
The preoperative hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for non-small cell lung cancer patients undergoing adjuvant chemotherapy: a retrospective study
Introduction
Lung cancer is the leading cause of cancer deaths worldwide, with an estimated 1.8 million deaths annually (1). Statistically, approximately 85% of lung cancer patients exhibit a group of histological subtypes, collectively known as non-small cell lung cancer (NSCLC) (2). Adjuvant chemotherapy produces a substantial positive survival rate for resectable NSCLC patients (3); however, there are considerable differences in the survival rates of NSCLC patients who undergo adjuvant chemotherapy at the same stage. Although numerous clinicopathological parameters, such as tumor size (4), lymph node metastasis (LNMets) (5), margin status (6), and tumor node metastasis (TNM) system (7), can generally predict prognosis, simpler, more reliable, and lower-cost biomarkers are essential to forecast the prognosis of NSCLC patients receiving adjuvant chemotherapy.
Studies have demonstrated that a series of hematological indicators reflecting the inflammation or nutritional state of the body, including albumin (8), hemoglobin (9), and lymphocytes (10), are related to NSCLC prognosis. However, the disadvantage of these single indicators is that each indicator reflects only one aspect of inflammation or nutrition. Previous reports have confirmed that the combination of these hematological indicators can forecast the prognosis of patients more accurately than any single indicator. For example, predictors related to inflammation or nutrition, including the platelet to lymphocyte ratio (PLR) and the neutrophil to lymphocyte ratio (NLR), have been applied to predict multiple cancer types, including NSCLC (11-13). Furthermore, programmed cell death-ligand 1 (PD-L1) expression and tumor mutation burden (TMB) have been reported as popular biomarkers for NSCLC prognosis in recent years (14). However, these biomarkers are expensive and technically complex, making it difficult to be applied universally in clinical practice. Recently, the score combining hemoglobin and albumin levels and lymphocyte and platelet counts (HALP score) has been confirmed to be closely related to the prognosis of gastric carcinoma (15), esophageal cancer (16), pancreatic cancer (17), and colorectal cancer (18). However, researches focusing on the prognostic role of the HALP score in NSCLC patients is lacking.
This study primarily aimed to investigate the prognostic role of the HALP score in patients with NSCLC undergoing adjuvant chemotherapy. Furthermore, we also sought to build a prognostic model for NSCLC patients undergoing adjuvant chemotherapy. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1097/rc).
Methods
Study population
A retrospective design, consecutive, hospital-based study was conducted. We enrolled patients who received adjuvant chemotherapy at the Affiliated Tumor Hospital of Nantong University from June 2013 to October 2015. Patients who met the following criteria were included: (I) diagnosis of NSCLC and receipt of adjuvant chemotherapy; (II) no upper respiratory tract infection 30 days before surgery; (III) routine plasma tests for lymphocyte, monocyte, hemoglobin, albumin, and platelet levels before surgery; and (IV) sufficient clinical, pathological, and follow-up indicators. Individuals were excluded if they had blood diseases, autoimmune diseases, persistent uncooperative respiratory diseases, or heart disease. Ultimately, 362 NSCLC patients undergoing adjuvant chemotherapy were included. Procedures involving human subjects in the current research were approved by the Institutional Review Board of the Affiliated Tumor Hospital of Nantong University (No. LW2021003), and all participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection and follow-up
Demographic characteristic, including age, sex, smoking status, and drinking history, were collected from case report forms at admission. Standard blood samples and related clinicopathological features, including pathologic stage, tumor size, LNMets, monocyte, and HALP scores, were collected through the hospital’s medical record management system. The HALP score was computed according to the following formula: hemoglobin (g/L) × albumin (g/L) × lymphocytes (g/L)/platelets (g/L). Serum biochemical parameters were analyzed using an autoanalyzer and automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA).
Overall survival (OS) was defined as the time to death from any cause, and disease-free survival (DFS) was defined as the time to the first NSCLC recurrence or progression, or death from any cause. Patient follow-ups were conducted primarily through outpatient review, readmission records, and mobile phone conferences employed to obtain feedback according to the routine procedure in our institution. The last follow-up was conducted on October 10, 2020.
Statistical analysis
Categorical variables were presented as frequency (n) and percentages (%), and were compared using the Chi-square test. The optimal HALP score cutoff values were determined using X-tile software version 3.6.1 (Yale University). To assess the associations between the HALP score and OS/DFS outcomes, hazard ratios (HRs) with 95% confidence intervals (CIs) were assessed by using univariate and multivariate Cox regression analysis. In multivariate Cox regression model, we adjusted age and covariates that were significant in univariate analysis, including sex, pathological stage, tumor size, and LNMets. The Kaplan-Meier method was also used to assess OS and DFS outcomes, and the log-rank test was used to identify significant differences. Data analysis was conducted through SPSS version 21.0 software (Chicago, IL, USA), R version 3.3.2, and GraphPad Prism version 8.0. A two-sided P<0.05 was considered to be statistically significant.
Results
Baseline characteristics
A total of 362 patients with NSCLC undergoing adjuvant chemotherapy were included in our analysis (Table 1). Among this study population, 217 (59.94%) patients were men, and 158 (43.65%) were aged ≥65 years. Patients with a low HALP score were more likely to have a smaller tumor compared to those with a high HALP score (P=0.009). The median follow-up period was 64 months, during which 135 (37.29%) patients died.
Table 1
| Variables | Total patients, n (%) | HALP low, n (%) | HALP high, n (%) | P value |
|---|---|---|---|---|
| Age (years) | 0.208 | |||
| <65 | 204 (56.35) | 77 (52.38) | 127 (59.07) | |
| ≥65 | 158 (43.65) | 70 (47.62) | 88 (40.93) | |
| Gender | 0.847 | |||
| Female | 145 (40.05) | 58 (39.46) | 87 (40.47) | |
| Male | 217 (59.94) | 89 (60.54) | 128 (59.53) | |
| Smoking history | 0.298 | |||
| No | 221 (61.05) | 85 (57.82) | 136 (63.26) | |
| Yes | 141 (38.95) | 62 (42.18) | 79 (36.74) | |
| Drinking history | 0.245 | |||
| No | 289 (79.83) | 113 (76.87) | 176 (81.86) | |
| Yes | 73 (20.17) | 34 (23.13) | 39 (18.14) | |
| Pathologic stage | 0.177 | |||
| I–II | 272 (75.14) | 105 (71.43) | 167 (77.67) | |
| III–IV | 90 (24.86) | 42 (28.57) | 48 (22.33) | |
| Tumor size (cm) | 0.009 | |||
| <3 | 145 (40.06) | 47 (31.97) | 98 (45.58) | |
| ≥3 | 217 (59.94) | 100 (68.03) | 117 (54.42) | |
| LNMets | 0.342 | |||
| No | 220 (60.77) | 85 (57.82) | 135 (62.79) | |
| Yes | 142 (39.23) | 62 (42.18) | 80 (37.21) |
HALP, hemoglobin and albumin levels and lymphocyte and platelet counts; LNMets, lymph node metastasis.
Association between HALP score and OS/DFS
We conducted univariate Cox regression analysis to evaluate the potential associations between clinicopathological features and OS/DFS, and found the significant associations with sex, pathological stage, tumor size, and LNMets (Tables 2,3). According to the results of multivariate analysis, individuals with a high HALP score were associated with lower OS (HR: 0.707; 95% CI: 0.503–0.995) and DFS (HR: 0.671; 95% CI: 0.491–0.916) (Tables 2,3). The Kaplan-Meier analysis showed that a low HALP score predicted poorer OS (P=0.02) and DFS (P<0.01) outcomes (Figure 1A,1B).
Table 2
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| <65 | Reference | Reference | |||
| ≥65 | 1.039 (0.740, 1.459) | 0.827 | 1.162 (0.819, 1.648) | 0.399 | |
| Gender | |||||
| Female | Reference | Reference | |||
| Male | 1.397 (0.980, 1.991) | 0.065 | 1.428 (0.999, 2.040) | 0.051 | |
| Smoking history | |||||
| No | Reference | ||||
| Yes | 1.210 (0.859, 1.704) | 0.276 | |||
| Drinking history | |||||
| No | Reference | ||||
| Yes | 1.002 (0.653, 1.537) | 0.994 | |||
| Pathologic stage | |||||
| I–II | Reference | Reference | |||
| III–IV | 2.690 (1.906, 3.796) | <0.001 | 1.741 (1.105, 2.742) | 0.017 | |
| Tumor size | |||||
| <3 | Reference | Reference | |||
| ≥3 | 1.951 (1.345, 2.832) | <0.001 | 1.319 (0.886, 1.963) | 0.172 | |
| LNMets | |||||
| No | Reference | Reference | |||
| Yes | 2.555 (1.816, 3.595) | <0.001 | 1.793 (1.153, 2.789) | 0.010 | |
| HALP | |||||
| Low | Reference | Reference | |||
| High | 0.672 (0.479, 0.942) | 0.021 | 0.707 (0.503, 0.995) | 0.048 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; LNMets, lymph node metastasis; HALP, hemoglobin and albumin levels and lymphocyte and platelet counts.
Table 3
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| <65 | Reference | Reference | |||
| ≥65 | 0.888 (0.654, 1.205) | 0.444 | 1.045 (0.764, 1.430) | 0.781 | |
| Gender | |||||
| Female | Reference | Reference | |||
| Male | 1.558 (1.133, 2.143) | 0.006 | 1.680 (1.219, 2.316) | 0.002 | |
| Smoking history | |||||
| No | Reference | ||||
| Yes | 1.329 (0.980, 1.802) | 0.067 | |||
| Drinking history | |||||
| No | Reference | ||||
| Yes | 1.152 (0.794, 1.671) | 0.456 | |||
| Pathologic stage | |||||
| I–II | Reference | Reference | |||
| III–IV | 3.114 (2.290, 4.233) | <0.001 | 1.798 (1.210, 2.673) | 0.004 | |
| Tumor size | |||||
| <3 | Reference | Reference | |||
| ≥3 | 2.152 (1.537, 3.013) | <0.001 | 1.394 (0.975, 1.992) | 0.068 | |
| LNMets | |||||
| No | Reference | Reference | |||
| Yes | 3.088 (2.272, 4.198) | <0.001 | 2.125 (1.444, 3.127) | <0.001 | |
| HALP | |||||
| Low | Reference | Reference | |||
| High | 0.648 (0.475, 0.882) | 0.006 | 0.671 (0.491, 0.916) | 0.012 | |
DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; LNMets, lymph node metastasis; HALP, hemoglobin and albumin levels and lymphocyte and platelet counts.

Stratification analysis results
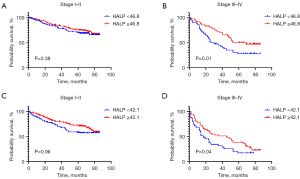
We performed stratification analysis by TNM stage, and the result indicated no significant difference in OS (P=0.38) or DFS (P=0.06) among patients with low and high HALP scores in stage I–II NSCLC (Figure 2A,2B). In contrast, a low HALP score predicted poor OS (P=0.01) and DFS (P=0.04) outcomes in stage III–IV NSCLC patients (Figure 2C,2D).
Discussion
Lung cancer is the leading cause of cancer-related mortality, accounting for approximately 19% of all cancer-related deaths, and results in a substantial disease burden (19). NSCLC accounts for the majority of all lung cancers and usually presents as an advanced metastatic disease. Despite therapeutic progress, the prognosis of NSCLC patients is still poor (12,20). Given the high cost of NSCLC treatment, the identification of reliable predictive biomarkers is crucial to predict treatment outcomes and identify patients who are most likely to benefit from therapy. Our study assessed the prognostic role of the HALP score in NSCLC patients undergoing adjuvant chemotherapy. Our results demonstrated that the HALP score could effectively predict poor OS and DFS outcomes. Furthermore, stratification analysis indicated that the HALP score could accurately predict OS and DFS outcomes for stage III–IV NSCLC patients.
The HALP score is a comprehensive index used to measure the nutritional and immune health state of patients. Studies have generally indicated that immune reaction and overall nutritional state are associated with the survival of cancer patients (21-23). It is well known that albumin and hemoglobin levels and lymphocyte and platelet counts are common clinical biomarkers. Since cancer is a chronic consumption disease with advanced tumors, patients’ hemoglobin levels are significantly correlated with their survival rates and tumor progression (24,25). Preoperative anemia or anemia is substantially associated with tumor recurrence and cancer-specific mortality (9,26). Using serum albumin as an indicator of nutritional status can be employed to assess the survival rate of patients with cancer. Djaladat et al. reported that lower blood albumin was independently associated with carcinoma recurrence and poor OS after radical cystectomy in bladder cancer patients (27). Liu et al. reported that a high serum albumin was exhibited a 45% reduced risk of death in patients with non-metastatic breast cancer (28).
A large number of studies have shown that an inflammatory microenvironment is a vital component of carcinogenesis. Lymphocytes and platelets, which are fundamental components of the systemic inflammatory response, are associated with persistent inflammation of the tumor microenvironment (14,29). For instance, lymphocytes play an important role in the anti-tumor immune response by inhibiting tumor cell growth (30). As an independent prognostic factor of OS and DFS in cancer patients, lymphopenia is more common in advanced cancer patients (10,31). Lymphocytes can release a series of cytokines and are an important part of anti-tumor immunity. Platelets play a role in regulating the tumor microenvironment by releasing factors that promote tumor growth, invasion, and angiogenesis (32). Tumor cells can an escape the recognition damage of the immune system by activating platelets and combining with platelets to form tumor thrombi. These studies have revealed that serum albumin, hemoglobin, and lymphocytes can be classified as advantage factors for the prognosis of NSCLC, and platelets may be a disadvantage factor (10,14,29-32).
The HALP score has a prognostic effect on a variety of gastrointestinal and urinary malignancies, including esophageal cancer (33), colorectal cancer (18,34), gastric cancer (15), and renal cell carcinoma (35). However, the current epidemiologic evidence for the prognostic role of the HALP score for NSCLC patients is limited. Our recent study of 238 patients demonstrated that the HALP score was independent predictor for NSCLC after radical surgery (36). Based on the above findings, we expanded the current study’s sample size and confirmed that the HALP score is an independent prognostic factor for NSCLC patients undergoing adjuvant chemotherapy. An improved HALP score can effectively predict the OS and DFS outcomes of NSCLC patients.
The present study had several limitations that should be noted. Firstly, since this was a retrospective study and the research data were collected from a single center, selection and information bias could not be excluded. Secondly, the limited sample sizes might have yielded statistical bias. Thirdly, stratification analysis indicated no significant difference in OS or DFS among patients with low and high HALP scores in stage I–II NSCLC. Therefore, the accuracy of the HALP score in predicting prognosis in early-stage NSCLC must be further verified. Nonetheless, the current study indicated that the HALP score is a valuable prognostic index for advanced NSCLC (stage III–IV). Additional high-quality, prospective, multicenter studies with large sample sizes are required to confirm these finding.
Conclusions
Our study demonstrated that the HALP score might be a suitable prognostic index for OS and DFS outcomes for NSCLC patients undergoing adjuvant chemotherapy. Given that the HALP score is a convenient, easily obtained, and low-cost biomarker, combining demographic and clinicopathological features with the HALP score may help clinicians predict survival and treatment outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1097/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1097/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1097/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) Procedures involving human subjects in the current research were approved by the Institutional Review Board of the Affiliated Tumor Hospital of Nantong University (No. LW2021003), and all participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Solomon B, Bunn PA Jr. Adjuvant chemotherapy for non-small cell lung cancer. Cancer Invest 2007;25:217-25. [Crossref] [PubMed]
- Bureau M, Chatellier T, Perennec T, et al. Baseline tumour size is an independent prognostic factor for overall survival in PD-L1 ≥50% non-small cell lung cancer patients treated with first-line pembrolizumab. Cancer Immunol Immunother. 2021; Epub ahead of print. [Crossref] [PubMed]
- Li ZM, Ding ZP, Luo QQ, et al. Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 2013;144:1253-60. [Crossref] [PubMed]
- Predina JD, Keating J, Patel N, et al. Clinical implications of positive margins following non-small cell lung cancer surgery. J Surg Oncol 2016;113:264-9. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Wu Y, Wu H, Lin M, et al. Factors associated with immunotherapy respond and survival in advanced non-small cell lung cancer patients. Transl Oncol 2022;15:101268. [Crossref] [PubMed]
- Chen C, Song Z, Wang W, et al. Baseline anemia and anemia grade are independent prognostic factors for stage IV non-small cell lung cancer. Mol Clin Oncol 2021;14:59. [Crossref] [PubMed]
- Bryant AK, Sankar K, Strohbehn GW, et al. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer 2022;163:35-41. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886-94. [Crossref] [PubMed]
- Kang J, Chang Y, Ahn J, et al. Neutrophil-to-lymphocyte ratio and risk of lung cancer mortality in a low-risk population: A cohort study. Int J Cancer 2019;145:3267-75. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1‒Positive, Advanced Non‒Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol 2020;38:1580-90. [Crossref] [PubMed]
- Chen XL, Xue L, Wang W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget 2015;6:41370-82. [Crossref] [PubMed]
- Feng JF, Wang L, Yang X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J Basic Med Sci 2021;21:773-81. [Crossref] [PubMed]
- Xu SS, Li S, Xu HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol 2020;26:828-38. [Crossref] [PubMed]
- Yalav O, Topal U, Unal AG, et al. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir 2021;92:283-92. [PubMed]
- Cheng TY, Cramb SM, Baade PD, et al. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol 2016;11:1653-71. [Crossref] [PubMed]
- Moro-Sibilot D, Smit E, de Castro Carpeño J, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer 2015;88:215-22. [Crossref] [PubMed]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223-6. [Crossref] [PubMed]
- Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243-74. [Crossref] [PubMed]
- Ryan AM, Power DG, Daly L, et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 2016;75:199-211. [Crossref] [PubMed]
- Belcher DA, Ju JA, Baek JH, et al. The quaternary state of polymerized human hemoglobin regulates oxygenation of breast cancer solid tumors: A theoretical and experimental study. PLoS One 2018;13:e0191275. [Crossref] [PubMed]
- Guo Y, Shi D, Zhang J, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score is a Novel Significant Prognostic Factor for Patients with Metastatic Prostate Cancer Undergoing Cytoreductive Radical Prostatectomy. J Cancer 2019;10:81-91. [Crossref] [PubMed]
- Jo JK, Jeong SJ, Hong SK, et al. The impact of preoperative anemia on oncologic outcome in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Int Urol Nephrol 2016;48:489-94. [Crossref] [PubMed]
- Djaladat H, Bruins HM, Miranda G, et al. The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int 2014;113:887-93. [Crossref] [PubMed]
- Liu X, Meng QH, Ye Y, et al. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis 2015;36:243-8. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Lohinai Z, Bonanno L, Aksarin A, et al. Neutrophil-lymphocyte ratio is prognostic in early stage resected small-cell lung cancer. PeerJ 2019;7:e7232. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Contursi A, Grande R, Dovizio M, et al. Platelets in cancer development and diagnosis. Biochem Soc Trans 2018;46:1517-27. [Crossref] [PubMed]
- Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol 2017;46:75-9. [Crossref] [PubMed]
- Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget 2016;7:72076-83. [Crossref] [PubMed]
- Peng D, Zhang CJ, Tang Q, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol 2018;18:20. [Crossref] [PubMed]
- Zhai B, Chen J, Wu J, et al. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann Transl Med 2021;9:976. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


