
Melatonin exerts anti-angiogenic and anti-inflammatory effects in alkali-burned corneas
Introduction
The avascular status of the cornea is vital for light refraction and visual acuity (1). Corneal neovascularization (CNV) involves the formation of new vascular structures in the cornea and can reduce corneal transparency and lead to impaired vision (2-4). It is estimated that about 1.4 million people experience CNV each year in the United States (5); however, the available treatment options for CNV are limited. Thus, the development of effective measures to prevent CNV is necessary. Multiple studies have confirmed that inflammation is the core mechanism of CNV, and is caused by chemical injury, infection, immune diseases, and degenerative disorders (6-8). In particular, corneal injury leads to a rapid inflammatory response involving the elevation of pro-angiogenic factors. The imbalance of pro-angiogenic and anti-angiogenic factors ultimately leads to neovascularization. Inflammation and angiogenesis are intertwined, with each process influencing the other (9). Thus, inhibiting the inflammatory response has become a strategy for treating CNV (6,10,11).
After corneal injury, neutrophils and macrophages infiltrate the cornea. Compared with other inflammatory cells, macrophages are presumed to be an important source of pro-angiogenic molecules, and they can support vascular endothelial sprouting (9,12,13). Furthermore, recent studies have revealed that the development of CNV can be suppressed by inhibiting macrophage infiltration but not neutrophil infiltration (14,15). Therefore, understanding the regulatory mechanisms of macrophages is crucial to the treatment of pathologic neovascularization.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone mainly released by the pineal gland in a circadian rhythm. This neurohormone has been reported to have multiple biologic functions, such as maintaining circadian rhythmicity (16), participating in sexual maturation and gonadal development (17), regulating sleep (18) and immunity (19), inhibiting tumor progression (20), protecting against oxidative damage (21), and exerting anti-angiogenetic (22,23) and anti-inflammatory effects (24,25). Furthermore, melatonin receptors have been identified in many ocular structures, including the cornea, ciliary body, lens, retina, choroid, and sclera (17). This evidence indicates that melatonin may play a role in ocular physiology. Accumulating evidence shows that melatonin could potentiate lacrimation, accelerate corneal wound healing, regulate intraocular pressure, protect against cataracts, reduce retinal vascular leakage, and prevent retinal and choroidal vascular proliferation (26-30). However, the effects of melatonin on CNV have not yet been elucidated. In this study, we utilized alkali injury models to examine whether melatonin could suppress inflammation-induced CNV. Moreover, we investigated the role of melatonin regarding pro-angiogenic factors and inflammation-related molecules in vivo and in vitro macrophages. We present the following article in accordance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4927/rc).
Methods
Animals
Male C57BL/6 mice aged 6 to 8 weeks were purchased from the Animal Laboratory of the Zhongshan Ophthalmic Center (Guangzhou, China). All mice were reared in specific-pathogen-free (SPF) conditions, at 20–25 °C and 40–60% humidity, with a 12-hour light/12-hour dark cycle. Mice with abnormal corneas, including corneal ulcers, corneal scars, and corneal dysplasia, were excluded from this study. A total of 111 mice were randomly allocated to 3 groups: the normal group, the vehicle control group, and the melatonin-treated group. The normal group was defined as wild type mice without any injury. The corneal injury model was induced by sodium hydroxide (NaOH). In the vehicle control group, mice with corneal injury were treated with phosphate-buffered saline (PBS). In the melatonin-treated group, mice with corneal injury were treated with melatonin. During the different stages of the experiment, the researchers (Huang and Meng) were aware of the group allocation. A protocol was prepared before the study without registration. Animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in Ophthalmology and Vision Research and approved by the Animal Ethical Committee at Zhongshan Ophthalmic Center of Sun Yat-sen University (Permit Number 2020-149).
Alkali injury-induced CNV
The vehicle control group and the melatonin-treated group of mice were intraperitoneally anesthetized with 1.5% pelltobarbitalum natricum at a dose of 50 mg/kg body weight. Tetracaine was applied to the right eye of each mouse after anesthesia. Then, a 2 mm diameter filter paper saturated with 1 M NaOH was placed gently on the right eye for 40 seconds, and the eye was immediately rinsed with 20 mL PBS. After alkali injury, the corneal epithelium was removed using a swab. Mice in the vehicle control group and the melatonin-treated group were treated with 5 µL PBS and melatonin, respectively, followed by tobramycin ophthalmic ointment. The left eye of each mouse remained uninjured. Then, the mice were treated with 5 µL PBS or melatonin twice daily for either 7 or 14 days. During the experiment, if mice experienced corneal perforation, they were euthanized and replaced by new mice. At the indicated times (days 7 and 14), the mice were sacrificed, and their eyes were enucleated for additional evaluation.
Biomicroscopic examination
Slit lamp (ZEISS, Jena, Germany) examinations and in vivo photographs of corneas were performed on days 7 and 14 after alkali burn injury. Each group contained 6 eyes at each time point. The CNV area was quantified, as previously reported (15). On day 7, 0.5% fluorescein sodium salt was used to detect corneal epithelial defects. Each epithelial defect area was evaluated using ImageJ software (imagej.nih.gov/il/; National Institutes of Health, Bethesda, MD, USA). The percentage of epithelial defect area referred to the area of epithelial defect of each eye as compared to the area of the entire cornea.
Corneal whole mounts
The mice were sacrificed on days 7 and 14, and the right eyes were harvested and fixed for 30 minutes at 4 °C in 4% paraformaldehyde. The corneas were separated and blocked with 0.5% Triton X-100 in PBS for 20 minutes at −80 °C and then for 10 minutes at room temperature. Corneas were incubated overnight at 4 °C with goat anti-mouse CD31 (1:20; catalog no. AF3628; R&D Systems, Minneapolis, MN, USA), 2% bovine serum albumin (BSA), 5% normal donkey serum (NDS), and 2% Triton X-100 in PBS. After rinsing in PBS, the corneas were incubated with Alexa Fluor 555-conjugated donkey anti-goat IgG antibody (1:500; catalog no. A-21432; Thermo Fisher Scientific, Waltham, MA, USA) for 2 hours at room temperature. Under the microscope, the corneas were flat mounted on glass slides using mounting medium and coverslips after 3 washes in PBS. In the vehicle control group and the melatonin-treated group, 6 cornea flat mounts were detected at each time point, and in the normal group, a total of 6 corneas were examined. All the flat mounts were captured with a confocal microscope (Carl Zeiss LSM 880, Germany). The degree of CNV was expressed as a percentage of the corneal area by using the confocal microscope built-in software (ZEN 2.3 blue edition, Carl Zeiss Microscopy GmbH, Germany).
Histopathologic examination
At 7 days after alkali injury, the right eyes were harvested and fixed in 10% neutral buffered formalin overnight, then embedded in paraffin. Each eye was cut into 5 µm vertical sections through the corneal central area and stained with hematoxylin and eosin (H&E) according to standard techniques (31). Histopathologic analyses of corneal tissues were performed under a microscope (Carl Zeiss LSM 510, Germany) to detect inflammatory cells and central corneal thickness. The number of inflammatory cells was counted on 5 randomly chosen fields of 1 cornea at 200-fold magnification. Then, the numbers of inflammatory cells per square millimeter were calculated. The central corneal thickness was calculated as the average of 3 measurements in the central region of the cornea. In each group, 3 corneas were detected. The data were measured by 2 independent examiners blinded to the experimental procedures.
Immunohistochemical (IHC) analysis
On day 7 after corneal injury, right eyes were harvested and fixed in 4% paraformaldehyde for 2 hours. After rinsing in PBS, the eyes were embedded in optimal cutting temperature compound (OCT) and frozen at −80 °C. We prepared 8 µm-thick corneal sections and blocked them with 2% BSA −0.5% Triton for 1 hour at room temperature. For the detection of macrophages, the sections were incubated with anti-F4/80 antibody (1:200; catalog no. ab6640; Abcam, Cambridge, Cambridge, UK) overnight at 4 °C. Then, Alexa Fluor 488-conjugated donkey anti-rat lg G antibody (1:500; catalog no. A-21208; Thermo Fisher Scientific, USA) was used as the secondary antibody. The sections were further counterstained with 4', 6-diamidino-2-phenylindole (DAPI) and mounted. Images were captured with a confocal microscope (Carl Zeiss LSM 880, Germany) at 400-fold magnification. In each group, 6 corneas were detected, and the number of F4/80+ cells per square millimeter was calculated, as described above.
Murine peritoneal macrophage extraction and culture
The SPF, 8-week-old, male mice were sacrificed, and murine peritoneal macrophages were harvested, as described previously (32). Briefly, sterile PBS was injected in the peritoneal cavity and then the macrophage suspension was removed from the peritoneum. After centrifugation, the cells were resuspended in Roswell Park Memorial Institute (RPMI) 1,640 medium containing 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, and 100 IU/mL penicillin (Thermo Fisher Scientific, USA) and incubated in 6-well cell culture plates under 5% carbon dioxide (CO2) at 37 °C. After 2 hours, the medium was changed, and the non-adherent cells were discarded. Flow cytometry confirmed that more than 95% of cells were F4/80+ macrophages. Then, murine macrophages were pre-stimulated with lipopolysaccharide (LPS) (100 ng/mL) for 1 hour and treated with either 20 μM melatonin or PBS in the presence of LPS for 18 hours.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
The total RNA of the cornea samples (n=3 corneas per sample) and cultured cells were extracted using the RNA Purification Kit (Yishan Biotechnology, Shanghai, China) and miRNeasy Micro kit (Qiagen, Wolfsburg, Germany), respectively, according to the manufacturers’ instructions. An Evo M-MLV RT kit (Accurate Biology, Hunan, China) was used for reverse transcription into complementary DNA (cDNA). Subsequently, qRT-PCR was carried out with a SYBR Premix Pro Taq HS (Accurate Biology, Hunan, China). To determine the relative expression of the target gene, all target messenger RNAs (mRNAs) were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The qRT-PCR analysis was performed using the Light Cycler 480 Real-Time PCR System (Roche Molecular Systems, Inc., Pleasanton, CA, USA). The primer sequences used in this study are listed in Table 1.
Table 1
| Gene | Forward primer 5'-3' | Reverse primer 5'-3' |
|---|---|---|
| VEGF | GCACATAGAGAGAATGAGCTTCC | CTCCGCTCTGAACAAGGCT |
| MMP-9 | GCAGAGGCATACTTGTACCG | TGATGTTATGATGGTCCCACTTG |
| MCP-1 | TAAAAACCTGGATCGGAACCAAA | GCATTAGCTTCAGATTTACGGGT |
| NLRP3 | ATCAACAGGCGAGACCTCTG | GTCCTCCTGGCATACCATAGA |
| IL-1β | TTCAGGCAGGCAGTATCACTC | GAAGGTCCACGGGAAAGACAC |
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
qRT-PCR, quantitative real-time reverse transcription-polymerase chain reaction; VEGF, vascular endothelial growth factor; MMP-9, matrix metallopeptidase-9; MCP-1, monocyte chemoattractant protein-1; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western blotting
At 7 days after alkali injury, corneas of the right eyes were harvested and homogenized in a lysis buffer containing protease and phosphatase inhibitors (Keygen Biotech Co., Ltd., Jiangsu, China). After the total protein was isolated from the cornea samples (n=3 corneas per sample), the protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Shanghai, China). Equal amounts of protein samples were run on 8% polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Darmstadt, Germany). The PVDF membranes were blocked with 5% BSA in phosphate-buffered saline with tween (PBST) for 1 hour at room temperature and subsequently incubated with primary antibodies overnight at 4 °C. Primary antibodies in this study, including anti-vascular endothelial growth factors (VEGF; 1:1,000; catalog no. NB100-644; Novus Biologicals, Centennial, CO, USA), anti-nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 [NLRP3; 1:1,000; catalog no.15101; Cell Signaling Technology (CST), Beverly, MA, USA], β-actin (1:1,000; catalog no. 3700; CST, USA). Then, horseradish peroxidase (HRP)-conjugated secondary antibody (CST, USA) was diluted to 1:2,000 and added to the membranes for 1 hour at room temperature. The expression of target proteins was normalized to β-actin and then quantified using ImageJ software (33).
Cell counting kit-8 (CCK-8) assay
The cells were seeded at 1×105 cells/mL in a 96-well plate with 100 µL media in each well. Subsequently, the cells were treated with different concentrations of melatonin (0, 5, 10, 20, 40, 80, and 160 µM). After 24 hours, the cells were treated with 10 µL of CCK-8 (AbMole Bioscience, Houston, TX, USA) and further incubated for 1 hour at 37 °C. Cell viability was evaluated through the measurement of absorbance at 450 nm using a microplate reader (Thermo Fisher Scientific, USA).
Enzyme-linked immunosorbent assay (ELISA)
Murine macrophages were seeded onto 6-well plates and cultured with growth medium. After stimulation with LPS (100 ng/mL) for 1 hour, the cells were treated with 20 μM melatonin or PBS. After 24 hours, the supernatants were collected and detected with a mouse VEGF ELISA kit (MMV00; R&D Systems, USA) according to the manufacturer’s instructions.
Statistical analysis
The qRT-PCR and western blot analyses were repeated 3 times. Data were presented as mean ± standard error of mean (SEM). A one-way analysis of variance (ANOVA) was performed, followed by Bonferroni’s post-hoc test using Stata software (StataCorp LLC, College Station, TX, USA). Statistical significance was set at P<0.05.
Results
Topical administration of melatonin reduced CNV in the alkali injury model
To investigate the effect of melatonin on CNV, we applied different concentrations of melatonin solution (30, 50, or 70 µM) to alkali-burned corneas. In the control group, new blood vessels had sprouted in over half of the cornea on day 7 and reached the central area of the cornea on day 14 (Figure 1A). However, compared with the control group, the CNV was significantly decreased in a dose-dependent manner in the melatonin-treated group (Figure 1A,1B), and the most significant inhibitory effect was observed at a concentration of 70 µM. Thus, we selected 70 µM melatonin to treat corneas in the following study. Corneal whole-mounts were further stained with anti-CD31, a specific endothelial cell marker, to identify blood vessels. The CNV area was also markedly reduced in the melatonin-treated eyes on days 7 and 14 after an alkali injury (Figure 1C,1D).
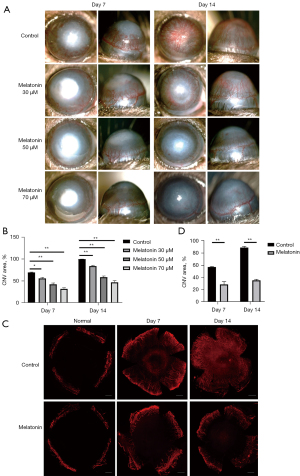
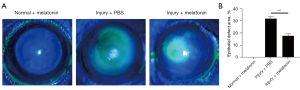
After an alkali burn, the rapid repair of corneal epithelium is of great importance in avoiding further damage to the cornea (4,34). Therefore, we next used fluorescein staining to detect the regeneration of the corneal epithelium. We observed that melatonin had no toxic effect on the epithelium and could promote epithelial wound healing on day 7 (Figure 2). These results demonstrated that melatonin could markedly attenuate CNV and epithelial defects area in alkali-burned corneas.
Melatonin inhibited the infiltration of inflammatory cells and macrophages in alkali-injured corneas
Corneal alkali burn can lead to the infiltration of inflammatory cells. Compared with other inflammatory cells, macrophages are known to play a key role in the development of CNV (4). To further understand the effect of melatonin in the process of CNV, we examined the infiltration of inflammatory cells and macrophages in the alkali-injured corneas using H&E staining and immunofluorescence staining, respectively. On day 7 after alkali burn injury, numerous inflammatory cells were observed in the corneas. However, the number of cells was significantly decreased in the corneas treated with melatonin (Figure 3A,3B). Also, the number of F4/80+ cells noticeably increased in the alkali-injured corneas but significantly decreased after melatonin treatment (Figure 3C,3D). These results suggest that melatonin inhibited the alkali-induced infiltration of macrophages and other inflammatory cells.
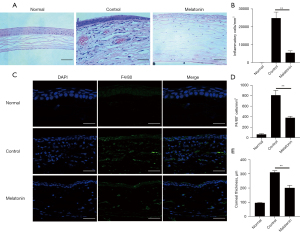
Further examination revealed that corneal thickness in the melatonin-treated corneas was significantly reduced compared to that in the control group (Figure 3E). This indicated that melatonin protected the eyes from visual loss due to corneal edema (1).
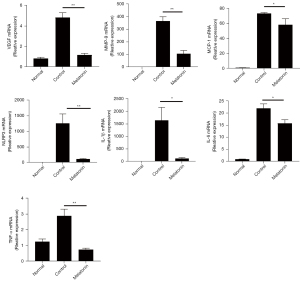
Melatonin reduced the expression of pro-angiogenic factors, chemokines, and inflammation-related molecules in alkali-burned corneas
The previous results have demonstrated that inflammation is of vital importance in alkali-induced CNV. Macrophages can be a rich source of numerous angiogenic factors, chemokines, and inflammatory cytokines, and the abnormal release of these factors can lead to angiogenetic processes (12,15). Therefore, we next examined the effect of melatonin on the expression of these factors in alkali-burned corneas. Pro-angiogenic factors, such as VEGF and matrix metalloproteinase (MMP)-9, were up-regulated in corneas after alkali injury. Melatonin treatment resulted in a significant decrease in the intracorneal mRNA expression of pro-angiogenic factors. Similar to the expression of pro-angiogenic genes, chemokine [monocyte chemotactic protein-1 (MCP-1)] and inflammation-related molecules [NLRP3, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)] were also significantly decreased after melatonin treatment in alkali-burned corneas (Figure 4).
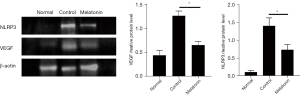
As VEGF and NLRP3 are important pro-angiogenic and inflammatory-related factors, respectively, we further confirmed the protein expression of these 2 factors. The results of the western blot analysis showed that VEGF and NLRP3 were up-regulated in the alkali-injured cornea and down-regulated after melatonin treatment (Figure 5). These data indicated that the topical application of melatonin inhibited angiogenesis and inflammation induced by corneal alkali injury.
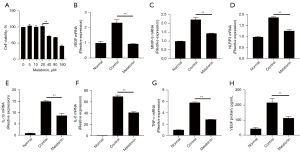
Melatonin inhibited pro-angiogenic factors and inflammation-related molecules in vitro in murine peritoneal macrophages
Our previous analysis revealed that melatonin could down-regulate the infiltration of macrophage and the expression of CNV-related genes in vivo. We next examined the effects of melatonin on pro-angiogenic and inflammation-related molecules in murine peritoneal macrophages. The result of the CCK-8 assay showed that melatonin did not affect cell viability at concentrations of 20 µM or lower (Figure 6A). When cells were treated with melatonin, the mRNA expression levels of LPS-induced angiogenic and inflammatory factors were significantly decreased (Figure 6B-6G). Furthermore, the results of ELISA also showed that melatonin inhibited the secretion of VEGF in vitro in murine peritoneal macrophages (Figure 6H). These data indicated that melatonin may attenuate CNV by down regulating the expression of pro-angiogenic and inflammatory factors in macrophages.
Discussion
The role of melatonin in controlling angiogenesis has been extensively studied under various conditions. In tumor tissue, melatonin has been shown to be effective in inhibiting angiogenesis by reducing VEGF, vascular endothelial growth factor receptor 2 (VEGFR2), and hypoxia-inducible factor 1 alpha (HIF-1α) expression (35). In the retina, melatonin and its metabolites play protective roles against retinal neovascularization by reducing the levels of VEGF, nitric oxide, and free radicals (23,36). Xu et al. demonstrated that melatonin attenuates choroidal neovascularization by regulating macrophage polarization (30). Moreover, multiple studies have shown that melatonin can markedly inhibit cell viability of human umbilical vein endothelial cells (HUVEC) and disrupt the process of cell migration, invasion, and tube formation (35,37,38). However, previous studies have not investigated the role of melatonin in corneal inflammatory neovascularization. In the present study, our results showed that the topical administration of melatonin is effective in inhibiting alkali-induced CNV (Figure 1).
In the early stages of corneal alkali injury, epithelial defects appear. To reduce the risk of corneal ulceration, perforation, CNV, and opacification, rapid re-epithelialization of the damaged area is extremely important (39). Previous studies have indicated that melatonin is involved in normal corneal growth (40) and can enhance the defense ability of corneal epithelial cells (41). Similarly, we found that topical administration of melatonin could accelerate corneal wound healing (Figure 2), which is consistent with previous studies (27). Other experiments have revealed the role of melatonin in promoting tear secretion (26), which can help create an environment conducive to corneal healing. These findings, as well as previous reports, indicate that melatonin has a protective effect on alkali corneal injury. Moreover, in our study, normal corneas that received melatonin for 7 days had no corneal epithelial defects (Figure 2), indicating the safety of melatonin for use in vivo.
Angiogenesis is an essential process in wound repair because it provides oxygen, nutrients, and immune cells to the damaged area. However, excessive and dysfunctional angiogenesis may cause detrimental effects on repair outcomes (9). The level of angiogenesis in wounds often correlates with the inflammatory response. Numerous mediators, including angiogenic molecules, chemokines, and inflammatory cytokines, have been reported to play a major role in inflammation-associated angiogenesis (15). The most bioactive endogenous proangiogenic factor identified thus far, VEGF, is also a chemoattractant for macrophages and stimulates pathologic positive inflammation feedback during CNV formation (9). The MMPs are also involved in the process of angiogenesis, which degrade the extracellular matrix and allow for endothelial cell differentiation, migration, and survival (23). Previous studies have shown that VEGF and MMP-9 gene silencing are both beneficial in terms of reducing CNV (31,42). Our experiments demonstrated that the topical application of melatonin markedly down-regulated the expression of VEGF and MMP-9 in alkali-burned corneas, suggesting that melatonin attenuates CNV by suppressing the expression of VEGF and MMP-9.
The NLRP3 inflammasome is the most extensively studied inflammasome, and it promotes the maturation of IL-1β and activates a more robust inflammatory response (43). As an intracellular sensor, NLRP3 can detect a broad range of stimuli, including pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) (44). In resting macrophages, the expression of NLRP3 is low and inadequate for initiating inflammasome activation. When cells are exposed to priming stimuli, the up-regulation of NLRP3 promotes inflammasome activation (45). Recently, mounting evidence has demonstrated that NLRP3 is involved in the pathogenesis of corneal diseases (43,46,47). In particular, the inhibition of NLRP3 improves wound healing and corneal clarity in an alkali burn model by reducing inflammation (46,48). The NLRP3 inflammasome is also considered as a target of melatonin. By inhibiting NLRP3, melatonin can reduce inflammation and affect various molecular pathways (49). Consistent with these studies, we found that melatonin reduced the expression of NLRP3 and other proinflammatory molecules, suggesting that the anti-angiogenic effects of melatonin also involve non-VEGF-dependent mechanisms.
Currently, the exact mechanism of CNV is unclear, but it is generally believed that the development of inflammatory corneal angiogenesis is directly related to macrophages. In this study, we observed that the number of F4/80+ cells in alkali-burned corneas were markedly reduced after melatonin treatment (Figure 3). Additionally, as a chemoattractant molecule for macrophages, MCP-1 was also decreased in melatonin-treated corneas. Previous studies have revealed that the production of pro-angiogenic factors (VEGF and MMP-9), NLRP3, and pro-inflammatory factors (IL-1β, IL-6, and TNF-α) are mainly associated with macrophages (4,11,50). To further understand the role of melatonin in macrophage function, we used murine peritoneal macrophages. Similar to the expression in alkali-injured corneas, melatonin was able to reduce the expression of VEGF, MMP-9, NLRP3, IL-1β, IL-6, and TNF-α in macrophages (Figure 6), which is in accordance with previous studies (51). However, further studies are needed to explore the mechanism via which melatonin modulates cell activity and regulates the release of cytokines and growth factors. In addition, the diversity and plasticity of macrophages make it possible for them to polarize into distinct functional phenotypes according to the micro-environment. It is worth pursuing further study of the effect of melatonin on macrophage polarization in corneal alkali burn model.
Interestingly, some other studies have suggested that melatonin could have an opposite effect on angiogenesis. In gastric ulcers, skin lesions, and some physiologic processes, melatonin promotes neovascularization. Ganguly et al. reported that implanting melatonin in normal rat corneal micropockets could increase blood vessels (52). This seems to contradict our results. However, melatonin exerts different functions (for example, it stimulates or inhibits neovascularization) according to specific pathophysiological processes and conditions (23). Specifically, corneal alkali burn injuries and corneal micropockets have totally different mechanisms in terms of blood vessel formation. In contrast to an alkali injury model, a corneal micropocket is an incisional wound that often heals without CNV (5). Furthermore, the corneal alkali injury model is a well-known model of inflammation-induced CNV, while the corneal micropocket assay is not affected by the inflammatory response (50). Collectively, our findings may support a mechanism via which melatonin directly inhibits the inflammatory response and thus results in the reduction of CNV.
In conclusion, we found that melatonin has efficacy in suppressing angiogenesis in alkali-injured corneas. The effect of melatonin is mediated, at least partially, through inhibiting macrophage infiltration and the secretion of proangiogenic factors and inflammatory molecules. These results suggest that melatonin may be useful in the prevention and treatment of inflammatory CNV.
Acknowledgments
We thank the staffs of Core Facilities at the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center for their technical support.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81371051).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4927/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4927/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4927/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2020-149) granted by the Animal Ethical Committee at Zhongshan Ophthalmic Center of Sun Yat-sen University, in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in Ophthalmology and Vision Research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun MM, Chan AM, Law SM, et al. Epithelial membrane protein-2 (EMP2) antibody blockade reduces corneal neovascularization in an in vivo model. Invest Ophthalmol Vis Sci 2019;60:245-54. [Crossref] [PubMed]
- Wan SS, Pan YM, Yang WJ, et al. Inhibition of EZH2 alleviates angiogenesis in a model of corneal neovascularization by blocking FoxO3a-mediated oxidative stress. FASEB J 2020;34:10168-81. [Crossref] [PubMed]
- Lu P, Li L, Liu G, et al. Enhanced experimental corneal neovascularization along with aberrant angiogenic factor expression in the absence of IL-1 receptor antagonist. Invest Ophthalmol Vis Sci 2009;50:4761-8. [Crossref] [PubMed]
- Kim JW, Jeong H, Yang MS, et al. Therapeutic effects of zerumbone in an alkali-burned corneal wound healing model. Int Immunopharmacol 2017;48:126-34. [Crossref] [PubMed]
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:264-302.
- Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and emerging therapies for corneal neovascularization. Ocul Surf 2018;16:398-414. [Crossref] [PubMed]
- Vivanco-Rojas O, García-Bermúdez MY, Iturriaga-Goyon E, et al. Corneal neovascularization is inhibited with nucleolin-binding aptamer, AS1411. Exp Eye Res 2020;193:107977. [Crossref] [PubMed]
- Nicholas MP, Mysore N. Corneal neovascularization. Exp Eye Res 2021;202:108363. [Crossref] [PubMed]
- DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol 2016;100:979-84. [Crossref] [PubMed]
- Lennikov A, Mirabelli P, Mukwaya A, et al. Selective IKK2 inhibitor IMD0354 disrupts NF-κB signaling to suppress corneal inflammation and angiogenesis. Angiogenesis 2018;21:267-285. [Crossref] [PubMed]
- Wolf M, Clay SM, Zheng S, et al. MMP12 inhibits corneal neovascularization and inflammation through regulation of CCL2. Sci Rep 2019;9:11579. [Crossref] [PubMed]
- Lu P, Li L, Kuno K, et al. Protective roles of the fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal neovascularization through enhanced antiangiogenic factor expression. J Immunol 2008;180:4283-91. [Crossref] [PubMed]
- Ueta T, Ishihara K, Notomi S, et al. RIP1 kinase mediates angiogenesis by modulating macrophages in experimental neovascularization. Proc Natl Acad Sci U S A 2019;116:23705-13. [Crossref] [PubMed]
- Lu P, Li L, Mukaida N, et al. Alkali-induced corneal neovascularization is independent of CXCR2-mediated neutrophil infiltration. Cornea 2007;26:199-206. [Crossref] [PubMed]
- Li Z, Chen J, Lei L, et al. Laquinimod inhibits inflammation-induced angiogenesis in the cornea. Front Med (Lausanne) 2020;7:598056. [Crossref] [PubMed]
- Cipolla-Neto J, Amaral FGD. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev 2018;39:990-1028. [Crossref] [PubMed]
- Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther 2007;113:507-22. [Crossref] [PubMed]
- Slomski A. Melatonin improves sleep in patients with circadian disruption. JAMA 2018;320:749. [Crossref] [PubMed]
- Luo J, Zhang Z, Sun H, et al. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci 2020;242:117191. [Crossref] [PubMed]
- Zhang J, Jiang H, Du K, et al. Pan-cancer analyses reveal genomics and clinical characteristics of the melatonergic regulators in cancer. J Pineal Res 2021;71:e12758. [Crossref] [PubMed]
- Wang B, Li J, Bao M, et al. Melatonin attenuates diabetic myocardial microvascular injury through activating the AMPK/SIRT1 signaling pathway. Oxid Med Cell Longev 2021;2021:8882130. [Crossref] [PubMed]
- González A, Alonso-González C, González-González A, et al. Melatonin as an adjuvant to antiangiogenic cancer treatments. Cancers (Basel) 2021;13:3263. [Crossref] [PubMed]
- Ma Q, Reiter RJ, Chen Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020;23:91-104. [Crossref] [PubMed]
- Zhang D, Xu S, Wang Y, et al. The potentials of melatonin in the prevention and treatment of bacterial meningitis disease. Molecules 2021;26:1419. [Crossref] [PubMed]
- Soni JM, Sardoiwala MN, Choudhury SR, et al. Melatonin-loaded chitosan nanoparticles endows nitric oxide synthase 2 mediated anti-inflammatory activity in inflammatory bowel disease model. Mater Sci Eng C Mater Biol Appl 2021;124:112038. [Crossref] [PubMed]
- Hoyle CH, Peral A, Pintor J. Melatonin potentiates tear secretion induced by diadenosine tetraphosphate in the rabbit. Eur J Pharmacol 2006;552:159-61. [Crossref] [PubMed]
- Crooke A, Guzman-Aranguez A, Mediero A, et al. Effect of melatonin and analogues on corneal wound healing: involvement of Mt2 melatonin receptor. Curr Eye Res 2015;40:56-65. [Crossref] [PubMed]
- Lundmark PO, Pandi-Perumal SR, Srinivasan V, et al. Role of melatonin in the eye and ocular dysfunctions. Vis Neurosci 2006;23:853-62. [Crossref] [PubMed]
- Lledó VE, Alkozi HA, Sánchez-Naves J, et al. Modulation of aqueous humor melatonin levels by yellow-filter and its protective effect on lens. J Photochem Photobiol B 2021;221:112248. [Crossref] [PubMed]
- Xu Y, Cui K, Li J, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J Pineal Res 2020;69:e12660. [Crossref] [PubMed]
- Fu YC, Xin ZM. Inhibited corneal neovascularization in rabbits following corneal alkali burn by double-target interference for VEGF and HIF-1α. Biosci Rep 2019;39:BSR20180552. [Crossref] [PubMed]
- Rios FJ, Touyz RM, Montezano AC. Isolation and Differentiation of Murine Macrophages. Methods Mol Biol 2017;1527:297-309. [Crossref] [PubMed]
- Wan P, Su W, Zhang Y, et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ 2020;27:176-91. [Crossref] [PubMed]
- Chen M, Matsuda H, Wang L, et al. Pretranscriptional regulation of Tgf-beta1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol Ther 2010;18:519-27. [Crossref] [PubMed]
- Talib WH. Melatonin and cancer hallmarks. Molecules 2018;23:518. [Crossref] [PubMed]
- Crooke A, Huete-Toral F, Martínez-Águila A, et al. Ocular disorders and the utility of animal models in the discovery of melatoninergic drugs with therapeutic potential. Expert Opin Drug Discov 2012;7:989-1001. [Crossref] [PubMed]
- Alvarez-García V, González A, Alonso-González C, et al. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc Res 2013;87:25-33. [Crossref] [PubMed]
- Cheng J, Yang HL, Gu CJ, et al. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int J Mol Med 2019;43:945-55. [PubMed]
- Wirostko B, Rafii M, Sullivan DA, et al. Novel therapy to treat corneal epithelial defects: a hypothesis with growth hormone. Ocul Surf 2015;13:204-212.e1. [Crossref] [PubMed]
- Wahl C, Li T, Takagi Y, et al. The effects of light regimes and hormones on corneal growth in vivo and in organ culture. J Anat 2011;219:766-75. [Crossref] [PubMed]
- Ciuffi M, Pisanello M, Pagliai G, et al. Antioxidant protection in cultured corneal cells and whole corneas submitted to UV-B exposure. J Photochem Photobiol B 2003;71:59-68. [Crossref] [PubMed]
- Torrecilla J, Gómez-Aguado I, Vicente-Pascual M, et al. MMP-9 downregulation with lipid nanoparticles for inhibiting corneal neovascularization by gene silencing. Nanomaterials (Basel) 2019;9:631. [Crossref] [PubMed]
- Wei C, Ma L, Chi H, et al. The NLRP3 inflammasome regulates corneal allograft rejection through enhanced phosphorylation of STAT3. Am J Transplant 2020;20:3354-66. [Crossref] [PubMed]
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 2019;20:3328. [Crossref] [PubMed]
- Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009;183:787-91. [Crossref] [PubMed]
- Shimizu H, Sakimoto T, Yamagami S. Pro-inflammatory role of NLRP3 inflammasome in experimental sterile corneal inflammation. Sci Rep 2019;9:9596. [Crossref] [PubMed]
- Dai Y, Zhang J, Xiang J, et al. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol 2019;21:101093. [Crossref] [PubMed]
- Bian F, Xiao Y, Zaheer M, et al. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int J Mol Sci 2017;18:562. [Crossref] [PubMed]
- Ashrafizadeh M, Najafi M, Kavyiani N, et al. Anti-inflammatory activity of melatonin: a focus on the role of NLRP3 inflammasome. Inflammation 2021;44:1207-22. [Crossref] [PubMed]
- D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 1994;91:4082-5. [Crossref] [PubMed]
- Xia Y, Chen S, Zeng S, et al. Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res 2019;66:e12547. [Crossref] [PubMed]
- Ganguly K, Sharma AV, Reiter RJ, et al. Melatonin promotes angiogenesis during protection and healing of indomethacin-induced gastric ulcer: role of matrix metaloproteinase-2. J Pineal Res 2010;49:130-40. [Crossref] [PubMed]
(English Language Editors: K. Gilbert and J. Jones)


