T cells, CD20+ B cells, CD163+ macrophages and S100+ dendritic cells are all present in this tissue, suggesting that they represent a potential therapeutic target. Furthermore, higher CD4+, CD20+ and S100+ cells are positive prognostic factors highlighting that even in this advanced tumor context there still are important players in the tumor immune microenvironment. The prognostic significance of CD4+ cells and S100+ cells was retained in multivariate analysis adjusting for the histological type and other immune cells populations, further reinforcing the role of these populations. Also, we show that the two compartments, intratumoral and stromal, behave differently in terms of immune cell counts and prognostic significance, reinforcing the notion that they need to be differentiated when evaluating tumor-infiltrating immune cells (15), and highlighting the necessity of tissue specimens in this context. When comparing lung to breast cancer metastases, we noted higher CD163 and S100 counts, however, the rest of the immune cells’ populations did not differ significantly. These findings suggest that the influx of T and B cells is probably a characteristic of the pleural cavity itself not depending on metastasis’ characteristics, whereas M2 macrophages and dendritic cells probably depend on primary tumors characteristics, such as its antigenicity (4). In previous studies, B cells have been rarely evaluated compared to T cells but suggest a positive prognostic role in MM (16). Our study confirms this role of B cells in pleural metastases. The positive prognostic significance of B cells could be attributed to their role in humoral immunity and/or their antigen-presenting role. As for dendritic cells, these are also rarely studied in pleural malignancy. In our study, despite the dendritic cells’ relative limited number, they are strong prognostic factors.
This is also the first study associating the thoracoscopic macroscopic findings with these markers, probably reflecting a change in the immune cells counts with the macroscopic extent of the disease, since the presence of masses was correlated with lower counts of cytotoxic T cells and B cells, but higher M2 macrophages. This decrease of anti-tumor and the increase of pro-tumor immune cells in more advanced local disease further supports the role of these cells in locally controlling the disease. In a study with thoracoscopic evaluation of tumor burden in relation to the presence of adhesions, Bielsa and collaborators (17) found that the higher the grade of pleural adhesions was, the greater the tumor burden existed, and the presence of pleural adhesions implied a poor prognosis. In another thoracoscopic study, the authors (18) found that mixed pleural endoscopic findings (nodules, masses, pachypleuritis, inflammation) was related to visceral pleural invasion (P=0.015), which was the only predictor of pleural invasion and positive cytology (P<0.001). The findings of our study regarding the differences observed in the microenvironment of the intrapleural various modes of tumor invasion/aspects open new horizon to explain the complicated mechanisms of pleural invasion (19,20).
Despite not finding sufficient in situ studies on tissues from pleural metastases, studies on pleural fluid regarding immune cells do exist. These studies mainly focused on T cells, many of them on the subset of T regulatory cells, with only rare reports for other cell types. Two studies (5,6) evaluating the counts of CD4+ and CD8+ cells, the first (5) in 9 pleural and 10 peritoneal metastatic effusions, the second (6) in 6 mesotheliomas, 9 lung carcinomas and 7 tuberculosis pleural, showed higher CD4+ cells counts and unchanged CD8+ counts for all these diseases compared to peripheral blood (5,6), while no differences were seen between the three disease groups (6). Interestingly, in two studies, one (7) with metastatic pleural effusions from variable primaries, and another from lung ADCs (8), T cells were mostly naive or of central memory, and not of effector type, thus suggesting a milieu of immune system escape in this tumor compartment. It seems that there is a tendency for metastatic pleural fluid to have higher levels of CD4+ cells compared to MM, and less important defect in recruiting CD8+ in the pleural cavity (2). Nieto and associates (21) showed that T cells are rather migrating than proliferating in the pleural cavity of lung cancer patients. Although in our study CD163 was not found to have a prognostic role, in a previous study CD163 higher counts in pleural fluid were associated with worse prognosis in 30 patients with lung cancer malignant pleural effusion (11). It is important noting that pleural fluid counts do not always match pleural tissue counts in paired specimens (22), suggesting that the pathophysiology of the pleura cavity is dynamic and differences may exist between parietal tissue, pleural space, and visceral tissue (18). The prognostic role of immune cells in pleural fluid is also rarely reported in these studies; their main finding is the greater CD4 than CD8 influx, which we also confirm on tissue. We further reported the important presence of other cell types but also the prognostic role of each population.
In contrast to the pleural metastatic disease, there is larger literature with MM tissues investigating immune microenvironment. Mesothelioma is infiltrated by immune effector cells but also contains cytokines and regulatory T-cells that suppress an efficient immune response (23). In a study (22) of tissues from 33 pleural MMs but only 5 pleural metastases, and pleural fluids from 49 MM and 32 metastases, CD4+ T cells showed the same counts in all samples, while CD8+ T cells were low in pleuritis samples and high in malignancies when examining the pleural fluid, but high in pleuritis and low in malignancies when examining the pleural tissues (22). Regulatory CD4+CD25+ cells were higher in MM fluid and tissue compared to metastases (22). B cells were rare in fluid and tissue (22), which is in contrast with our results. M2 macrophages were higher in MM and metastases compared to pleuritis (22). Prognostic information is provided only for MM patients: the counts of immune cells in pleural fluid did not reveal prognostic significance; their counts in tissue showed no significance for CD8+ T cells and M2 macrophages, similar to the current cohort of pleural metastases, but a negative role for high T regulatory cells (22). In a tissue microarray study of 230 epithelioid MM, immune cells were studied by immunohistochemistry and counted in a semi-quantitative manner, revealing that high intratumoral CD4+ and CD20+ cells were associated with better survival, with CD20 retaining its role in multivariate analysis (16), similarly to our cohort. CD4+ cells were also a positive prognostic factor in another study of 54 MM studied by immunohistochemistry (24). Two large studies (25,26), with immunohistochemistry in microarrays from MM tissues showed that low CD4 cells and high CD8 stromal cells were poor prognostic factors, high CD20 cells and low CD68 cells were positive prognostic factors (26), while B cells had no prognostic significance in another study (25). In 52 MM tissues, CD68 staining comprised 27% of the tumor area, and was negatively correlated with survival in non-epithelioid only tumors (27). Thus, our findings are generally in line with MM tissue studies revealing that CD4 and CD20 are positive prognostic factors, while CD8 and macrophages usually do not reveal prognostic significance.
Indeed, a recent analysis of primary lung ADCs and their precursors (28), showed that CD4+ cells increased, while CD8+ cells decreased when moving from normal tissues to ADC precursors and finally invasive lesions. CD8+ T cells are generally considered cytotoxic, however the functions and phenotypes of CD4+ T cells are more heterogenous; there are T helper cells which promote other immune cells and are antitumoral, but also T regulatory cells, which are immune suppressive, favoring tumors (28). Given that most previous studies in pleural fluid show low levels of T regulatory cells and the fact that the current as well as other studies reveal a positive prognostic role for CD4+ cells, it is very probable that the majority of CD4+ cells detected correspond to T helper cells boosting anti-tumoral activity.
The main limitation of our study is the retrospective character, and especially the possible impact in prognosis of factors such as performance status, number of metastatic sites and treatments. However, patients with pleural metastatic disease have a poor overall median survival very few affected by the different treatments applied (29,30). Also, despite applying a broad spectrum of immune cell markers, this is far from being an exhaustive list and further research should focus on prospective evaluation including these markers comparing pleural biopsies to effusions, to better define the place of each in the process of therapeutic targeting.
To conclude, this is the first study investigating immune cell populations in the metastatic pleural cavity, taking advantage of whole tissue sections from large thoracoscopic specimens, and providing association with clinical characteristics. Our findings show that the immune microenvironment may be important in this advanced tumoral setting and that possible targets of the nowadays numerous treatment strategies implicating the immune system may merit further exploration in this poor prognosis disease.
Acknowledgments
The authors would like to thank Philippe Cosmo from the Tumorothèque/Centre de Ressources Biologiques de CHU Saint-Etienne (BRIF No. BB-0033-00041), as well as Christophe Bruyere for his excellent technical assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6326/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6326/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6326/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Terre d’Ethique (IRBN582021/CHUSTE). Patients’ consent was waived by the institutional review board given the retrospective nature of the study and the anonymization of all data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karpathiou G, Stefanou D, Froudarakis ME. Pleural neoplastic pathology. Respir Med 2015;109:931-43. [Crossref] [PubMed]
- Scherpereel A, Grigoriu BD, Noppen M, et al. Defect in recruiting effector memory CD8+ T-cells in malignant pleural effusions compared to normal pleural fluid. BMC Cancer 2013;13:324. [Crossref] [PubMed]
- Anevlavis S, Kouliatsis G, Sotiriou I, et al. Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration 2014;87:311-6. [Crossref] [PubMed]
- Désage AL, Karpathiou G, Peoc’h M, et al. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers (Basel) 2021;13:3205. [Crossref] [PubMed]
- Robinson E, Segal R, Vesely Z, et al. Lymphocyte subpopulations in peripheral blood and malignant effusions of cancer patients. Eur J Cancer Clin Oncol 1986;22:191-3. [Crossref] [PubMed]
- Lucivero G, Pierucci G, Bonomo L. Lymphocyte subsets in peripheral blood and pleural fluid. Eur Respir J 1988;1:337-40. [PubMed]
- Atanackovic D, Block A, de Weerth A, et al. Characterization of effusion-infiltrating T cells: benign versus malignant effusions. Clin Cancer Res 2004;10:2600-8. [Crossref] [PubMed]
- Prado-Garcia H, Aguilar-Cazares D, Flores-Vergara H, et al. Effector, memory and naïve CD8+ T cells in peripheral blood and pleural effusion from lung adenocarcinoma patients. Lung Cancer 2005;47:361-71. [Crossref] [PubMed]
- Chen YQ, Shi HZ, Qin XJ, et al. CD4+CD25+ regulatory T lymphocytes in malignant pleural effusion. Am J Respir Crit Care Med 2005;172:1434-9. [Crossref] [PubMed]
- DeLong P, Carroll RG, Henry AC, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther 2005;4:342-6. [Crossref] [PubMed]
- Yang L, Wang F, Wang L, et al. CD163+ tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patients. Oncotarget 2015;6:10592-603. [Crossref] [PubMed]
- Budna J, Kaczmarek M, Kolecka-Bednarczyk A, et al. Enhanced Suppressive Activity of Regulatory T Cells in the Microenvironment of Malignant Pleural Effusions. J Immunol Res 2018;2018:9876014. [Crossref] [PubMed]
- Budna J, Spychalski Ł, Kaczmarek M, et al. Regulatory T cells in malignant pleural effusions subsequent to lung carcinoma and their impact on the course of the disease. Immunobiology 2017;222:499-505. [Crossref] [PubMed]
- Tissot C, Gay P, Brun C, et al. Novel insights into the systemic treatment of lung cancer malignant pleural effusion. Clin Respir J 2019;13:131-8. [Crossref] [PubMed]
- Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol 2017;24:235-51. [Crossref] [PubMed]
- Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015;4:e1009285. [Crossref] [PubMed]
- Bielsa S, Martín-Juan J, Porcel JM, et al. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008;3:1251-6. [Crossref] [PubMed]
- Froudarakis ME, Plojoux J, Kaspi E, et al. Positive pleural cytology is an indicator for visceral pleural invasion in metastatic pleural effusions. Clin Respir J 2018;12:1011-6. [Crossref] [PubMed]
- Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989;2:366-9. [PubMed]
- Karpathiou G, Mobarki M, Stachowicz ML, et al. Pericardial and Pleural Metastases: Clinical, Histologic, and Molecular Differences. Ann Thorac Surg 2018;106:872-9. [Crossref] [PubMed]
- Nieto JC, Zamora C, Porcel JM, et al. Migrated T lymphocytes into malignant pleural effusions: an indicator of good prognosis in lung adenocarcinoma patients. Sci Rep 2019;9:2996. [Crossref] [PubMed]
- Salaroglio IC, Kopecka J, Napoli F, et al. Potential Diagnostic and Prognostic Role of Microenvironment in Malignant Pleural Mesothelioma. J Thorac Oncol 2019;14:1458-71. [Crossref] [PubMed]
- Hegmans JP, Hemmes A, Hammad H, et al. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Respir J 2006;27:1086-95. [Crossref] [PubMed]
- Marcq E, Siozopoulou V, De Waele J, et al. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2017;6:e1261241. [Crossref] [PubMed]
- Fusco N, Vaira V, Righi I, et al. Characterization of the immune microenvironment in malignant pleural mesothelioma reveals prognostic subgroups of patients. Lung Cancer 2020;150:53-61. [Crossref] [PubMed]
- Chee SJ, Lopez M, Mellows T, et al. Evaluating the effect of immune cells on the outcome of patients with mesothelioma. Br J Cancer 2017;117:1341-8. [Crossref] [PubMed]
- Burt BM, Rodig SJ, Tilleman TR, et al. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 2011;117:5234-44. [Crossref] [PubMed]
- Dejima H, Hu X, Chen R, et al. Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat Commun 2021;12:2722. [Crossref] [PubMed]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018;52:1800349. [Crossref] [PubMed]
- Brun C, Gay P, Cottier M, et al. Comparison of cytology, chest computed and positron emission tomography findings in malignant pleural effusion from lung cancer. J Thorac Dis 2018;10:6903-11. [Crossref] [PubMed]


